First-degree relatives of patients with colorectal cancer (CRC) are at high risk of this disease. For this reason, medical organizations and clinical guidelines recommend more intensive screening and surveillance for such first-degree relatives than for the average-risk population. Colonoscopy has been the cornerstone of CRC screening in this setting. Although colonoscopy is the most sensitive technique for the detection of neoplastic lesions (especially non-advanced adenomas), its role is less clear for CRC. In addition, screening colonoscopy has several limitations that may affect the success of a screening campaign, such as poor participant acceptance, the need for skilled endoscopists, participant access to screening colonoscopy, overburdened endoscopy units, potential complications, and procedure-related costs. In addition, recent evidence has cast doubt on the advantage of colonoscopy over other strategies for the detection of advanced neoplastic lesions. Despite being less sensitive in general, other screening methods frequently recommended in the average-risk population may be more acceptable and thus help increase CRC screening uptake. This review discusses recent evidence on the risk of CRC in first-degree relatives, the advantages and disadvantages of each screening technique, participation rates depending on the technique, patient preferences, and barriers to screening.
Los familiares de primer grado de pacientes con cáncer colorrectal (CCR) tienen mayor riesgo de CCR que la población general. Por este motivo, las organizaciones médicas recomiendan una vigilancia más intensiva en esta población. Aunque la colonoscopia ha sido considerada la piedra angular de cribado, tiene limitaciones que pueden afectar el éxito de una campaña de cribado, que incluyen: baja aceptación, necesidad de personal entrenado, sobrecarga de trabajo, complicaciones y costes relacionados con el procedimiento. Evidencias recientes cuestionan la ventaja de la colonoscopia sobre otras estrategias de cribado para la detección de lesiones neoplásicas avanzadas. Aunque otras estrategias son menos sensibles, pueden ser mejor aceptadas y podrían incrementar la participación del cribado del CCR en los familiares de primer grado.
Esta revisión discute la evidencia actual sobre: riesgo de CCR en familiares de primer grado, beneficios y contrapartidas de cada técnica de cribado, participación de cada estrategia, preferencias y las barreras para la participación.
Colorectal cancer (CRC) is the second leading cause of cancer-related death in developed countries and it ranks third among the most common cancers worldwide.1 Several factors such as age, sex, and family history, have been related to the risk of developing CRC. Family history is the most powerful risk factor after age, particularly when dealing with first-degree relatives (FDR) of a patient with CRC.2 In addition, the risk of developing CRC is directly related to the number of relatives affected and inversely related to the age of the youngest index case.2–4
The risk of developing CRC in these FDRs is 2- to 4-fold higher than that of the average-risk population. For this reason, current practice guidelines recommend more intensive surveillance in this population. Screening starting at the age of 40 years or 10 years before the youngest case affected is universally recommended.5,6 In FDRs of patients with CRC, the predominant screening strategy is colonoscopy every 5 or 10 years, depending on the number of relatives affected and age at diagnosis, on the basis that it is the most effective procedure to detect and remove pre-malignant lesions or early cancers.7 However, colonoscopy has various limitations, such as its inherent invasiveness, potential life-threatening complications, the need for skilled endoscopists, subject access to screening colonoscopy, low screening uptake, and procedure-related costs. Finally, it is not an infallible screening method and significant neoplasms may be missed.8,9 Other screening strategies, including flexible sigmoidoscopy and stool-based tests, are also recommended for a select group of FDRs with the lowest risk of CRC.5,6 These recommendations are empirically based on the higher risk of CRC in FDRs compared with average-risk participants, but there is limited evidence about what procedure should be recommended at each level of risk, because randomized controlled trials comparing different strategies in this setting are scarce.7 Because of the scientific vacuum, some guidelines recommend that the screening test should be selected based on a risk/benefit assessment of the procedure, availability of the screening test, and patient preferences.10 The present review aims to assess the pros and cons of each screening strategy for FDRs of CRC patients, taking into account effectiveness, factors related to participation and patient preferences.
Familial risk of colorectal cancerApproximately 30% of patients with CRC have a family history of colorectal neoplasia11 and only 5% show hereditary syndromes conferring an increased risk of CRC. Around 10% of the general population have a family history of CRC.12,13 Therefore, most FDRs are labeled as individuals at moderate risk for CRC, distinguishing them from subjects with average-risk (general population aged over 50 years without any family history) and high-risk (individuals belonging to families with genetic syndromes described above). In these subjects, environment–genetic background interaction would explain the familial aggregation of cases with CRC, probably due to other genes or low-penetrance polymorphisms.14
In the general population, the risk of CRC is approximately 5%,13 but it is increased when familial aggregation is present. Factors such as age of the index case, kinship, number of affected relatives or tumor location in the index case are associated with different risks.
Published evidence on family risk of CRC can be summarized from three meta-analyses including 27, 33 and 57 studies.2–4 Age less than 50 years in the index case significantly increases the risk of CRC: relative risk (RR) ranged between 1.84 and 6.83. In another study, relatives of subjects with CRC older than 40 years had a similar risk to those over 50 years with no family history of CRC.12
The greater the number of FDRs affected with CRC, the higher the risk of developing CRC (RR=2.2, ≈4 and 8.5 for 1, 2 or 3 affected relatives, respectively). The existence of second-degree relatives affected with CRC is also a risk factor for this disease (RR=1.73).3 Similarly, a meta-analysis that included 13 studies found an increased risk of colorectal adenomas in individuals with a family history of CRC (OR 1.7; 95% CI 1.4–3.5).15 Regarding CRC location, FDRs of patients with colon cancer have a higher risk of CRC than those with relatives whose tumors are located in the rectum (RR≈2.3 vs. ≈1.9). FDRs of patients with left-sided colon cancer are at greater risk than those of patients with right-sided tumors.12 In familial CRC, knowledge of the genes involved in etiopathogenesis is scarce. Certain low-penetrance genes or polymorphisms of genes involved in DNA repair processes might explain the presence of cases of familial aggregation that cannot be classified in well characterized hereditary CRC syndromes. Such low-penetrance genetic alterations could trigger the development of advanced adenomas and speed up the transition from adenoma to CRC, thus increasing the risk of CRC in this population.16 If this hypothesis is true, one would expect to find a higher prevalence of advanced adenomas at an earlier age in FDRs of patients with CRC than in average-risk individuals. However, previous studies have yielded contradictory results. Some studies have found increased prevalence of advanced adenoma, but the results are limited by the small sample size and retrospective design.17,18 The few prospective studies that have been performed had the drawback of insufficient sample size to stratify for familial risk or did not include appropriate controls and showed discordant results.17,19–22 A recent study aimed to determine the prevalence and risk factors for advanced colorectal neoplasia (ACN) in asymptomatic FDR of patients with non-hereditary CRC.23 Asymptomatic participants from two Spanish randomized controlled trials, COLONPREV and COLONFAM, were included.7,24 The COLONPREV trial compared two screening strategies: one-time colonoscopy and biennial fecal immunochemical test (FIT) in an average risk population, whilst the COLONFAM trial compared colonoscopy with yearly FIT in a family risk population (excluding hereditary syndromes). Overall, 1564 subjects were included (686 average-risk participants and 878 FDRs). In this study, the prevalence of ACN was comparable between the average-risk and the familial-risk populations, and only those participants with two or more FDRs with CRC showed a marked increase in the prevalence of ACN. However, the risk of participants with just one affected relative did not differ from that of the average-risk population. On multivariate analysis, advanced age, male gender and the presence of two or more FDRs with CRC were associated with an increased risk of ACN. These data suggested that screening strategies for the familial-risk population should be revised.
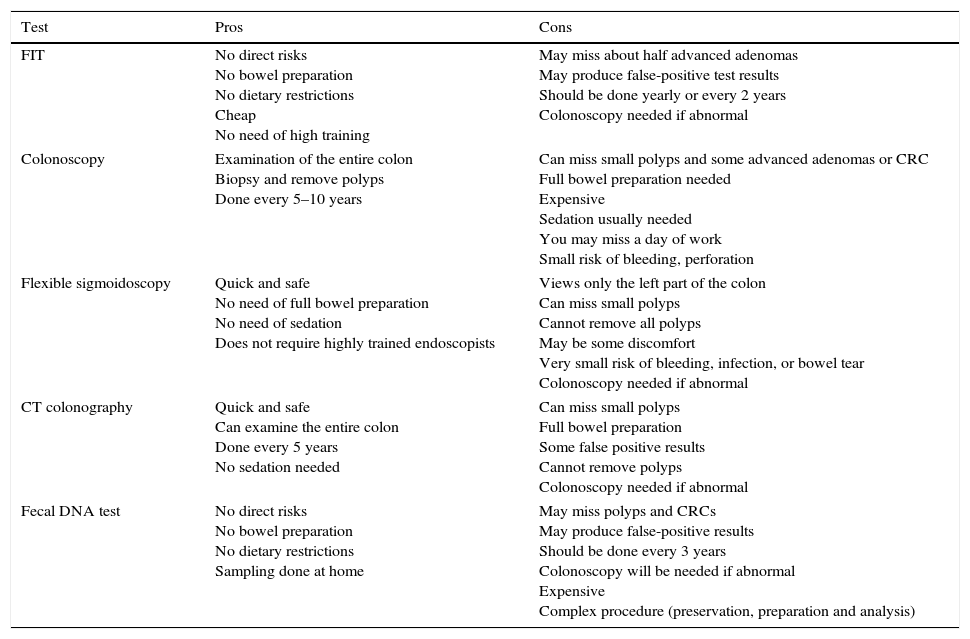
Colorectal cancer screening strategies in first-degree relatives of patients with colorectal cancer. Pros and cons (Table 1)As mentioned, family history and age are the main risk factors for developing CRC. Based on these findings, different screening strategies have been postulated by scientific societies in order to reduce the incidence of CRC. Although there is no uniform approach among scientific societies, most recommendations are based on colonoscopy, especially in the familial risk population, but other strategies are also recommended for specific risk groups.5,25–27
Pros and cons of each test.
| Test | Pros | Cons |
|---|---|---|
| FIT | No direct risks No bowel preparation No dietary restrictions Cheap No need of high training | May miss about half advanced adenomas May produce false-positive test results Should be done yearly or every 2 years Colonoscopy needed if abnormal |
| Colonoscopy | Examination of the entire colon Biopsy and remove polyps Done every 5–10 years | Can miss small polyps and some advanced adenomas or CRC Full bowel preparation needed Expensive Sedation usually needed You may miss a day of work Small risk of bleeding, perforation |
| Flexible sigmoidoscopy | Quick and safe No need of full bowel preparation No need of sedation Does not require highly trained endoscopists | Views only the left part of the colon Can miss small polyps Cannot remove all polyps May be some discomfort Very small risk of bleeding, infection, or bowel tear Colonoscopy needed if abnormal |
| CT colonography | Quick and safe Can examine the entire colon Done every 5 years No sedation needed | Can miss small polyps Full bowel preparation Some false positive results Cannot remove polyps Colonoscopy needed if abnormal |
| Fecal DNA test | No direct risks No bowel preparation No dietary restrictions Sampling done at home | May miss polyps and CRCs May produce false-positive results Should be done every 3 years Colonoscopy will be needed if abnormal Expensive Complex procedure (preservation, preparation and analysis) |
This technique is currently the gold-standard for CRC screening, as it has demonstrated high sensitivity for the detection of CRC and polyps, and a protector effect after polypectomy in terms of incidence and mortality.28,29 Evidence of its benefits in FDRs comes from prospective and retrospective cohort studies.30,31 Niv et al.30, in a case–control study, assessed the history of CRC screening (fecal occult blood test, flexible sigmoidoscopy, barium enema, and colonoscopy) in FDRs selected from a colonoscopy screening program between 1998 and 2000. The cases included 40 FDRs themselves affected with CRC and the controls comprised 160 FDRs without CRC. Screening colonoscopy was performed in only 2.5% of the case subjects in the previous 10 years compared with 48.7% of controls (p<0.0001). Similarly, some type of screening procedure other than colonoscopy was performed in 12.5% and 73.7%, respectively (p<0.0001). Dove-Edwin et al.,31 in a prospective, observational study carried out in FDRs, with a 16-year follow-up period, demonstrated a reduction in CRC-related mortality in those accepting colonoscopy screening. The incidence of CRC was 80% lower in the FDRs with moderate risk (one, two or three index cases) who underwent colonoscopy than the expected incidence that would have occurred in the absence of surveillance.
To date, randomized controlled trials designed to demonstrate the usefulness of colonoscopy screening in terms of reduced mortality and incidence compared with other screening strategies in FDRs have not been performed. However, colonoscopy has several drawbacks. First, it is not infallible, missing as many as 12% of advanced adenomas.9 Second, it is an invasive procedure with potential complications, reported to occur in about 2.8 per 1000 procedures.32 Third, negative perception regarding colonoscopy may lead to suboptimal adherence to recommended testing,33 and finally, investment in facilities and trained personal are needed. One study estimated the number of colonoscopies generated by a CRC screening program according to the screening strategy used.34 Considering a hypothetical participation rate of 60%, screening with colonoscopy could double the annual workload of endoscopy units.
Fecal occult blood testScreening with guaiac-based fecal occult blood test (FOBT) followed by diagnostic colonoscopy in positive cases has been shown to reduce CRC incidence and mortality in the average-risk population.35–39 FIT has demonstrated better accuracy for CRC and advanced adenoma than the old FOBT,40,41 as well as better adherence, because it requires fewer tests and no dietary restrictions. A recent meta-analysis41 including 19 studies in the average-risk population suggested that these tests had high accuracy, with high specificity and acceptable sensitivity for the detection of CRC. In this meta-analysis, a threshold lower than 20μg Hb/g feces yielded the best sensitivity/specificity ratio (89% and 91% respectively).
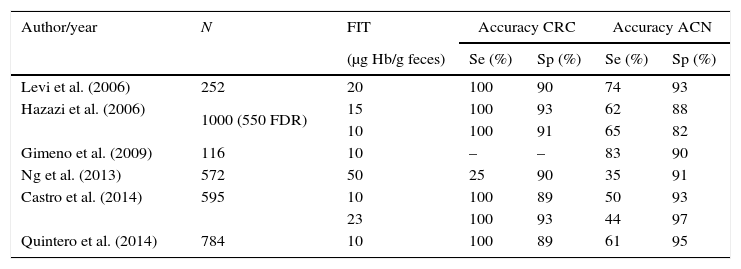
Several studies (Table 2)7,42–45 have assessed the accuracy of FIT for CRC detection in FDRs referred for screening colonoscopy, showing acceptable sensitivity and specificity for the diagnosis of CRC. In a retrospective study, Levy et al.45 included 252 FDRs who provided three FIT results before colonoscopy (cut-off 20μg Hb/g feces). The FIT was positive in 31 patients (12.3%). Sensitivity, specificity, positive and negative predictive values for CRC were: 100%, 90%, 16% and 100% respectively, and for ACN: 74%, 93%, 45% and 98% respectively. All the CRCs and 74% of the ACN were detected with FIT at the selected threshold of 20μg Hb/g feces. This study suggested that FIT-based screening would save up to 88% of colonoscopies. The same group designed another prospective double-blind study, which included 1000 ambulatory asymptomatic high-risk patients44 (555 with a family history of CRC, 445 surveillance for past neoplasm), tested by three FITs before elective colonoscopy. In this study, different thresholds were compared (10, 15 and 20μg Hb/g feces). Sensitivity for CRC and ACN was highest (65.3%; 95% CI 54.3–76.3) when the threshold was equal or lower than 10μg Hb/g feces (15.4%), with high specificity too (87.5%; 95% CI 86.4–90.5). At this positive cut-off level, all CRCs and 62% of ACN were detected.
Screening with fecal immunochemical test in familial CRC screening.
| Author/year | N | FIT | Accuracy CRC | Accuracy ACN | ||
|---|---|---|---|---|---|---|
| (μg Hb/g feces) | Se (%) | Sp (%) | Se (%) | Sp (%) | ||
| Levi et al. (2006) | 252 | 20 | 100 | 90 | 74 | 93 |
| Hazazi et al. (2006) | 1000 (550 FDR) | 15 | 100 | 93 | 62 | 88 |
| 10 | 100 | 91 | 65 | 82 | ||
| Gimeno et al. (2009) | 116 | 10 | – | – | 83 | 90 |
| Ng et al. (2013) | 572 | 50 | 25 | 90 | 35 | 91 |
| Castro et al. (2014) | 595 | 10 | 100 | 89 | 50 | 93 |
| 23 | 100 | 93 | 44 | 97 | ||
| Quintero et al. (2014) | 784 | 10 | 100 | 89 | 61 | 95 |
FIT: fecal immunology test; CRC: colorectal cancer; ACN: advanced colorectal neoplasia; FDR: first-degree relatives; Se: sensitivity; Sp: specificity.
Another study included 169 asymptomatic FDRs who were invited to return one FIT (positive threshold ≥10μg Hb/g feces) before a screening colonoscopy.43 FIT was able to detect 10 out of 12 (83%) advanced adenomas, with sensitivity, specificity, positive predictive value and negative predictive value of 83%, 91%, 53% and 98%, respectively. No cancers were detected in this study.
Castro et al.42 carried out a multicenter, prospective, double-blind study to assess the accuracy of FIT in this familial-risk population. They included 595 FDRs who delivered two consecutive FITs and underwent a screening colonoscopy. FIT showed high accuracy for the detection of CRC in FDRs, and the best sensitivity/specificity ratio for CRC diagnosis was achieved with a threshold of 23μg Hb/g feces. All CRCs were detected with the first FIT at the threshold of 20μg Hb/g feces. Two FITs did not improve the diagnostic accuracy for CRC, increased costs and the number of patients needed to scope (NNS) to detect a lesion. More disappointing were the results found by Ng et al. in 576 FDRs who had FIT and colonoscopy.46 Diagnostic performance of FIT was modest (sensitivities for ACN and CRC were 35.1% and 25%, respectively). However, the threshold used was much higher than in the rest of the studies (50μg Hb/g feces).
Recently, the first randomized study comparing yearly FIT with one-time colonoscopy in 1566 FDRs has been published. One FIT (<10μg/g feces) was offered during three consecutive years.7 After the last screening round, a colonoscopy was offered regardless of FIT result to all participants assigned to the FIT group. This study showed that FIT-based screening was equivalent to colonoscopy in detecting ACN in FDRs if adherence to FIT was at least 5% higher than that of colonoscopy.
Other strategies in familial colorectal cancer screeningComputed tomographic (CT) colonography has been shown to be useful in detecting ACN (larger than 10mm).47 As it is less invasive and possibly better tolerated than colonoscopy, CT colonography is now considered a valid alternative for CRC screening in the general population.48 In a multicenter, a cross-sectional study including 982 individuals at increased risk of CRC (FDRs, personal history of colorectal adenomas, or positive FOBT), CT colonography showed acceptable diagnostic yield for detecting ACN in this high-risk population (sensitivity 85.3% and specificity 90.8%), suggesting that it might be a valid option for screening or surveillance in high-risk individuals.49
Video capsule colonoscopy is a minimally invasive technique with proven usefulness in CRC screening as a safe and effective method for visualizing the colon and detecting colon lesions.50,51 In a randomized, prospective study that compared conventional colonoscopy and video capsule endoscopy in 320 FDRs of CRC patients, the rate of ACN detection was similar between the two techniques. However, participant acceptance of video capsule endoscopy was worse than that of conventional endoscopy in this population, because of the need to undergo conventional therapeutic colonoscopy in the event of a lesion being identified by the capsule.52
Other strategies, such as rectosigmoidoscopy or fecal DNA, have already proved their utility in average-risk CRC screening,53,54 but randomized studies in FDRs have not been performed.
ParticipationHigh participation rates are mandatory for the success of any screening campaign. Although the accuracy of colonoscopy for the detection of colorectal lesions is superior to that of other strategies, at least in a single screening round,7 it remains uncertain whether colonoscopy is superior to other screening strategies in the particular setting of a screening program. Indeed, although colonoscopy is the gold standard for CRC screening in FDRs, it is limited by suboptimal adherence.55–57 Low uptake to colonoscopy screening seems to be influenced by different factors: first, screening initiatives in FDRs are mostly opportunistic, as FDRs are usually excluded from average-risk population screening programs, and second, there is underutilization of colonoscopy at the recommended intervals in this population, as shown in various studies.56,57
Rees et al.58 reviewed thirty studies in order to assess the level of screening participation in a high-risk population. Screening uptake defined as “having ever had” sigmoidoscopy or colonoscopy ranged from 16% to 69%. Studies that included FDRs with complex family history (index case <60 years old, two or more relatives affected with CRC) found over 50% screening uptake. Screening uptake of FOBT varied considerably across studies, ranging from 22% to 88% when “having ever had a FOBT” was used as the definition of participation. When the definition was “having had a FOBT in the past year”, it ranged from 15% to 42%. So wide ranges in the rates of screening uptake might be explained by several factors. First, the authors did not provide a precise estimate of screening participation stratifying for genetic risk factors. Because they included people with inherited syndromes and familial risk, screening uptake may have been overestimated. Second, studies with different designs were included, such as community surveys, audit of screening programs, prospective studies of screening behavior and qualitative studies. In addition, most studies relied on self-reported family history and screening behavior.
More recently, AIT Ouakrim et al.55 conducted a systematic review and meta-analysis of observational studies to assess participation rates in FDRs, to overcome the limitations of the review by Rees et al., and to update the information on this topic. They included a total of seventeen studies involving 13,269 FDRs in total. Seven studies, which included 6901 FDRs, showed a pooled FOBT screening participation rate (defined as “having ever had” a FOBT) of 25% (95% CI: 12–38%). The five studies (with 5091 participants in total) that assessed having ever had sigmoidoscopy screening found a pooled participation rate of 16% (95% CI: 7%–27%). In seven studies (with 9965 FDRs in total) the pooled participation rate for colonoscopy screening was 40% (95% CI: 26%–54%). Five studies (with 2154 participants) reported screening participation based on guidelines recommendations. Compliance, defined as 5 yearly colonoscopy screening, was 31% (95% CI: 12–51%).
The authors concluded that CRC screening uptake in FDRs is suboptimal and initiatives are required to increase participation rates. Given the low participation rates of colonoscopy, current CRC screening guidelines should be adapted in accordance with the true state of screening in this population.
Predictors of screening uptake in FDRsSociodemographic data have not been consistently associated with screening uptake across studies.58,59 However, a recent systematic review aimed to assess predictors of screening in FDR, found higher screening uptake among adults aged 50–60 years compared with younger age groups.59 Participation may be influenced by the screening test offered. Indeed, colonoscopy participation was higher in younger age groups in two studies compared with older adults.60,61 High educational level was associated with greater screening participation in three out of eight studies, but the remaining others found no such association.59 Strong family history has been consistently associated with CRC screening behavior. In fact, FDRs with more than one relative with CRC were 3.7-fold more willing to enroll in screening programs compared with those with a single relative.59,62 Most studies also found that physician recommendation was a powerful method of engagement in screening, resulting in a 5- to 27-fold increase in the likelihood of undergoing CRC screening.59
Factors susceptible to modification by interventional strategies have aroused great interest. Theories of health behavior or theoretical models have been developed in order to identify independent predictors of screening uptake. The Health Belief Model is one of the best known theoretical models.63 This model allows one to identify factors that could be targets of intervention to increase compliance. Based on this model, the motivation needed to change health behaviors is associated with psychological factors, such as, knowledge of a disease, self-perceived susceptibility, perceived severity, positive and negative attitudes (i.e. benefits of screening and barriers) and motivation to preserve health. Psychological factors may be responsible, at least in part, for participation rates. Several studies have shown that FDRs were more likely to take part in screening than average-risk individuals.58,64 In addition, higher risk perception of CRC has been found in FDRs compared with average-risk individuals and it has been reported as an independent predictor of screening uptake in other studies.43,65 A large recent study involving 10,078 Chinese participants (42% were relatives of patients with CRC) and using a validated questionnaire about risk factors and symptoms of CRC found that self-perceived risk and a family history of CRC were independently associated with higher knowledge scores.66
The relationship between positive and negative attitudes and CRC screening was investigated in 6 studies included in a systematic review by AIT Ouakrim et al.59 Four of them found positive associations. Social pressure to take part in screening, the person's level of awareness and positive attitudes about how to keep healthy were associated with increased screening uptake. However, negative attitudes were not consistently associated with screening participation.
Most studies did not separately analyze predictors of screening participation using a specific test. In a recent systematic review,59 only three studies specifically assessed factors associated with screening colonoscopy in FDR. Age <60 years, complex family history of CRC (case index <60 years or ≥2 index cases), living in a rural area,67 physician recommendation,68,69 low negative attitudes,69 perception of easy access to colonoscopy,69 having discussed benefits and drawbacks with relatives,69 and being a member of an association were all factors associated to colonoscopy screening on multivariate analysis. In another recent study performed in FDR, lower negative attitudes (i.e. fear, embarrassment or pain/discomfort), expressed intention to undergo screening and advanced age, were significantly associated with colonoscopy uptake.70
Screening preferencesGiven the suboptimal participation of FDR, some authors have suggested that the goal in this population should be to increase screening uptake without regard for the screening test used. To achieve this goal, patient choices should be taken into account according to some authors.71 A large recent study, involving 7845 participants, assessed stated preferences for CRC screening between colonoscopy and FIT.72 In this study, family history and self-perception of high risk increased the chance of choosing screening colonoscopy, whilst advanced age, single marital status and a negative perception of CRC screening decreased the choice of colonoscopy. Schroy et al.71 assessed screening preferences in a group of FDR of a single index case. They used a shared decision-making process that involved an educational intervention which included information about CRC epidemiology and risk factors, the importance of screening and available strategies, and advantages and drawbacks of each test. Factors influencing the choice of the participant were assessed. Although colonoscopy ranked first in screening preferences, 44% of the participants preferred other screening strategies, in most cases FOBT. Accuracy was named as the main factor influencing the decision, followed by discomfort. The age of the index case ≥60 years and a previous FOBT were independently associated with colonoscopy preference. Barriers, costs and self-perceived risks were not properly tested. It should be emphasized that two-thirds of the participants thought that the decision should be either a consensus between patient and provider or it should completely fall on the screener. This percentage was even higher among those participants who ranked colonoscopy in first place. A recent systematic review about preferences in the average-risk population failed to conclude which test was preferred because of a high heterogeneity across the studies.73 However, most studies agree that the preferred test should be sufficiently sensitive, it should last a short time, it should not require bowel preparation and it should entail no risk of complications. As this type of test does not exist, there was no single strategy that was unanimously preferred.
ConclusionRecent evidence suggests that risks of ACN could be similar in average-risk individuals and FDRs with less than two relatives affected, regardless of the age of the index case at diagnosis. Although colonoscopy is the most sensitive screening tool for the diagnosis of pre-neoplastic lesions and CRC, it may be limited because of poor subject acceptance, difficulties in access, availability, complications, discomfort and costs. In the screening setting, a prerequisite is to achieve high participation since the screening program would otherwise have a low impact. In addition, taking into account participation rates and accuracy, FIT may be equivalent to colonoscopy for the detection of ACN. Interventions targeting barriers to screening in general and specific techniques are required to increase participation. Since participation with any screening technique is suboptimal, the primary objective should be to ensure screening without regard for the strategy used. To achieve this goal, patient preferences should be taken into account and guidelines should be more flexible.
Conflict of interestThe study received no financial support. The authors declare they have no conflict of interest.