Granulocyte–monocyte apheresis (GMA) has shown to be safe and effective in ulcerative colitis (UC), also in combination with biologics, mainly with anti-TNF. The aim of this study was to evaluate the efficacy and safety of combining GMA after primary non-response (PNR) or loss of response (LOR) to ustekinumab (UST) in patients with UC.
Patients and methodsA retrospective study was performed in 12 IBD Units, including all patients with refractory UC or unclassified IBD (IBD-U) who received combined GMA plus UST. The number and frequency of GMA sessions, filtered blood volume and time of each session were registered. Efficacy was assessed 1 and 6 months after finishing GMA by partial Mayo score, C-reactive protein (CRP) and fecal calprotectin (FC). Descriptive statistics and non-parametric tests were used in the statistical analysis.
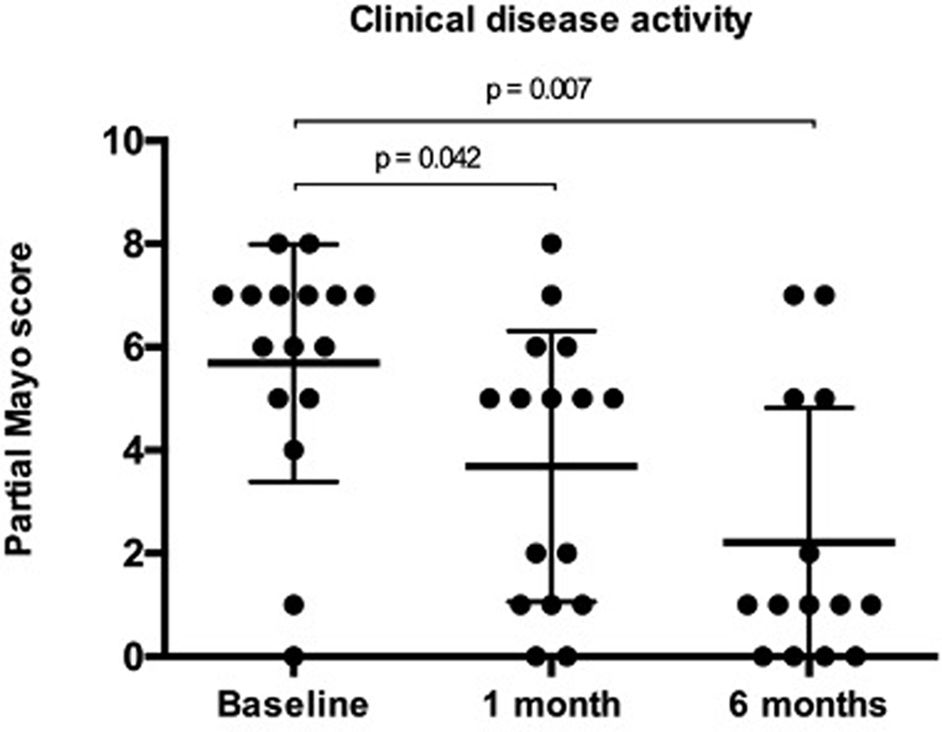
ResultsSeventeen patients were included (15 UC, 2 IBD-U; median age 47 years [IQR, 35–61]; 59% male; 53% E3). Most patients (89%) had prior exposure to anti-TNF agents and 53% to vedolizumab; 65% were also receiving steroids at baseline. Median partial Mayo score at baseline was 6 (IQR, 5–7) and it significantly decreased after 1 and 6 months (p=0.042 and 0.007, respectively). Baseline FC significantly decreased after 6 months (p=0.028) while no differences were found in CRP. During follow-up, 18% patients started a new biologic therapy and 12% required surgery; 64% of patients under steroids were able to discontinue them. Adverse events were reported in one patient.
ConclusionGMA can recapture the response to UST in selected cases of UC after PNR or LOR to this drug.
La granulocito-monocito aféresis (GMA) ha demostrado ser segura y eficaz en el tratamiento de la colitis ulcerosa (CU), incluso en combinación con fármacos biológicos, especialmente anti-TNF. El objetivo de este estudio fue evaluar la eficacia y la seguridad de la combinación de GMA y ustekinumab (UST) tras falta de respuesta primaria o pérdida de respuesta a este fármaco en pacientes con CU.
Pacientes y métodosEstudio retrospectivo realizado en 12 Unidades de EII que incluyó a todos los pacientes con CU refractaria o EII no clasificada que recibieron GMA más UST. La eficacia se evaluó 1 y 6 meses después de finalizar la GMA mediante la puntuación de Mayo parcial, la proteína C reactiva (PCR) y la calprotectina fecal (CF). El análisis estadístico se realizó mediante análisis descriptivos y pruebas no paramétricas.
ResultadosSe incluyeron 17 pacientes (mediana 47 años [RIC: 35-61]; 59% mujeres; 53% E3). La mayoría de los pacientes (89%) habían recibido previamente anti-TNF y un 53% vedolizumab; el 65% también recibían esteroides concomitantes. La mediana de la puntuación de Mayo parcial basal fue de 6 (RIC: 5-7), y disminuyó significativamente tras 1 y 6 meses (p=0,042 y 0,007, respectivamente). La CF disminuyó significativamente tras 6 meses (p=0,028) y no se encontraron diferencias en la PCR. Durante el seguimiento, el 18% de los pacientes iniciaron un nuevo fármaco biológico y un 12% requirieron cirugía; el 64% de los pacientes tratados con esteroides pudieron suspenderlos. Se registraron efectos adversos en un paciente.
ConclusiónLa GMA podría recuperar la respuesta a UST en casos seleccionados de CU tras la falta de respuesta primaria o pérdida de respuesta a este fármaco.
Granulocyte–monocyte apheresis (GMA) has shown clinical efficacy in patients with inflammatory bowel disease (IBD).1,2 This therapy consists in a column of cellulose acetate beads that enables the interaction between blood components and selectively depletes activated neutrophils, monocytes and macrophages. The blood is then reinfused to the patient.3–7
Several studies and case series have shown positive results in patients with ulcerative colitis (UC) when combining GMA with different biologics, as well as with anti-integrin therapies1,6,8–10 and, in a lesser extent, with small molecules.11 Moreover, GMA has been shown to have a dose-sparing effect on steroids in steroid-dependent UC3,11 including in those failing advanced therapies.
Ustekinumab (UST), a fully human IgG1 monoclonal antibody targeting the p40 subunit of interleukin (IL)-12 and IL-23, is approved for moderate to severe UC.12 UST has demonstrated its safety and efficacy on the induction and maintenance of clinical remission in UC.13,14 The long-term extension of the UNIFI trial and recent real-world evidence corroborate its efficacy and safety even after 3 years of treatment.15–19 However, more than 40% of UST-treated UC patients have an inadequate response,20 although reinduction may be an option in case of loss of response (LOR) to UST.21 In contrast, two case series have shown the possibility of regaining response after combining GMA with UST in refractory patients.22,23 Hence, considering the limited evidence and treatment options in patients failing UST therapy, we investigated the combination therapy with GMA plus UST after PNR or LOR to this drug in patients with refractory UC.
Patients and methodsStudy populationA retrospective, multicentre study was performed in 12 IBD Units in Spain. We included all patients with refractory UC or unclassified IBD (IBD-U) who received the combination of GMA and UST between April 2019 and December 2022.
Treatment and assessmentsWe collected data on age at diagnosis, disease location according to Montreal classification,24 previous IBD-related medications, concomitant medical therapy, past relevant disease history, smoking habits, body mass index, disease duration, and extraintestinal manifestations. All IBD Units involved in this study follow the current clinical practice guidelines from the European Crohn's and Colitis Organisation (ECCO)25 and the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU).26
The number and frequency of GMA sessions, the filtered blood volume and the length of each session were compiled, along with clinical data. Efficacy was assessed 1 and 6 months after finishing GMA by partial Mayo score, including also C-reactive protein (CRP) and fecal calprotectin (FC). The proportion of patients with FC values <250mg/kg was calculated, Data regarding the need of UST dose escalation, use of new immunomodulators or biologics and colectomy during follow-up were also registered.
The use of GMA plus UST is not an established treatment for IBD, so the decision to start this combined therapy and its schedule were made on a case-by case basis based on the individual characteristics and clinical situation. All patients were treated with the same GMA device (Adacolumn®, JIMRO, Takasaki, Japan)5 receiving weekly, twice-weekly or three times per week sessions. The filtered volume and the duration of the sessions were chosen at the physician's discretion (the recommended volume is 1800mL per session of 60min, though a more intensive treatment schedule has been also described).11,23 Before starting GMA, patients received a bolus of weight-based low molecular-weight heparin.
All adverse events (AEs) were recorded during follow-up. Serious AEs were defined as any AE leading to treatment discontinuation, hospitalization, disability, colectomy, or death.
DefinitionsPNR was defined as the persistence of symptoms with a partial or complete absence of improvement in the partial Mayo score during the first 8–16 weeks of UST therapy. Secondary LOR was defined as a clinical relapse with a partial Mayo score≥2 and a bleeding subscore>1 after 16 weeks of starting UST after a period of clinical remission during the induction period.
Statistical analysisDescriptive statistics of the sample were determined, using frequencies and percentages for categorical variables and means and SD for continuous variables. Variables with biased distributions were expressed as median and interquartile ranges (IQRs). The Wilcoxon test was used to assess differences in continuous variables between the different time points. Statistical significance was defined as p<0.05.
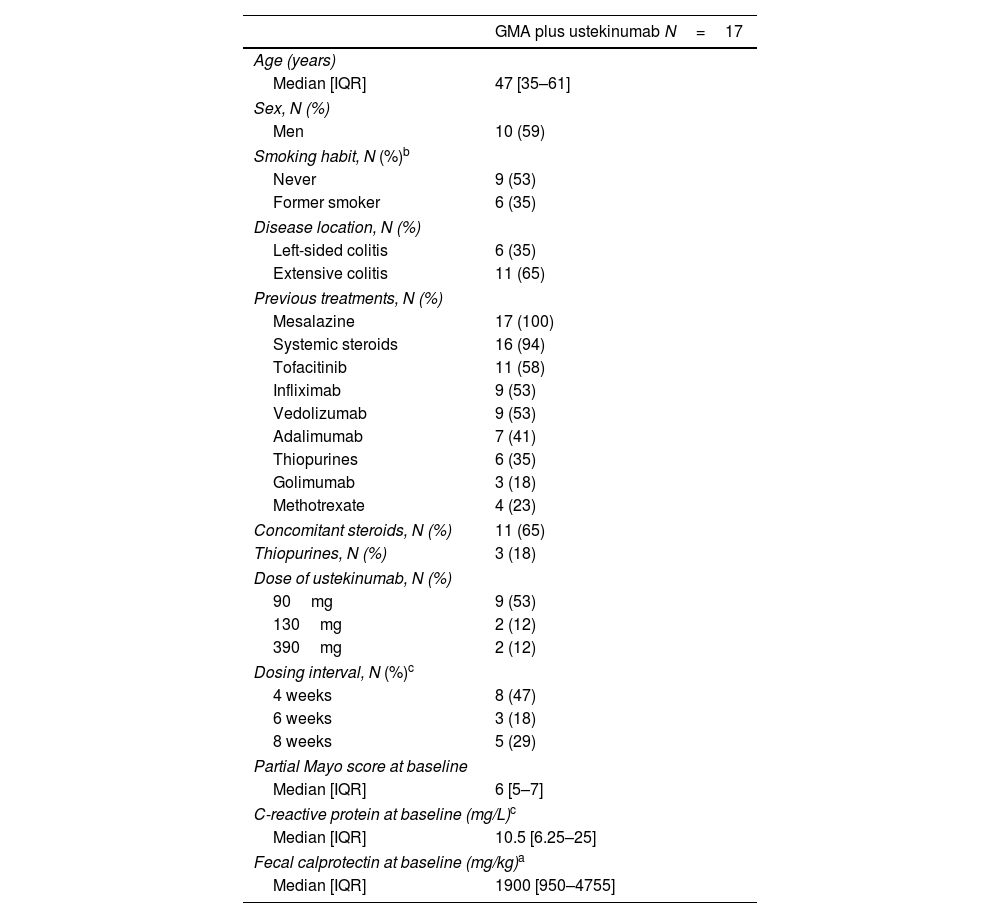
ResultsSeventeen patients were included, 15 with UC and 2 with IBD-U. Patient characteristics are summarized in Table 1. Median age at the moment of starting GMA was 47 years (IQR, 35–61) and 59% of patients were male. UC was classified as extensive (E3) in 11 cases (65%) and left-sided in 6 (35%). Median disease duration was 73 months (IQR, 23–164).
Patient characteristics.
| GMA plus ustekinumab N=17 | |
|---|---|
| Age (years) | |
| Median [IQR] | 47 [35–61] |
| Sex, N (%) | |
| Men | 10 (59) |
| Smoking habit, N (%)b | |
| Never | 9 (53) |
| Former smoker | 6 (35) |
| Disease location, N (%) | |
| Left-sided colitis | 6 (35) |
| Extensive colitis | 11 (65) |
| Previous treatments, N (%) | |
| Mesalazine | 17 (100) |
| Systemic steroids | 16 (94) |
| Tofacitinib | 11 (58) |
| Infliximab | 9 (53) |
| Vedolizumab | 9 (53) |
| Adalimumab | 7 (41) |
| Thiopurines | 6 (35) |
| Golimumab | 3 (18) |
| Methotrexate | 4 (23) |
| Concomitant steroids, N (%) | 11 (65) |
| Thiopurines, N (%) | 3 (18) |
| Dose of ustekinumab, N (%) | |
| 90mg | 9 (53) |
| 130mg | 2 (12) |
| 390mg | 2 (12) |
| Dosing interval, N (%)c | |
| 4 weeks | 8 (47) |
| 6 weeks | 3 (18) |
| 8 weeks | 5 (29) |
| Partial Mayo score at baseline | |
| Median [IQR] | 6 [5–7] |
| C-reactive protein at baseline (mg/L)c | |
| Median [IQR] | 10.5 [6.25–25] |
| Fecal calprotectin at baseline (mg/kg)a | |
| Median [IQR] | 1900 [950–4755] |
At baseline, 65% of the patients were receiving steroids. Most patients had prior exposure to anti-TNF agents, 65% to tofacitinib, and 53% to vedolizumab (VDZ). Median partial Mayo score at baseline was 6 (IQR, 5–7), with a median CRP of 10.5mg/L (IQR, 6.25–25) and FC of 1900mg/kg (IQ, 950–4755).
The main characteristics of GMA therapy are presented in Table 2. GMA was started mostly after PNR in 70% of the patients; 47% of patients started maintenance GMA. Sessions were twice-weekly in two thirds of patients (65%). The median clinical follow-up was 14 months (IQR, 5–29).
Characteristics of the granulocyte and monocyte apheresis (GMA) treatment (n=17) and duration of follow-up.
| GMA characteristics | GMA plus ustekinumab N=17 |
|---|---|
| Indication of apheresis, N (%) | |
| Primary nonresponsea | 12 (71) |
| Secondary loss of response | 2 (12) |
| Type of primary nonresponse, N (%)a | |
| Partial response | 10 (83) |
| No response | 2 (17) |
| Number of sessions at induction | |
| Median [Q1, Q3] | 10 [8–10] |
| Range | 6–14 |
| Total number of sessions | |
| Median [Q1, Q3] | 16 [11–24] |
| Frequency of sessions, N (%) | |
| Weekly | 2 (12) |
| Twice-weekly | 11 (65) |
| Three times per week | 1 (6) |
| Filtered volume, mL | |
| Median [IQR] | 2780 [2610–3200] |
| Range | 1800–4800 |
| Duration of sessions, min | |
| Median [IQR] | 90min (90–90) |
| Range | 60–100 |
| Maintenance apheresis, N (%) | 8 (47) |
| Follow-up, months | |
| Median [IQR] | 14 [5–29] |
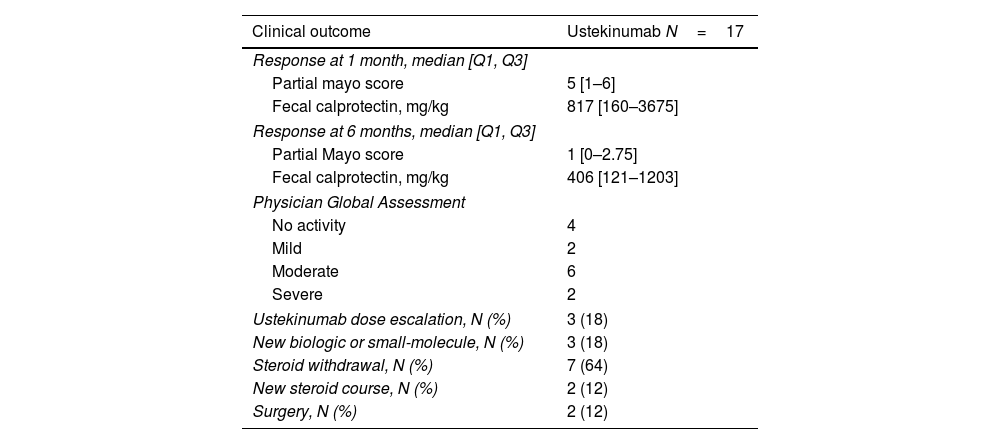
Follow-up data at 1 month was available for all patients and for 10 patients (83%) after 6 months. Partial Mayo score significantly decreased to a median of 5 (IQR, 1–6; p=0.042) 1 month after the last GMA session, and to a median of 1 (IQR, 0–2.75; p=0.007) at 6 months (Fig. 1 and Table 3). Clinical remission was achieved by 6 and 9 patients after 1 (35%) and 6 months (53%), respectively. CF values<250mg/kg were observed in 3 and 4 patients at 1 and 6 months (data available in 10 and 11 patients, respectively). There were no statistically significant changes in CRP, while FC levels numerically decreased at 1 month (p=0.401) and they were significantly reduced only after 6 months (p=0.028). Three patients receiving UST required dose or interval escalation and no patients were able to reduce the dose or increase the interval. Among those patients receiving steroids at baseline, 64% were able to stop them completely. During follow-up, 2 patients (12%) required a new course of steroids. Three patients (18%) stopped UST and started a new biologic therapy (1 tofacitinib, 1 filgotinib, and 1 vedolizumab) and 2 (12%) required surgery. AEs were reported in one patient (6%) who developed headache but this did not lead to changes in the medical therapy or GMA regimen.
Clinical outcomes (n=17).
| Clinical outcome | Ustekinumab N=17 |
|---|---|
| Response at 1 month, median [Q1, Q3] | |
| Partial mayo score | 5 [1–6] |
| Fecal calprotectin, mg/kg | 817 [160–3675] |
| Response at 6 months, median [Q1, Q3] | |
| Partial Mayo score | 1 [0–2.75] |
| Fecal calprotectin, mg/kg | 406 [121–1203] |
| Physician Global Assessment | |
| No activity | 4 |
| Mild | 2 |
| Moderate | 6 |
| Severe | 2 |
| Ustekinumab dose escalation, N (%) | 3 (18) |
| New biologic or small-molecule, N (%) | 3 (18) |
| Steroid withdrawal, N (%) | 7 (64) |
| New steroid course, N (%) | 2 (12) |
| Surgery, N (%) | 2 (12) |
We herein have reported the efficacy of combining GMA plus UST after PNR or LOR to this agent in patients with multi-refractory UC or IBD-U. Most patients of our cohort, where GMA was mostly started after PNR, had prior exposure to anti-TNF agents, mainly infliximab and adalimumab. More than half of them had also received tofacitinib and/or VDZ and were under steroids at baseline. Our findings show that this combination therapy might enable disease control and reduce the need of switching in these patients with limited therapeutic options.
GMA with Adacolumn® (JIMRO, Takasaki, Japan) is an effective and safe therapeutic option for patients with mild-to-moderate UC refractory to conventional medical therapy.6–9 It has shown long-term benefit in patients with active UC and insufficient response or intolerance to immunosuppressants or biologics.27 GMA is approved in Europe for inducing remission in patients with active UC or CD, and it has been commonly used in steroid-dependent UC.5 The usual GMA protocol consists in 1–2 sessions per week up to a total of 10 sessions,8 with a duration of 60–90min per session.4,6,28–30 Nonetheless, intensive GMA (two sessions per week) appears superior to weekly GMA in terms of both remission rate and time to remission among patients with refractory UC.31,32 In our cohort, 78.6% of the patients received twice-weekly GMA sessions with a median number of 16 sessions. In addition, almost half of the patients received maintenance GMA, suggesting a trend to choose intensive therapy in these highly refractory patients, even though this use remains controversial.8
GMA may act synergistically when combined with biologics or small molecules used for UC. The mechanism by which GMA improves UC involves selective adsorption of activated granulocytes and monocytes, decreased leukocyte-endothelial adhesion molecule-1, decreased chemotaxis, induction of qualitative changes in leukocytes and elimination of inflammatory cytokines by increasing soluble TNF-α receptors, resulting in a better control of gut inflammation.8 Furthermore, in the event of secondary LOR to anti-TNFs, GMA may help restoring the therapeutic effect in a proportion of patients,7,33 probably by reducing anti-drug antibodies without altering trough levels.9,34 Nevertheless, the precise mechanism of action of the combination of GMA with UST deserves more research.
In our cohort, partial Mayo score significantly decreased 1 and 6 months after the last GMA session. Notably, only two patients required colectomy during follow-up; most patients under steroids at baseline where able to discontinue them and only two required a new course of steroids. This confirms previous studies showing the steroid-sparing effect of GMA,3 which might be also applicable in the event of steroid-dependency in patients receiving biologics and small molecules.8
Two recent meta-analyses on the real-world outcomes of UST in UC35,36 have reported clinical remission rates of 24–60% after 4–16 weeks, 40–58% at 6 months and 33–79% at one year.36 At three years, the outcomes were better in the treatment-naïve population.13 The low complete response achieved with UST monotherapy (15.5% at 8 weeks)14 is consistent with the substantial proportion of UC patients that require successive lines of therapy due to a deficient response or a relapse after an initial response.37
Only two case series have described the feasibility of combining GMA with UST in patients with refractory IBD.22,23 Tanida et al. in 2018 retrospectively examined the efficacy and safety of combining intensive GMA plus UST during induction therapy, in refractory CD in three consecutive cases including two patients who were refractory to maintenance anti-TNF-α biologics.22 Clinical remission was achieved at week 10 in all cases, while serum CRP levels were not normalized in two cases and no endoscopic improvements were observed. In 2021, the same group retrospectively evaluated the effectiveness of UST plus intensive GMA on refractory UC patients including two corticosteroid-dependent patients, two corticosteroid-refractory patients and one patient with LOR to tacrolimus.23 Of the four patients who received this combination therapy, two (50%) achieved clinical remission at 10 weeks. The rate of patients achieving endoscopic improvement at 10 weeks was also 50%. In all cases, steroids were discontinued within 10 weeks. In both studies,22,23 this combination therapy showed a trend toward reducing CRP levels, though no significant differences were observed, as is the case of our cohort. It should be noted that, in our study, we did not combine GMA with UST during the induction, but after PNR or LOR to this agent. In any case, these data highlight the potential of this combination therapy in improving the outcomes at different clinical scenarios of IBD refractory patients.
Regarding safety, GMA appears to be safe and well tolerated in the treatment of IBD in steroid-dependent cases and also in combination with biologics.7,38 In our cohort, one patient reported an AE related to the therapy. This low rate is consistent with the findings of both studies of Tanida et al., where no EAs were observed during intensive GMA and UST therapy.22,23 Again, the low rate of surgery and AEs and the ability to reduce steroids may allow a better control the inflammatory process without switching the therapy in these refractory patients.
Our study has certain limitations in terms of the sample size, the retrospective design and the variability of UST and GMA regimens, which may have limited the robustness of our conclusions. Though, our results contribute to a field where data are scarce and could be the basis for future clinical trials exploring the potential of combining GMA plus UST in refractory UC patients. Large prospective studies are required to elucidate the mechanisms underlying the beneficial effect of this strategy and define the profile of patients with the highest probability of response.
ConclusionsDespite the benefits of UST therapy, a significant proportion of patients with UC develop PNR or LOR to this drug. Our results suggest that adding GMA to UST after PNR or LOR to this drug could be effective for selected refractory patients with active UC. Further research in larger cohorts of patients is needed to support these findings.
Authors’ contributionsIR-L and JLC: Study design, data analysis and drafting the manuscript.
CH-G, MB-W, JIG, EL, MC, FC, SC, CC, AE, EG, EI, DS, MB-A, DG: data collection.
DG and JLC: revised the manuscript for important intellectual content.
Ethical considerationsInformed consent was obtained from all patients included in the study. The study was approved by the local Ethics Committee of the coordinating center (EPA2017050).
FundingIR-L is funded by a research grant from Gobierno Vasco – Eusko Jaurlaritza [grant number 2020111061] and by a research grant from Biobizkaia Health Research Institute [Grant No BCB/I/LIB/22/008].
Conflict of interestIR-L has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Adacyte, Celltrion, Chiesi, Danone, Ferring, Faes Farma, Janssen, Galapagos, MSD, Pfizer, Roche, Takeda, and Tillotts Pharma. Financial support for research: Tillotts Pharma.
EL has received financial support for traveling and educational activities from or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Ferring, Dr Falk Pharma, Adacyte y Otsuka Pharmaceutical.
MCI has received financial support for traveling and educational activities from Abbvie, Janssen, Pfizer, Adacyte, Takeda, Kern Pharma and Tillots Pharma.
SC has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Janssen, Pfizer, Aldacyte, Dr Falk Pharma, Galápagos.
MB-A has received financial support for traveling and educational activities from or has served as an advisory board member for Pfizer, MSD, Takeda, AbbVie, Kern, Janssen, Fresenius Kabi, Galapagos, Lilly, BMS, Faes Farma, Chiesi and Adacyte.
JLC has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Adacyte, Chiesi, Ferring, Janssen, MSD, Pfizer, Takeda, and Tillotts Pharma.
The remaining authors have no conflicts of interest to declare.
We thank Adacyte for supporting the development of the database for this study. The company did not have an influence on the study design, interpretation of the results or drafting the manuscript.