High-resolution manometry (HRM) is a diagnostic tool for surgeons, gastroenterologists and other healthcare professionals to evaluate esophageal physiology. The Chicago Classification (CC) system is based on a consensus of worldwide experts to minimize ambiguity in HRM data acquisition and diagnosis of esophageal motility disorders. The most updated version, CCv4.0, was published in 2021; however, it does not provide step-by-step guidelines (i.e., for beginners) on how to assess the most important HRM metrics. This paper aims to summarize the basic guidelines for conducting a high-quality HRM study including data acquisition and interpretation, based on CCv4.0, using Manoview ESO analysis software, version 3.3 (Medtronic, Minneapolis, MN).
La manometría esofágica de alta resolución (MAR) es una herramienta de diagnóstico que permite a los cirujanos, gastroenterólogos y otros profesionales de la salud evaluar la fisiología esofágica en tiempo real. El sistema de Clasificación de Chicago (CC) está basado en el consenso global de expertos, y tiene por objetivo minimizar la ambigüedad en la adquisición de datos de la MAR y el diagnóstico de trastornos de la motilidad esofágica. La versión más actualizada, CCv4.0, se publicó en 2021; sin embargo, esta actualización no incluye recomendaciones paso a paso sobre cómo analizar e interpretar las métricas más importantes obtenidas a partir de la MAR. Este artículo tiene como objetivo proveer las pautas necesarias para que principiantes en el tema puedan realizar una MAR de alta calidad, así como adquirir e interpretar los datos obtenidos usando la CCv4.0 y el software de análisis ManoView® ESO, versión 3.3 (Medtronic, Minneapolis, MN, EE.UU.).
High-resolution manometry (HRM) is the current gold standard for evaluating esophageal motility. It was first conceptualized by Ray Clause in 1990 as a replacement for conventional esophageal manometry, and its clinical introduction occurred during the early 2000s.1 However, it was not until 2008 that it began to be recognized as a diagnostic tool after its predictive clinical value in patients with achalasia was demonstrated.2 Subsequently, its use was introduced in the evaluation of peristalsis and gastroesophageal junction outflow obstruction (EGJOO) disorders, functional dysphagia, and non-cardiac chest pain as well as to assess esophageal peristalsis before antireflux surgery.3
The benchmark for clinical use of HRM was the incorporation and progressive advancements in pressure sensor technology and the computational capabilities of software systems that facilitated the real-time plotting of spatiotemporal pressure topography.4 Esophageal pressure topography (EPT) has allowed for a better understanding of esophageal motility disorders; however, this critical development also brought with it the need to standardize the interpretation of these motility patterns, which led to the Chicago Classification (CC).1
Although CC was initially introduced in 2007, it was not until 2008 that CCv1.0 was published.5 The most recent version of CC was updated in 2021 (CCv4.0), and it serves as the most commonly used guideline for classifying esophageal motility disorders.6 CCv4.0 provides recommendations for the standardization of HRM study protocols, diagnostic thresholds, HRM metrics definitions, use of additional testing and provocative maneuvers, and finally, an update in the classification of esophagogastric junction (EGJ) disorders, peristalsis disorders, and EGJ morphology.6
HRM systemsTo date, there are two types of HRM systems available on the market (provided by multiple vendors). The first is a 22-, 24-, or 36-channel water-perfusion system (WPS), and the second is a solid-state manometry (SSM) system incorporating 36 channels (sensors). The WPS is comparatively less expensive, but it has some drawbacks. It requires tedious equipment maintenance, has a limited frequency response (i.e., range of frequencies at which the pressure changes in the esophagus are recorded during the test), and is sometimes challenging to set up and use; also, it is susceptible to artifacts brought on by movement of the connecting tubing or air bubbles in the system. SSM is the most frequently used. It has a high-frequency response and is easier to use; however, it is more expensive, fragile, and has a lower tolerability among patients.7
CCv4.0 recommends using any SSM with less than 2cm of sensor spacing; using WPS is possible but presents several limitations (i.e., assessment of supine swallows and provocative maneuvers).6 At our esophageal disease center, we use the 36-sensor SSM from Medtronic (Minneapolis, MN, USA); alternatives include Diversatek Healthcare system (Milwaukee, WI, USA), Alacer Biomedica (São Paulo, Brazil), AlbynMedical (Inverness, UK), Laborie Medical Technologies (Portsmouth, NH, USA), and EB Neuro S.p.A (Florence, Italy), among others. This paper details only the setup and use of the Medtronic system. A typical SSM catheter from Medtronic has a 4.2mm diameter and 36-channel pressure sensors at 1cm intervals. Each sensor measures pressure over a length of 2.5mm by an array of 12 circumferential solid-state micro-transducers, and the measurements from these 12 circumferential transducers are averaged to give a single value at each channel level.8
Multiple closely positioned pressure sensors enable the simultaneous measurement of pressure throughout the entire esophagus, spanning from the upper esophageal sphincter (UES) to the proximal stomach. The current software systems process the extensive data sets and present the pressure distribution, known as pressure topography, in an easy-to-understand manner. At our institution, we currently employ Manoview ESO analysis software, version 3.3 (Medtronic) for this purpose.
Furthermore, the advancement of impedance technology has facilitated the development of high-resolution impedance manometry (HRIM). The HRIM catheter used at our lab is a solid-state probe with a diameter of 4.2mm, containing 36 pressure sensors positioned at 1cm intervals around its circumference, along with 18 impedance segments spaced 2cm apart (Medtronic). HRIM allows for the visualization of the “movement” of a bolus within the esophagus, using impedance sensors. According to CCv4.0, using HRIM is recommended, though not required.6
Motility lab settingIn order to conduct HRM studies, the motility laboratory requires trained staff and adequate equipment.9,10 The manometry room setup varies by center; however, recommendations include a designated HRM tower, a fully adjustable reclining chair with a variety of pillows of different sizes, designated areas for waste disposal and hand washing, ample space for device storage, a chair to accommodate the patient's companion, basic life support equipment, and a readily accessible emergency system activation mechanism. Additionally, the environment within the room should be characterized by a tranquil and comfortable ambiance, promoting relaxation in the patient. Consideration may be given to incorporating elements such as low-volume meditation music and aroma diffusers emitting subtle fragrances. It is essential to consult with the patients about their preferences to create the scenario that generates the most comfort, allowing a better study record.
In addition, the operator or person responsible for performing the HRM must be a registered nurse or physician. This professional must have a certain level of training or continuous experience in motility studies to identify and manage the essential aspects related to a high-quality study in real time (e.g., correct identification of the essential anatomical landmarks, need for catheter repositioning, identification of common artifacts, complications or any equipment malfunction).10
Indications, contraindications, and patient preparationHRM has become the preferred technique for assessing esophageal motility disorders but is not limited to this purpose. Common indications for referrals include symptoms such as dysphagia, non-cardiac chest pain, odynophagia, nausea/vomiting, or typical gastroesophageal reflux symptoms (i.e., heartburn, regurgitation, chronic cough, etc.). However, almost all patients should undergo a barium swallow study or an upper GI endoscopy as an initial step to rule out any structural abnormalities such as strictures, masses, or fistulas that contraindicate HRM.11 The main indications and contraindications for the procedure are presented in Supplementary Table 1.
Before HRM, adequate patient counseling by a physician is expected. Clinicians should discuss the rationale and expectations of the procedure with the patient as well as educate them about the potential discomfort that may arise during the procedure.10 Before the HRM, the operator (i.e., usually a registered nurse specializing in GI motility) should explain step-by-step how the procedure will be done and confirm the adequate preparation of the patient for the study; informed consent must be obtained.
The patient should have fasted for at least 4h6; however, based on our experience, we recommend a longer period of fasting, especially for those patients with suspected achalasia or a history of delayed gastric emptying, to reduce the vomiting and aspiration risk during the catheter placement.9,11 Furthermore, some factors can interfere with the study's interpretation and results. Ideally, HRM should be done in the absence of consumption of any medications that may have an impact on esophageal peristalsis (e.g., anticholinergic agents, calcium channel blockers, nitrates, opioids, etc.)10,12; the suspension of these medications has to be done according to their half-life.9,12 However, if certain medications cannot be stopped in specific patients, HRM can still be performed. Other potential confounding factors in the interpretation of HRM include a history of previous foregut surgery (i.e., myotomy, fundoplication, hiatal hernia repair, etc.), hence, it is critical to document the patient's surgical history.11 Additionally, patients can be asked to complete a symptom questionnaire (e.g., GERD-Q, Eckardt score, The Mayo dysphagia questionnaire, etc.).
Data recording and study protocolAfter confirming the absence of potential allergic reactions, a topical anesthetic can be optionally applied inside the nostril and surrounding the tip of the catheter (e.g., 2% lidocaine hydrochloride topical jelly) to improve patient comfort and tolerance of the procedure. After calibration, the HRM catheter is passed transnasally and is positioned to include the upper esophageal sphincter (UES) and the proximal stomach. The catheter should first be inserted beyond the proximal stomach and then gradually pulled back to the proper position based on landmarks noted. Real-time assessment and confirmation of the UES, lower esophageal sphincter-crural diaphragm (LES-CD) complex, and proximal stomach are facilitated by the pressure topography display. A minimum of 3–4 channels should be positioned below the CD to ensure adequate gastric pressure for analysis.13,14 Once correctly positioned, the catheter is secured by taping it to the nose.
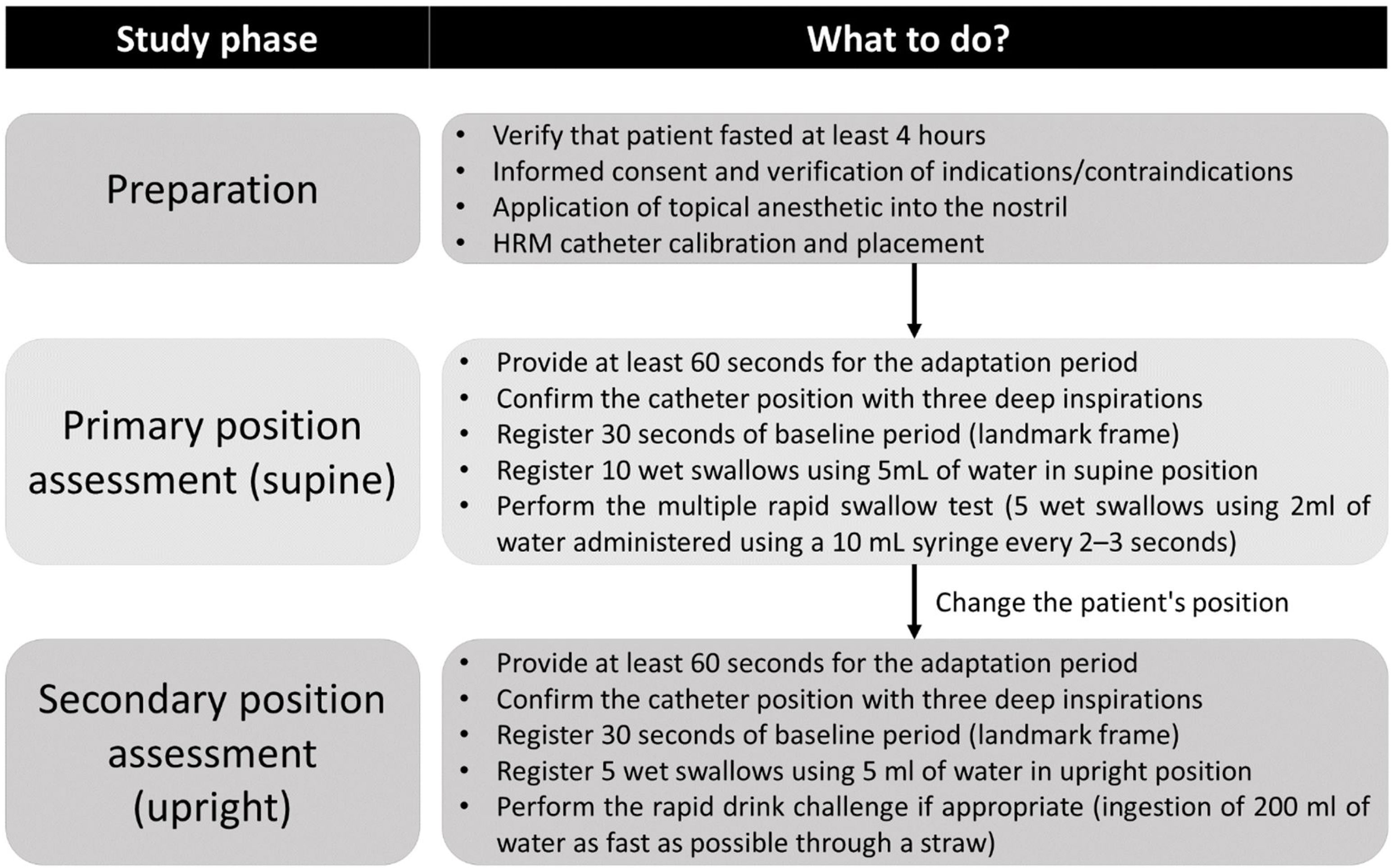
Subsequently, the patient is positioned supine or semi-supine at an approximate 30-degree elevation (generally a slight lateral rotation with a pillow support on the back helps prevent aspiration with swallows). To initiate the recording, the operator selects the “start recording button” and registers the catheter depth from the nostril into the software. The subject is allowed several minutes to acclimate to the presence of the catheter. A minimum of 60s of quiet rest is recommended, and the correct catheter position can be confirmed by three deep inspirations.6
The baseline period is recorded for 30 consecutive seconds without swallowing, secondary peristalsis, transient LES relaxation, cough, or belching. After this, ten single swallows of 5mL of water (or saline solution in cases including impedance) are recorded, each at least 30–45s apart. Before each swallow, the esophageal body and the EGJ are allowed to return to a resting state (there should be at least 30s between the swallows to avoid deglutitive inhibition artifacts). CCv4.0 incorporated the use of provocative maneuvers such as the multiple rapid swallows (MRS) test, which can be performed after the ten single swallows in the supine position.6
After completing the assessment in the primary position, the patient is asked to adopt the secondary position (upright if supine was the primary or the opposite). After a 1-min period of adaptation, the catheter position should be confirmed again with three deep inspirations and recording of a second baseline period (30s); subsequently, at least five swallows using 5mL of water each is performed in the secondary position. A rapid drink challenge (RDC) using 200mL of water can be done if indicated; moreover, additional provocative maneuvers such as solid test swallows and a solid test meal can be performed to assess other suspected conditions like rumination or a belching disorder.6 Once the study concludes, the HRM catheter should be removed, and the atmospheric pressure recorded for several seconds to perform adequate thermal compensation; then, select the “finish recording” button and save the raw data.
Notably, although the diagnosis classification is based only on the primary position in which the first ten swallows are performed, either supine or upright, the CCv4.0 encourages performing HRM in both positions.6 Some authors have reported that the body position can influence esophageal motility data in HRM studies.15–17 Normative values of the integrated relaxation pressure (IRP), distal contractile integral (DCI), and contractile front velocity (CFV) can decrease significantly in the sitting position.17 Assessment of swallows in the secondary position and provocative maneuvers are supportive data; hence, it should be carefully assessed if the HRM study is performed in a sitting position. Fig. 1 summarizes the study recommendations and protocol.
Summary of HRM study protocol following CCv4.0 recommendations.
While the Manoview ESO analysis software v3.3 offers valuable analytical capabilities, it is essential to note that the ultimate interpretation of results should be conducted by a trained clinician. Moreover, the HRM data analysis and interpretation must be systematic and organized, considering also the patient's medical history if available.
Thermal compensationThe warming of pressure sensors often results in thermal pressure drift secondary to the thermal expansion of metallic sensors after catheter removal. The error caused by the temperature effect can be adjusted by setting the thermal compensation. Before starting the analysis, using the navigation bar, display the end of the study where the catheter was removed and place the red vertical time bar immediately after the waterfall image and click the “Set Thermal Compensation” button located in the Tools Menu.
Assessment of the baseline frameUsing an adequate 30-s baseline period (without pressurization artifacts such as secondary peristalsis or cough), the anatomical markers can be placed and then the baseline parameters can be interpreted.
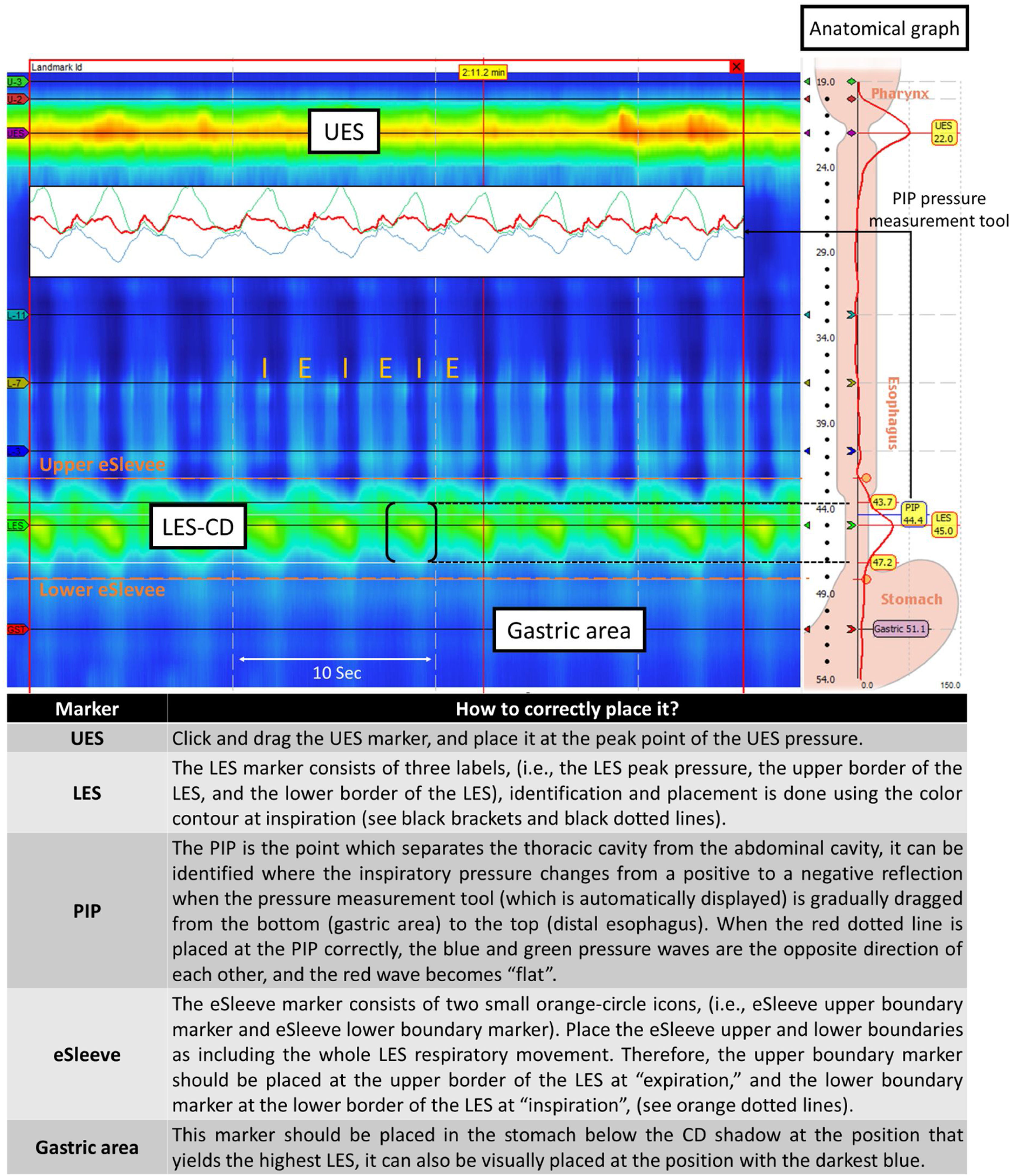
Anatomical and spatial markersSelect the landmark frame and confirm the frame color changed from green to red. The pressure change depending on respiratory cycle is seen in the esophageal body (i.e., dark blue color is seen at inspiration and light blue at expiration in the color contour scale). Place the red vertical time bar at inspiration. Then, adjust the position of the spatial markers including UES, LES, pressure inversion point (PIP), eSleeve, and gastric marker (Fig. 2). The software provides an anatomical reference graph on the right side to facilitate this step.
Anatomical markers placement. Accurate placement of landmarks or reference points within the HRM software is essential for the analysis of basal pressures and the evaluation of swallows. Abbreviations: CD: crural diaphragm; E: expiration; I: inspiration; LES: lower esophageal sphincter; UES: upper esophageal sphincter.
The baseline parameters including overall LES length (OL), abdominal LES length (AL), and resting pressure (LESP) can provide information regarding the LES barrier function against gastroesophageal reflux. Parameters of LES competence were reported using conventional water-perfused manometry by Zaninotto et al.18 in 1988, and were confirmed using HRM by Hoshino et al.19 in 2011. These parameters include OL ≥2cm, AL ≥1cm, and LESP >6mmHg. Further, the commonly accepted normal range of LESP is 10–45mmHg, although the manufacturer's instructions for HRM describe the normotensive LESP as 13–43mmHg.20 As an alternative to LESP, other measures, such as the LES pressure integral (LESPI) or EGJ contractile integral (EGJ-CI) can be obtained to assess the pressure of the EGJ.6,20
Recommendations provided by CCv4.0 for the EGJ assessment include the measurement during the baseline period avoiding swallowing or recording artifacts.6 Moreover, it is important to describe evidence of LES-CD separation ≥1cm during rest as this indicates the presence of manometric hiatal hernia. In a normal state (without hiatal hernia), the PIP localizes at the proximal margin of the LES-CD (EGJ) complex. In patients with a hiatal hernia, the PIP can localize either between the LES and CD or proximal to the LES; however, when the LES-CD separation is greater than 3cm, the PIP location and relation to LES can be unreliable. CCv4.0 accepts the use of either the classical classification system (type I, II, IIIa, and IIIb) or the physiological classification system proposed by Akimoto et al. (type A, B, and C; Supplementary Table 2).6,20,21
Assessment and interpretation of the swallow framesAfter selecting the swallow frame, the placement of the anatomical markers should be reviewed and placed appropriately if necessary. The main HRM diagnostic thresholds and basic definitions used in the CCv4.0 include the integrated relaxation pressure (IRP), distal contractile integral (DCI), distal latency (DL) and pressurization patterns. Fig. 3 summarizes how to identify and calculate these metrics using the HRM software.
The IRP is determined by measuring the lowest average LES or CD-LES pressure (if there is no evidence of a hiatal hernia). This measurement is obtained using the eSleeve method for 4s, continuously or intermittently, during a 10-s window following the relaxation of the UES after swallowing. Under normal circumstances, the upper limit of the IRP value is 15mmHg (using the Medtronic HRM system); however, the cut-off point for the IRP varies depending on the manufacturer. This value demonstrates a high sensitivity of 98% and specificity of 96% in identifying patients with achalasia.13 In CCv4.0, the median IRP value from ten wet swallows in the primary position serves as the diagnostic algorithm's main criterion and the initial point of evaluation.6 An elevated IRP exceeding 15mmHg is associated with EGJ outflow disorders. As a novelty, CCv4.0 also includes the concept of borderline median IRP, which corresponds to a value between 10 and 15mmHg in the supine position.6
Distal contractile integralThe DCI is a measure of the peristaltic vigor which represents the amplitude, duration, and length (mmHg s cm) of the esophageal contraction at the isobaric contour of 20mmHg. The DCI allows the classification of the contraction vigor into four subcategories: failed (DCI <100mmHgscm), weak (DCI >100 and <450mmHgscm), normal (DCI 450–8000mmHgscm), and hypercontractile (>8000mmHgscm). Importantly, CCv4.0 defines swallows presenting a failed, weak, or fragmented contraction (i.e., swallows with DCI >450mmHgscm and peristalsis breaks >5cm) as ineffective.6
Distal latencyThe DL is the time between the relaxation of the UES and the contractile deceleration point (CDP) of the distal esophagus placed along the 30-mmHg isobaric contour. The DL provides an indirect assessment of the inhibition of swallowing and, consequently, the presence of abnormal peristalsis.9 Premature contractions are defined when a DL <4.5s is documented.6
Pressurization patternsCCv4.0 recognizes two main pressurization patterns. The first one is the intrabolus pressure pattern, which is related to poor bolus clearance enhanced by esophageal body contraction and is defined as pressurization exceeding the isobaric contour of ≥20mmHg in the supine position. The second one is panesophageal pressurization (PEP) which is used for diagnosing type II achalasia (with esophageal compression) and is defined as pressurization of the entire esophageal body with isobaric contour ≥30mmHg, or >20mmHg during a rapid drink challenge or solid test meal.
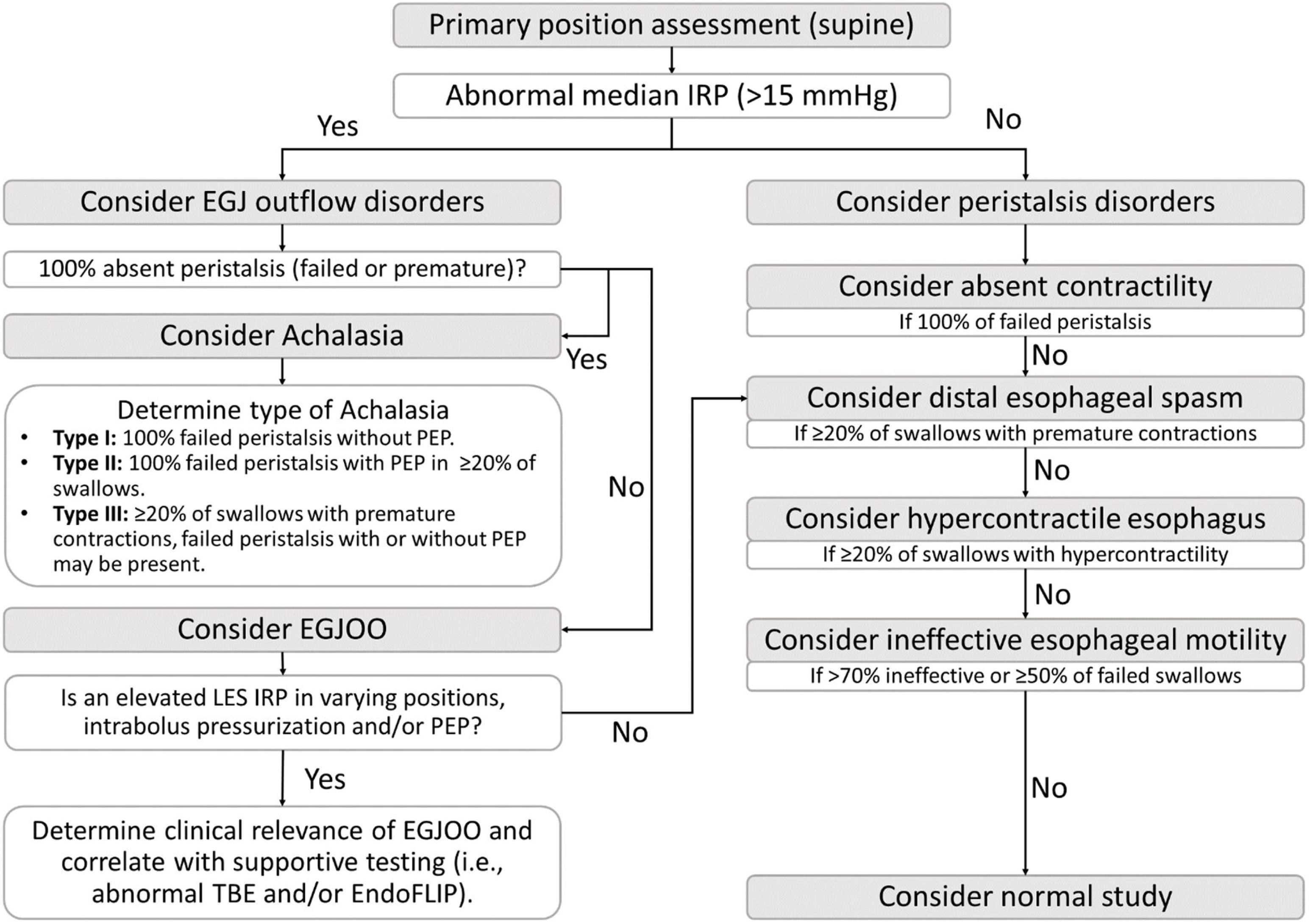
Data interpretation and diagnosis of motility disordersThe diagnostic process outlined in CCv4.0 follows a hierarchical algorithmic approach and is summarized in Fig. 4. The initial step involves the median IRP assessment. If the median IRP exceeds the threshold of 15mmHg, it indicates an abnormality related to the EGJ outflow (achalasia or EGJOO). If the median IRP is <15mmHg, further assessment is conducted to determine if the study meets the criteria for an esophageal peristalsis disorder. In cases where the study does not meet any diagnostic criteria, the study is considered normal. Fig. 3 presents a normal study and Fig. 5 shows examples of the manometric pattern of some motility disorders.
Summary of Chicago Classification v4.0 diagnostic algorithm for esophageal motility disorders. Abbreviations: EGJ: esophagogastric junction; EGJOO: esophagogastric junction outflow obstruction; EndoFLIP: endoluminal functional lumen imaging probe; IRP: integrated relaxation pressure; PEP: panesophageal pressurization pattern; TBE: timed barium esophagram.
Representative swallow frames of the different EGJ outflow and esophageal peristalsis disorders according to Chicago Classification v4.0. Abbreviations: DCI: distal contractile integral; DL: distal latency; EGJOO: esophagogastric junction outflow obstruction; IRP: integrated relaxation pressure; LES: lower esophageal sphincter; PEP: panesophageal pressurization pattern.
One of the most intricate diagnostic criteria in CCv4.0 pertains to normal esophageal motility, as its definition lacks explicit clarity. Nevertheless, it can be succinctly summarized as the evaluation of a minimum of ten swallows in the primary position meeting three criteria: (a) over 50% of swallows demonstrate effective or normal contractions (DCI ≥450mmHgscm and ≤8000mmHgscm without fragmented peristalsis), (b) less than 20% of swallows exhibit premature contractions (DL <4.5s), and (c) less than 20% of swallows display hypercontractile peristalsis (DCI >8000mmHgscm) (Fig. 3).
Esophagogastric junction outflow disordersAchalasiaThree subtypes of achalasia are recognized by CCv4.0. All of them require an abnormal elevated IRP (>15mmHg) and complete absent peristalsis, defined as 100% of swallows with either failed peristalsis or premature contraction.6 Type I (classic) is associated with absent contractility. Type II (with esophageal compression) is associated with evidence of panesophageal pressurization in 20% or more swallows. Type III (spastic) is associated with evidence of spasm (20% or more swallows with premature contraction without evidence of peristalsis).6
EGJ outflow obstructionThe diagnosis of EGJOO can be classified as a clinically relevant conclusive diagnosis or inconclusive using CCv4.0.6 In both cases, an abnormal median IRP (>15mmHg) without meeting achalasia criteria is needed. Clinically relevant conclusive EGJOO demands evidence of abnormal IRP in both the primary and secondary position, ≥20% of swallows with elevated intrabolus pressure in the supine position, evidence of peristalsis, documentation of clinically relevant symptoms such as dysphagia or chest pain, and supportive testing with timed barium esophagram or endoluminal functional lumen imaging probe (EndoFLIP [Medtronic]).6 Inconclusive EGJOO corresponds to the isolated abnormal elevation of the IRP only in one position.6 Furthermore, abnormal RDC or pharmacologic provocation tests (e.g., using amyl nitrite or cholecystokinin) can be useful supportive evidence of an obstruction.6
Peristalsis disordersAbsent contractilityA conclusive diagnosis of absent contractility is defined as normal median IRP (both supine and upright) with failed peristalsis (DCI <100mmHgscm) in 100% of swallows. On the other hand, if all swallows have failed peristalsis, but the IRP is borderline (10–15mmHg), type I achalasia must be ruled out.6
Distal esophageal spasm (DES)A conclusive manometric diagnosis of DES is made with a normal IRP and >20% of premature swallows (DL <4.5s and DCI >450mmHgscm); however, a clinically relevant diagnosis of DES requires the presence of symptoms such as dysphagia or chest pain. An inconclusive diagnosis of DES is made with a normal median IRP and >20% of swallows presenting spastic features with ineffective contraction (DL <4.5s and DCI <450mmHgs. cm).6,22
Hypercontractile esophagusA clinically relevant conclusive diagnosis of hypercontractile esophagus requires evidence of symptoms such as dysphagia and non-cardiac chest pain and manometric findings including a normal median IRP and >20% hypercontractile swallows (DCI >8000mmHgscm). An inconclusive manometric diagnosis is made with a normal IRP, and >20% of hypercontractile swallows are incidentally identified on evaluation for reflux symptoms.6,23
Ineffective esophageal motility (IEM)A conclusive diagnosis is made with a normal median IRP, and >70% ineffective swallows (failed, weak, or fragmented) or >50% swallows failed (DCI <100mmHgscm). In contrast, an inconclusive diagnosis of IEM is made when there are 50–70% ineffective swallows.
Additional materials for the readerA complete guide for analyzing high-resolution manometry based on Chicago Classification version 4.0© (1st ed.), a book written and published independently by the authors, comprehensively addresses more detailed aspects of the interpretation and analysis of HRM studies.24
Ethical considerationsThis work does not include the use of human or animal subjects.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflict of interest. However, part of the content was adapted from a previously independently published book by the authors, and it has been suggested as an additional/supplementary reading (i.e., including a link to printed version available for purchase).
The authors thank Kristine Nally for her editorial assistance.