To determine the risk and prognostic factors for Clostridioides difficile infection (CDI).
Patients and methodsProspective, case-control study with 61 cases and 64 controls, aged ≥ 2 years with diarrhoea, carried out in “Castilla-La Mancha” Health Care Area for 14 months. The diagnosis was made by immunochromatography technics (glutamate dehydrogenase and toxin A/B), confirming discordant cases by isothermal amplification. Demographic variables, comorbidities, type of acquisition, previous administration of antibiotics, antacids and immunosuppressants, and evolution were collected. The data were analysed using the chi-square test and the effect of risk and prognostic factors was quantified using an odds ratio with 95% confidence intervals.
ResultsHospital admission 4 weeks prior to infection, hypoalbuminemia, and previous administration of antibiotics were identified as independent risk factors for CDI. Presenting these 3 factors constitutes nearly 3-fold increase in the risk of becoming infected. A greater number of hospital admissions in the 4−12 weeks prior to CDI were found in the group of nosocomial acquisition. Although there was a greater tendency to recurrence and an unfavourable prognosis among nosocomial cases, these differences were not significant. We found that fever and hospital admission in the 4 weeks prior to infection were unfavourable prognostic factors of CDI.
ConclusionsThe independent risk factors for CDI were: hospital admission in the 4 weeks prior to infection, hypoalbuminemia, and previous administration of antibiotics. Fever and hospitalisation in the previous 4 weeks were also identified as prognostic factors of unfavourable evolution.
Determinar los factores de riesgo y factores pronósticos de la infección por Clostridioides difficile (ICD).
Pacientes y métodosEstudio prospectivo de casos-controles (61 casos y 64 controles) ≥2 años con diarrea, atendidos en un Área Sanitaria manchega durante 14 meses. El diagnóstico se realizó mediante inmunocromatografía (glutamato deshidrogenasa y toxina A/B), realizando amplificación isotérmica en los casos discordantes. Se recogieron variables demográficas, comorbilidades, tipo de adquisición, administración previa de antibióticos, antiácidos e inmunosupresores y evolución. Los datos se analizaron mediante la prueba de chi-cuadrado y el efecto de los factores de riesgo y pronósticos se cuantificó mediante odds ratio con intervalos de confianza del 95%.
ResultadosComo factores de riesgo independientes de ICD encontramos el ingreso hospitalario las 4 semanas previas a la infección, la hipoalbuminemia y la administración previa de antibióticos. Presentar estos 3 factores supuso un riesgo casi 3 veces mayor de infectarse. En el grupo de adquisición nosocomial se encontró mayor número de ingresos hospitalarios las 4−12 semanas previas a la ICD, y aunque hubo mayor tendencia a las recurrencias y pronóstico desfavorable entre los casos intrahospitalarios, estas diferencias no fueron significativas. Identificamos como factores de pronóstico desfavorable la fiebre y el ingreso hospitalario las 4 semanas previas a la infección.
ConclusionesLos factores de riesgo independientes de ICD fueron: ingreso hospitalario las 4 semanas previas a la infección, hipoalbuminemia y administración previa de antibióticos. La fiebre y la hospitalización las 4 semanas anteriores se identificaron además como factores pronósticos de evolución desfavorable.
Clostridioides difficile is a global public health problem, with C. difficile infection (CDI) being the main cause of nosocomial diarrhoea in industrialised countries1 and the origin of an increasing number of cases of diarrhoea in the community.2 It can cause conditions ranging from moderate diarrhoea to pseudomembranous colitis and severe complications, such as paralytic ileus and toxic megacolon. A lack of clinical suspicion, especially in those patients who do not exhibit the classic risk factors (advanced age, prolonged hospitalisation, previous use of antibiotics), means that CDI is still an underdiagnosed disease.3 This factor, together with its morbidity and mortality, raises the need for epidemiological studies to establish improved infection prevention and control strategies.
The aim of this study was to identify both the risk factors and prognostic factors for CDI in a rural health area, as well as to establish the main differences between nosocomial and community cases.
Patients and methodsDesignThis was an observational, prospective, case-control study of patients 2 years of age or over with diarrhoea (≥3 loose stools per day) treated in the La Mancha Centro Health Area, which serves a population of approximately 230,000 inhabitants, from 1 April 2015 to 30 May 2016. The patient follow-up period was extended for two more months.
Patient selectionCase definition: patient with a clinical and microbiological diagnosis of CDI treated in our health area. Hospital case: patient with CDI and hospital admission in the four weeks prior to infection, or whose infection developed when they had been hospitalised for more than 48h. Community case (CCDI): patient with CDI without hospital admission in the previous four weeks, or whose infection developed in the first 48h after admission.
Control definition: patient treated in our health area in the same period as the index case in which a stool culture was requested and the toxin test was negative. They were considered a hospital control if they had also been admitted in the previous four weeks, otherwise they were deemed a community control. The controls chosen were patients who were closest in time and age to the index case.
Sample sizeAll loose stools from patients aged 2 years or older received by the microbiology laboratory during the study period were consecutively included, regardless of the clinician's request. Number of cases: 61 (there were three cases that were ultimately excluded from the study because they were recurrences rather than new cases). Number of controls: 64.
Variables collected- □
Age, gender, acquisition (community/nosocomial).
- □
Short-form Charlson Index,4 which includes cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, heart failure/ischaemic heart disease, dementia, peripheral arterial disease, chronic kidney disease and cancer. In addition, the following comorbidities were collected: inflammatory bowel disease, immunosuppression, chemotherapy drugs, solid-organ and/or haematopoietic stem cell transplantation and hypoalbuminaemia.
- □
Symptoms: fever associated with diarrhoea (FAD), diarrhoea, abdominal pain and vomiting.
- □
Type of antibiotic, antacid or immunosuppressant previously administered.
- □
CDI type:
- o
Severe infection, if one or more of the following criteria are met: systemic signs of infection, leukocyte count ≥15,000/μl or creatinine increase >50% the baseline level.
- o
Severe-complicated infection, if one or more of the following criteria are met: hypotension or sepsis, paralytic ileus, toxic megacolon, intestinal perforation, ICU admission for CDI, surgery for the same reason or attributable death.
- o
Non-severe infection, if it does not meet the criteria for severe or severe-complicated infection.
- o
- □
Recurrence: patient who meets the CDI criteria again within three to 60 days after a previous clinically resolved episode, that is, within eight weeks after having correctly completed treatment for the infection.
- □
Concomitant intestinal infection and infection in other locations.
All loose stools received during the study period, regardless of the clinician's request, underwent an immunochromatographic test with simultaneous detection of glutamate dehydrogenase antigen and toxins A/B (C. Diff Quik-Chek Complete®, Alere). Discordant results were confirmed by loop-mediated isothermal amplification (Illumigene®, Meridian Bioscience).
Bivariate analysisThe response variable (case/control) was related to each of the explanatory variables by preparing contingency tables for the qualitative variables. To do this, statistical significance was estimated (χ2 test or Fisher's exact test if the number of expected values is less than 5) and a measure of the magnitude of the association in the form of the odds ratio and its corresponding confidence interval (95% CI). For the quantitative variables, the Student's t-test or the Mann–Whitney U test was used, depending on the distribution of the variable.
Subsequently, the cases were compared based on the type of infection acquisition (nosocomial versus community).
Multivariate analysisAll those explanatory variables that had a relationship with the response variable with a significance p<0.20, according to the criteria proposed by Maldonado and Greenland,5 formed part of the multivariate logistic regression models. Conditional (forward) logistic regression was used due to the paired nature of the controls.
To determine the unfavourable prognostic factors for CDI (appearance of complications, recurrences or attributable mortality), the following variables were evaluated, including symptoms and possible risk factors: advanced age (over 65 years), gender, type of acquisition, previous hospital admission, symptoms, previous antibiotic exposure, use of proton pump inhibitors, immunosuppression, enteral feeding, hypoalbuminaemia and concomitant infections.
Statistical significance (p<0.05) was used as a criterion for permanence in the final model. The calculations were made using the statistical program SPSS Statistics for Windows, version 18.0 (SPSS Inc., Chicago, USA).
Ethical considerationsThis study was approved by the Ethics Committee of the Hospital General La Mancha Centro. All the data collected throughout the conduct of the research project were processed in accordance with Spanish Organic Law 3/2018, of 5 December, on the protection of personal data and guarantee of digital rights. The database created for the conduct of this research was anonymised using codes and duly safeguarded by the principal investigator.
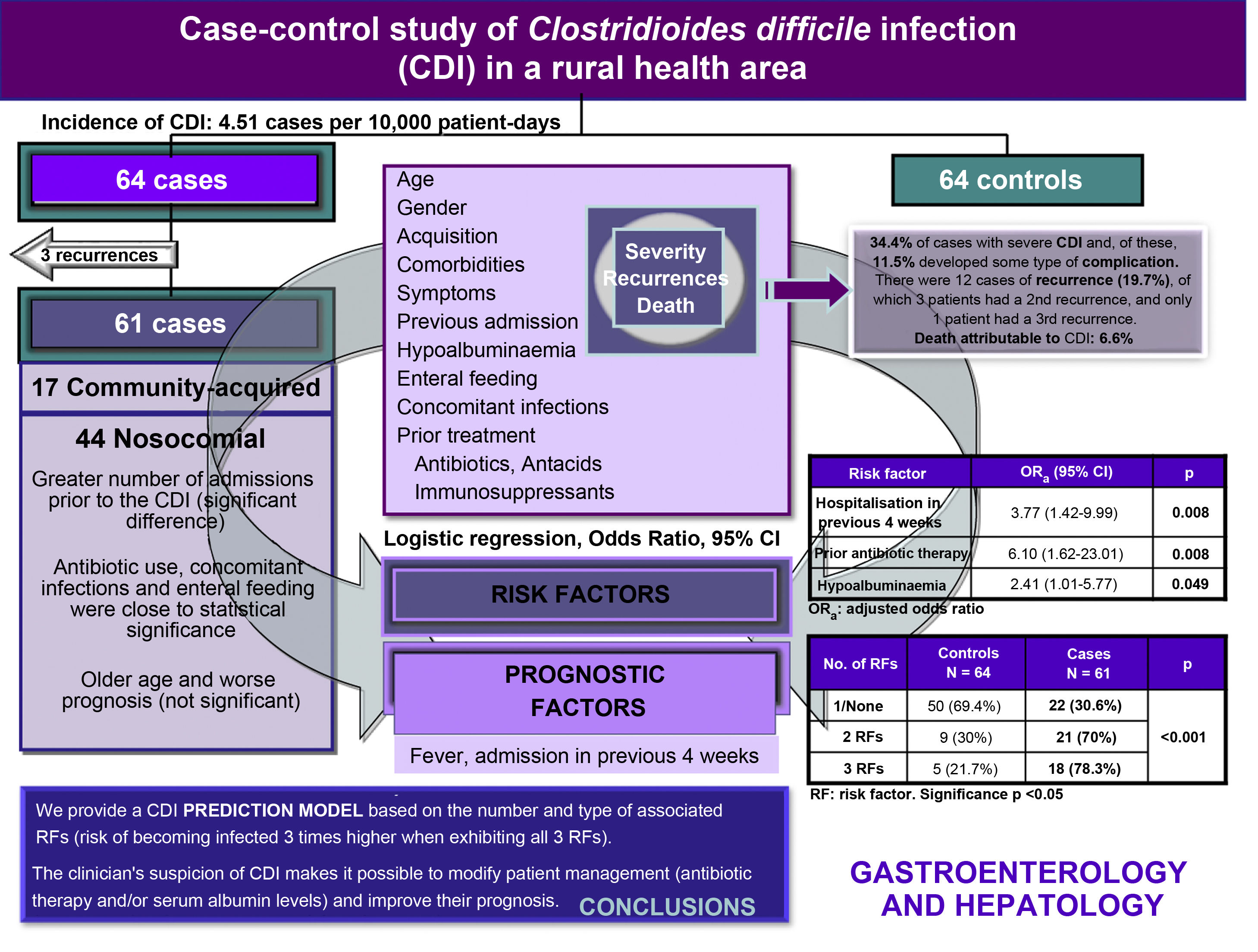
ResultsDescriptive analysisA total of 640 stool samples were analysed, 61 of which were toxin A/B positive, representing 9.5% of CDI cases. The incidence was 4.51 cases per 10,000 patient-days. In all, 61 cases (72.1% nosocomial acquisition and 27.9% community) and 64 controls were studied. The monthly distribution of CDI cases was heterogeneous; seasonality was observed (greater in autumn and winter) with an accumulation of 26 cases (42.6%) in the last quarter of 2015.
The median age of the cases was 80 years (IQR 13 years; range 28–95), with a slight predominance of females (57.4%). The age histogram shows a heterogeneous distribution, with a tendency to increase at advanced ages (70–90 years).
Of the total, 52% of the cases came from the Internal Medicine Department, 80% had some underlying disease and 56% some concomitant infection, of which urinary tract infection and respiratory infections were the most common. Meanwhile, 52% of the cases were associated with at least one gastrointestinal process: enteral feeding and gastrointestinal cancer were the most common. Additionally, 26% of the patients had a tumour.
The most common symptom was diarrhoea, which affected all patients except one (98.4%), followed by fever (42.6%), abdominal pain (24.6%) and vomiting (6.5%).
A total of 129 antibiotics were administered. Only three patients (5%) did not receive any antibiotic before the development of the infection. The most widely used family of antibiotics was beta-lactams (56.5%), followed by fluoroquinolones (20.1%). The most frequently administered antibiotics were: amoxicillin-clavulanate (20%), levofloxacin (13%) and imipenem (10%). Of the total, 94.4% consumed antacids continuously, of which omeprazole was the most used (74%) (Fig. 1).
Meanwhile, 34.4% of the cases developed a severe infection and, of these, 11.5% also had some type of complication; five patients (8.5%) required admission to the ICU. Overall mortality was 18%, while CDI-attributable mortality was 6.6%. Twelve patients (19.7%) had a first recurrence, and three of them suffered a second recurrence (5%). Only one patient had a third recurrence (1.6%).
Risk factorsBivariate analysisDiarrhoea was the most common symptom in both groups, although FAD was the only statistically significant symptom; it was more common in cases than in controls (Table 1). The risk factors associated with CDI were nosocomial acquisition, hospitalisation in the four weeks prior to infection, previous use of antimicrobials and antacids, hypoalbuminaemia and concomitant infections (Table 1).
Bivariate analysis: risk factors of patients with CDI.
| Characteristics n (%) | Overall (n=125) | Controls (n=64) | Cases (n=61) | p |
|---|---|---|---|---|
| Mean age (SD; range) | 73.2 (17.4; 11−98) | 71.4 (18.9) | 75.1 (15.8) | 0.242 |
| Gender | ||||
| Male | 57 (45.6%) | 31 (48.4) | 26 (42.6) | 0.514 |
| Female | 68 (54.4) | 33 (51.6) | 35 (57.4) | |
| Origin | ||||
| Hospital-acquired | 74 (59.2) | 30 (46.9) | 44 (72.1) | 0.004 |
| Community-acquired | 51 (40.8) | 35 (54.7) | 25 (41.0) | |
| Fever | 32 (25.6) | 10 (15.6) | 26 (42.6) | 0.001 |
| Diarrhoea | 119 (95.2) | 59 (92.2) | 60 (98.4) | 0.208 |
| Abdominal pain | 33 (26.4) | 18 (28.1) | 15 (24.6) | 0.654 |
| Previous hospital admission | 53 (42.4) | 16 (25.0) | 37 (60.7) | <0.001 |
| Admission in previous 4 weeks | 37 (29.6) | 8 (12.5) | 29 (47.5) | <0.001 |
| Admission in previous 4−12 weeks | 16 (12.8) | 8 (12.5) | 8 (43.1) | 0.918 |
| Corticosteroid therapy | 14 (11.2) | 8 (12.5) | 6 (9.8) | 0.637 |
| Immunosuppression | 28 (22.4) | 12 (18.8) | 16 (26.2) | 0.316 |
| Chemotherapy | 11 (8.8) | 3 (4.7) | 8 (13.1) | 0.096 |
| Hypoalbuminaemia | 46 (36.8) | 15 (23.4) | 31 (50.8) | 0.002 |
| Enteral feeding | 17 (13.6) | 9 (14.1) | 8 (13.1) | 0.877 |
| Previous antibiotic use | 102 (81.6) | 44 (68.8) | 58 (95.1) | <0.001 |
| Previous antacid use | 95 (76.0) | 41 (64.1) | 54 (88.5) | 0.001 |
| Type of antacid use | ||||
| Continuous | 89 (71.2) | 39 (95.1) | 50 (94.3) | >0.999 |
| Occasional | 5 (4.0) | 2 (4.9) | 3 (5.7) | |
| Previous use of immunosuppressants | 28 (22.4) | 12 (18.8) | 16 (26.2) | 0.316 |
| Charlson (comorbidity) | ||||
| Low | 81 (64.8) | 42 (65.6) | 39 (63.9) | 0.843 |
| High | 44 (35.2) | 22 (34.4) | 22 (36.1) | |
| Concomitant infections | 57 (45.6) | 23 (35.9) | 34 (55.7) | 0.026 |
| Associated intestinal infection | 4 (3.2) | 0 | 4 (6.6) | 0.200 |
| Death attributable to CDI | 4 (3.2) | 0 | 4 (6.6) | 0.054 |
SD: standard deviation.
Significant p<0.05.
Using logistic regression analysis, hospital admission in the four weeks prior to infection, hypoalbuminaemia and prior administration of antibiotics were identified as independent risk factors for CDI (Table 2). The probability of developing a CDI when a patient presents one or none of these risk factors is 30%, rising to 70% when presenting two factors and, finally, to 78% when presenting three factors, constituting an almost three times greater risk of acquiring the infection.
Prognostic factors for the clinical course of Clostridioides difficile infectionBivariate analysisFAD, hospital admission during the four weeks prior to infection, hypoalbuminaemia and the presence of concomitant infections were found to be unfavourable prognostic factors (Table 3).
Bivariate analysis: prognostic factors for unfavourable clinical course.
| Characteristics | Overall | Favourable clinical course | Unfavourable clinical course | p |
|---|---|---|---|---|
| Gender | ||||
| Male | 57 (45.6) | 48 (46.2) | 9 (42.9) | 0.782 |
| Female | 68 (54.4) | 56 (53.8) | 12 (57.1) | |
| Origin | ||||
| Hospital-acquired | 65 (52.0) | 51 (49.0) | 14 (66.7) | 0.140 |
| Community-acquired | 60 (48.0) | 53 (51.0) | 7 (33.3) | |
| Fever | 36 (28.8) | 24 (23.1) | 12 (57.1) | 0.002 |
| Diarrhoea | 119 (95.2) | 99 (95.2) | 20 (95.2) | >0.999 |
| Abdominal pain | 33 (26.4) | 26 (25.0) | 7 (33.3) | 0.429 |
| Previous hospital admission | 53 (42.4) | 41 (39.4) | 12 (57.1) | 0.134 |
| Admission in previous 4 weeks | 37 (29.6) | 25 (24.0) | 12 (57.1) | 0.002 |
| Admission in previous 4−12 weeks | 16 (12.8) | 16 (115.4) | 0 | 0.071 |
| Corticosteroid therapy | 14 (11.2) | 13 (12.5) | 1 (4.8) | 0.461 |
| Immunosuppression | 28 (22.4) | 24 (23.1) | 4 (19.0) | 0.782 |
| Chemotherapy | 11 (8.8) | 9 (8.7) | 2 (9.5) | 0.898 |
| Hypoalbuminaemia | 46 (36.8) | 33 (31.7) | 13 (61.9) | 0.009 |
| Enteral feeding | 17 (13.6) | 12 (11.5) | 5 (23.8) | 0.162 |
| Previous antibiotic use | 102 (81.6) | 82 (78.8) | 20 (95.2) | 0.120 |
| Previous antacid use | 95 (76.0) | 76 (73.1) | 19 (90.5) | 0.089 |
| Use of immunosuppressants | 28 (22.4) | 25 (24.0) | 3 (14.3) | 0.403 |
| Charlson (comorbidity) | ||||
| Low | 81 (64.8) | 68 (65.4) | 13 (61.9) | 0.761 |
| High | 44 (35.2) | 22 (34.4) | 22 (36.1) | |
| Concomitant infections | 57 (45.6) | 43 (41.3) | 14 (66.7) | 0.034 |
Significant p<0.05.
FAD and hospital admission in the four weeks prior to infection were found to be independent prognostic factors for an unfavourable clinical course (Table 4).
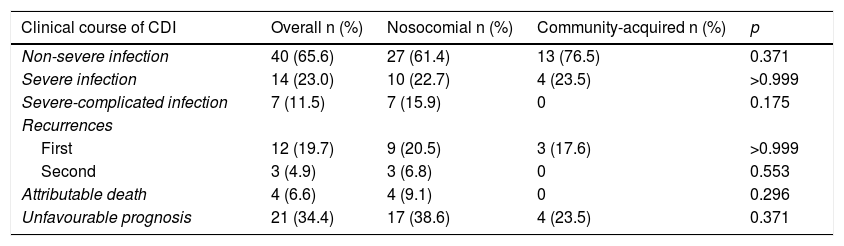
Comparison between hospital and community casesWe found that 40% of infections in patients under 65 years of age were community-acquired compared to 25% in patients 65 years of age or older. In all, 82.3% of community cases were hospitalised. In the nosocomial acquisition group, we found a greater number of hospital admissions in the 4−12 weeks prior to the CDI, a difference that was statistically significant (Table 5). Enteral feeding was almost statistically significant. However, although we observed greater severity and, in general, a worse prognosis among hospital-acquired cases, the differences were not statistically significant (Table 6).
Comparison between nosocomial and community-acquired cases.
| n (%) | Overall | Nosocomial (n=44) | Community-acquired (n=17) | p |
|---|---|---|---|---|
| Age (mean) | 77 | 72 | 0.228 | |
| Gender | ||||
| Male | 26 (42.6) | 19 (43.2) | 7 (41.2) | 0.887 |
| Female | 35 (57.4) | 21 (58.3) | 14 (56.0) | |
| Fever | 26 (42.6) | 18 (40.9) | 8 (47.1) | 0.663 |
| Diarrhoea | 60 (98.4) | 43 (97.7) | 17 (100) | >0.999 |
| Abdominal pain | 15 (24.6) | 6 (35.3) | 9 (20.5) | 0.228 |
| Previous admission | 37 (60.7) | 31 (70.5) | 6 (35.3) | 0.012 |
| 4−12 weeks | 8 (13.1) | 2 (4.5) | 6 (35.3) | 0.004 |
| Immunosuppression | 16 (26.2) | 11 (25.0) | 5 (29.4) | 0.725 |
| Hypoalbuminaemia | 31 (50.8) | 24 (54.5) | 7 (41.2) | 0.349 |
| Enteral feeding | 8 (13.1) | 8 (13.1) | 0 | 0.092 |
| Antibiotic therapy | 58 (95.1) | 43 (97.7) | 15 (88.2) | 0.185 |
| Antacids | 54 (88.5) | 40 (90.9) | 14 (82.4) | 0.386 |
| Charlson | ||||
| Low | 39 (63.9) | 28 (63.6) | 11 (64.7) | 0.938 |
| High | 22 (36.1) | 16 (36.4) | 6 (35.3) | |
| Concomitant infections | 34 (55.7) | 27 (61.4) | 7 (41.2) | 0.155 |
Significant p<0.05.
Clinical course of CDI cases according to the type of acquisition.
| Clinical course of CDI | Overall n (%) | Nosocomial n (%) | Community-acquired n (%) | p |
|---|---|---|---|---|
| Non-severe infection | 40 (65.6) | 27 (61.4) | 13 (76.5) | 0.371 |
| Severe infection | 14 (23.0) | 10 (22.7) | 4 (23.5) | >0.999 |
| Severe-complicated infection | 7 (11.5) | 7 (15.9) | 0 | 0.175 |
| Recurrences | ||||
| First | 12 (19.7) | 9 (20.5) | 3 (17.6) | >0.999 |
| Second | 3 (4.9) | 3 (6.8) | 0 | 0.553 |
| Attributable death | 4 (6.6) | 4 (9.1) | 0 | 0.296 |
| Unfavourable prognosis | 21 (34.4) | 17 (38.6) | 4 (23.5) | 0.371 |
Unfavourable prognosis: severe, severe-complicated infection, recurrences, or attributable death.
CDI: C. difficile infection.
Significant p<0.05.
In order to better understand the role of C. difficile in our health area, we conducted this research with 61 cases of CDI and 64 controls over a 14-month period. Hospital admission four weeks prior to infection, hypoalbuminaemia and prior antibiotic administration were risk factors for developing CDI. However, although other classic risk factors such as hospitalisation in the previous 4−12 weeks or the use of antacids and immunosuppressants were more common in cases than in controls, they were not significantly associated with a higher risk of infection.
Spain has one of the highest antibiotic consumption rates of any European country,6 which, together with an ageing population, with numerous comorbidities and continuous hospitalisations, creates the ideal breeding ground for acquiring CDI. In our study, we observed an incidence of 4.51 cases per 10,000 patient-days, lower than that found in Spain (6.5 episodes per 10,000 patient-days).7 We found a greater number of cases during the autumn/winter months, a time when respiratory infections and, therefore, antibiotic use increase, often unnecessarily because they are viral infections, such as the flu.
Community-acquired CDI has increased in the past decade,8 accounting for up to a third of new cases9,10 and affecting population groups previously considered low risk.11 This increase in community cases could be explained, in part, by the fact that they have been underdiagnosed because they are mild and self-limiting cases. Our results are consistent with these findings, as 28% of cases were community-acquired.
Our results showed that the CCDI group were younger patients, with a shorter length of hospital stay and a lower risk of having a severe infection. These findings are consistent with other published studies.9–11 Therefore, these data emphasise the need to find other possible sources of infection that justify the increase in the number of cases in the community, such as transmission of the disease through contact with animals and food.12
The use of antibiotics modifies the normal intestinal microbiota, which provides a “niche” for the overgrowth of C. difficile.13 It must be taken into account that both prolonged exposure to antibiotics and exposure to multiple antibiotics increase the risk of CDI.14 In total, 95% of our patients had received antibiotics in the previous three months. Since prior antibiotic exposure is the most important modifiable risk factor for the development of CDI, we stress the importance of developing antibiotic optimisation programmes in both hospital and community settings. Another modifiable CDI risk factor is hypoalbuminaemia, which may be a key point in preventing infection. Several studies show that hypoalbuminaemia is also a predictor variable of recurrent infection and higher mortality.15 According to our data, patients with hypoalbuminaemia have more than double the risk of acquiring a CDI.
The incidence of colonisation by C. difficile during hospitalisation varies between 2% and 20%, reaching up to 50% after being hospitalised for one month. In our study, hospitalisation in the previous four weeks resulted in an almost four times greater risk of infection. In addition, 35% of the community-acquired cases had had a previous hospital admission. These data confirm the importance of health centres as reservoirs of C. difficile spores, especially in emergency departments, since the high volume of patients that are treated in each shift and the continuous turnover of patients limit the capacity for adequate disinfection between one patient and another.16
We have not been able to identify age as an independent risk factor for CDI since, given the design of our study, the controls were matched by age and gender with the cases. The fact that the median age of the cases was 80 years indicates that age is indeed a potential risk factor for CDI. Because several of the recognised risk factors for CDI are often found in the elderly,17 the line between a risk factor and a confounding factor may become blurred, creating a certain degree of controversy in the results. For example, authors such as Khanafer et al.18 do not associate age with infection, while other authors, like us, do not find a statistically significant relationship with the administration of antacids19. However, we observed a high continuous consumption in our patients, so it would be advisable to reassess the need for their administration.
The selection of appropriate controls is one of the points that can most influence the results of a case-control study design. For this reason, to avoid possible biases, the controls closest in time and age to the cases were chosen. The information collected in the medical records may not accurately reflect the data, may be incomplete or even contain contradictory data. However, we believe that this information bias does not differentially affect cases and controls, so we assume that it would not invalidate the associations found. In addition, we have tried to control for possible confounding factors in the estimates of associations.
To provide a better understanding of the gaps in our knowledge regarding C. difficile transmission, we believe further studies are needed to determine whether animals are an important reservoir for CCDI20 and whether C. difficile can be transmitted after exposure to certain foods, such as red meat, poultry or fresh vegetables.21 In this way, the lifestyle or profession of a patient could influence the risk of acquiring the infection, either through their eating habits or through contact with domestic animals at home or at work in the case of veterinarians or farmers.
ConclusionsThe independent risk factors for acquiring a CDI in our health area are hospital admission in the previous four weeks, previous administration of antibiotics, and hypoalbuminaemia. Identification of these factors leads to a suspicion of infection. In addition, we identified fever and previous hospitalisation as unfavourable prognostic factors. By modifying or suspending antibiotic therapy whenever possible, adjusting albumin levels and prescribing specific therapy early, we can improve the prognosis of the infection.
FundingThis study received no specific funding from public, private or non-profit organisations.
Conflicts of interestNone.