Chagas disease, also known as American trypanosomiasis, is a zoonosis that affects about 8–12 million people in Latin America.1,2 It is estimated that some 40 million people are at risk of acquiring the infection.3
In its natural life cycle, Trypanosoma cruzi (T. cruzi) is transmitted through triatomine vectors. Traditionally, Chagas disease was regarded as being typical of poor rural areas, though at present, increased migratory trends have caused the disease to manifest in urban areas of endemic countries,2,4 as well as in countries that receive a growing number of immigrants.5,6 In such areas, where the vector is lacking, T. cruzi can be transmitted through blood transfusions and blood products,7 organ and tissue transplants,8 or vertically from infected mothers to their offspring.9
Following an acute phase that usually goes unnoticed because of its few or no symptoms, untreated Chagas disease enters an initially asymptomatic chronic phase known as the indeterminate form of the disease. After a prolonged period of time (20–30 years), 20–30% of all infected patients develop cardiac complications (cardiac form), 15–20%10 suffer digestive disorders (digestive form) or both (mixed form), and under 5% develop the neurological form of the disease. The rest of the patients continue to present the indeterminate form of Chagas disease, with no clinical manifestations at any point in life.11 Digestive manifestations are the second most common cause of complications of Chagas disease, and although the mortality rate is low, they can have a considerable impact upon patient quality of life. The prevalence of such digestive manifestations varies according to the geographical origin of the patients. In this sense, they are more common in central Brazil, less frequent in Bolivia, and practically non-existent in countries north of the Amazon basin, Central America and Mexico. In non-endemic areas, the prevalence of digestive manifestations associated with the disease depends on the origin of immigration. In Spain there have already been reports of patients with Chagas disease and digestive complications.12,13
As has been commented, the digestive complications of Chagas disease are more common in the central and southern regions of South America. Although all parts of the digestive system may be affected, the alterations with the greatest clinical expression correspond to the oesophagus and colon. In endemic countries, megacolon is usually the final manifestation, since onset of symptoms is slower than in the case of oesophageal involvement.14
Spain is one of the countries that receive most immigrants from Latin America. In 2001, Spain registered 49% of total immigration from Latin American within Europe, followed by Italy (13%), England (12%) and Germany (10%).15 Immigration from Ecuador was most significant in Spain, while in England 56% of the immigrants came from Jamaica, a non Chagas endemic area. These discrepancies can explain the differences in the epidemiology of Chagas disease in different European countries, and they have already published data on different aspects of the illness.16–18 At present, Spain has more than 2 million Latin American immigrants from endemic areas.19
Chagas disease is an emergent disease in Spain,5 and poses different challenges for the Spanish healthcare system.20 One such challenge is adequate management and treatment of the patients that develop complications of the disease. In line with the consensus document on the management of the cardiac complications of Chagas disease auspiced by the Sociedad Española de Medicina Tropical y Salud Internacional (SEMTSI),21 the present document addresses the diagnosis, management and treatment of the digestive manifestations of the disease in the Spanish setting.
Pathogenesis of digestive involvement in Chagas diseaseThe pathogenesis of the disease is subject to controversy, though current knowledge points to the existence of mixed mechanisms with direct participation by the parasite22,23 and associated autoimmune phenomena24,25 involving microvascular alterations and autonomous denervation.26–29
Thus, the acute phase of Chagas disease is characterized by direct parasite invasion of the muscle tissue of the digestive system, with lymphocytic infiltrates (ganglionitis) and degenerative neuronal lesions.30
However, the pathogenesis of the digestive involvement has been most studied in the chronic form of the disease. The fundamental damage that occurs as a result of peristaltic dysfunction and that ultimately results in megavisceral presentations is the destruction of the neurons of the enteric nervous system.31 Neuronal damage is variable in each case, and can affect all sections of the digestive tract.
A recent study in patients with megacolon suggests that such neuronal destruction may be selective, fundamentally affecting the nitric oxide (NO) and vasoactive intestinal peptide (VIP) producing neurons that are involved in smooth muscle relaxation.32 In cases of megacolon and megaoesophagus, an important reduction in the number of interstitial cells of Cajal has also been demonstrated33,34 These cells play an important role in the modulation of digestive tube motility; as a result, their destruction could constitute a key element in the pathogenesis of the digestive changes of Chagas disease.
From the histological perspective, and in additional to neuronal reduction, patients with Chagas disease and megacolon present focal areas of fibrosis in the smooth muscle and myenteric plexuses, together with lymphocytic infiltrates at submucosal, myenteric plexus and smooth muscle level. Probably as a compensating mechanism, muscle wall hypertrophy is observed. Such hypertrophy becomes less apparent in very advanced forms of the disease.35
The ultimate outcome of the motor disorders, with sphincter discoordination and increased intraluminal pressure, is progressive dilatation and a reduction of organ contractile capacity. In addition, the muscularis mucosa can become hypertrophic, and there have been reports of hyperplastic and dysplastic alterations of the oesophageal epithelium favouring an increase in the number of cases of carcinoma.36
Although the oesophagus and colon (particularly the rectum, sigmoid colon and descending colon) are the portions of the digestive tube with the most evident clinical manifestations, all gastrointestinal segments can be affected. At gastric level there have been reports of altered myoelectrical activity (gastric dysrhythmias), alterations in gastric emptying and distension, hypoperistalsis,37 hypotonus, hypochlorhydria and eventually pyloric hypertrophy – though gastric dilatation is not frequent. Involvement of the small bowel has also been documented (Chagas enteropathy), manifesting either with or without dilatation.38
Involvement of the small intestine may favour the development of bacterial overgrowth, pseudo-obstructive syndromes and dyspeptic syndrome.35 Patients with gastric or small intestine involvement normally also present oesophageal or colon involvement.35,38
Diagnosis of chronic infection due to T. cruziThe diagnosis of Chagas disease has already been addressed in the first consensus document5 and in other reviews.39 In addition to the considerations of the mentioned consensus document, and in the context of the study of the digestive manifestations of chronic Chagas disease, it is advisable to screen for Trypanosoma cruzi in all patients coming from Latin America and presenting with constipation, dysphagia or any of the digestive symptoms attributable to the disease. Mention will only be made here of the fact that the diagnosis is based on two criteria:
- •
Compatible epidemiological antecedents.
- •
Serological tests. In order to accept infection, the patient must yield two positive results with two serological techniques involving the use of different antigens. In the event of doubts or discrepancies between them, a third technique is indicated.
The aim is to detect symptoms indicative of probable digestive involvement. To this effect, attention should focus on the associated signs and symptoms reflected in Table 1. When compiling the medical history, a number of aspects must be taken into account:
- 1.
Coexisting heart disease may be found in up to 30% of all patients.40
- 2.
Patients may also present digestive disorders unrelated to Chagas disease.
- 3.
There may be variations in the way in which the symptoms are described, due to the different cultural origins of the patients.
- 4.
Anxiety associated with the immigration process may be related to some symptoms or may condition them.
- 5.
Patients should be questioned about their current dietary habits. Immigration causes many Latin American immigrants to change their diets in comparison with what they were used to eating in their home countries. There are basically two reasons for this: economic and market-related (i.e., certain foods typically found in Latin American countries are difficult to find in Spain). These changes in diet can in turn induce changes in bowel habit, and may modify the symptoms related with Chagas disease.41
- 6.
Drug treatment. It is important to know whether the patient is taking certain drugs that can intensify or lessen the digestive symptoms (e.g., codeine, laxatives, psychoactive drugs, etc.42)
- 7.
As in all clinical histories, it is important to establish previous illness, as this will help us to correlate the symptoms to other disorders. The geographical origin of the patient must be established, in view of the different incidences of the digestive manifestations according to the place of origin.
- 8.
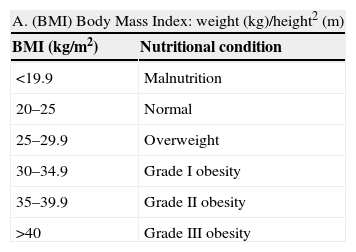
Nutritional evaluation (Table 2) in patients with Chagas disease is of great importance for a number of reasons. Firstly, and given the possible existence of cardiovascular involvement, the early diagnosis and management of vascular risk factors is particularly relevant. On the other hand, altered nutritional status is a marker of potential digestive involvement.
Table 2.Evaluation of nutritional condition based on anthropometric parameters and plasma protein measurements
A. (BMI) Body Mass Index: weight (kg)/height2 (m) BMI (kg/m2) Nutritional condition <19.9 Malnutrition 20–25 Normal 25–29.9 Overweight 30–34.9 Grade I obesity 35–39.9 Grade II obesity >40 Grade III obesity B. Determination of plasma proteins Normal Mild depletion Moderate depletion Severe depletion Albumin (g/dl) 3.5–4.5 2.8–3.5 2.1–2.7 <2.1 Transferrin (mg/dl) 250–350 150–250 100–150 <100 Prealbumin (mg/dl) 18–28 15–18 10–15 <10
Chagas disease. Signs and symptoms of digestive involvement
|
A complete physical examination (including body weight and body mass index (BMI)) is required.
Salivary gland hypertrophy and sialorrhoea may be present as manifestations of oesophageal disorders.
The abdominal exploration should be thorough, including the evaluation of tympanism and abdominal distension and/or asymmetry. In advanced phases it is possible to detect enlarged organs, with palpation of some distended portion of the colon.
Complementary testsElectrocardiogram (ECG)All patients diagnosed with Chagas disease should undergo an ECG study even if no cardiological symptoms have been reported. The ECG tracing may help identify early cardiological alterations or may suggest that certain patient symptoms are of coronary – not digestive – origin.43
A 12-lead ECG tracing is to be obtained, including at least a 30-second lead DII recording.
Radiological studyAll patients diagnosed with Chagas disease should undergo a posteroanterior and lateral projection chest X-ray study, in order to detect cardiomegalia (despite the low sensitivity in diagnosing Chagas cardiopathy) and mediastinal alterations secondary to megaoesophagus.
All subjects with upper and/or lower digestive tract symptoms should undergo an oesophagogram and barium enema (Annex A). Any of the organs that comprise the digestive system may be affected; consequently, radiological alterations may be evidenced at any level.14 The complete radiological study (colon and oesophagus) may be carried out on the same visit – in which case the first step should involve a barium enema.
Given the existence of digestive tract alterations in up to 11% of all asymptomatic patients,14 radiological evaluation is recommended in all patients with T. cruzi infection.
Diagnosis of Helicobacter pylori infectionAlthough the relationship between non-ulcerative dyspepsia and H. pylori infection is the subject of controversy,44–46 all the national47 and international consensus documents48 consider the “test and treat” strategy to be indicated from the cost-effectiveness perspective in adult patients with non-investigated dyspepsia under 45–50 years of age without clinical alarm signs or symptoms. Since the prevalence of H. pylori infection in this population is moderate to high,49–53 we recommend the evaluation of such infection in all patients presenting dyspepsia, pyrosis, or signs of postprandial abdominal distension. It is advisable to confirm H. pylori eradication at least four weeks after the end of treatment.54
There are different techniques for diagnosing Helicobacter pylori infection; of the existing options, we recommend the breath test with 13C-urea, since it is noninvasive and offers high sensitivity and specificity,55,56 provided the technique is available.
Study of parasites in stoolsThe digestive symptoms attributable to Chagas disease can also be caused by other intestinal parasitoses, with a high prevalence among patients from Latin America. Serial evaluations of parasites in stools are advised, based on three samples on alternate days, and including a fresh sample analysis.
Oesophageal manometryChagas oesophageal disease is manometrically characterized by changes in oesophageal body peristalsis and lower oesophageal sphincter (LES) relaxation. These alterations are variable, and range from nonspecific motor disturbances to diffuse oesophageal spasms and even manifestations analogous to those of idiopathic achalasia.
We recommend manometry for all those patients who, even in the presence of a normal oesophagogram, present oesophageal symptoms and in whom there are diagnostic doubts that condition the perceived quality of life.
In the case of non-advanced disease (0–II), variants of normal behaviour can be observed, such as partial LES relaxation and intermittent and/or segmental aperistalsis. In the case of severe disease (III–IV), complete aperistalsis with low oesophageal body pressure values are observed.57
In addition, manometry may be useful for evaluating the response to different treatments.
EndoscopyEndoscopy is indicated in selected cases of chronic Chagas disease:
- •
In the presence of megaoesophagus, to assess the condition of the oesophageal mucosa.
- •
When underlying oesophageal/colon disease is suspected due to the existence of other signs or symptoms reported by the patient or established from the family history, with the criteria applied to the general population (Table 3).
Table 3.Indications of upper digestive tract endoscopy (modified from the clinical guidelines of the Asociación Española de Gastroenterología)
Organ Indications Oesophagus - •
Study of gastrooesophageal reflux
- •
Portal hypertension
- •
Achalasia
- •
Suspected neoplasm
- •
Oesophageal dilatations
- •
Extraction of foreign bodies
- •
Peptic acid disease
- •
Hiatal hernia
- •
Oesophageal stenosis (dysphagia or odynophagia)
- •
Barrett oesophagus
- •
Toxic substances ingestion (acid or alkaline
- •
Varicose vein sclerotherapy
- •
Polypectomy
- •
Study of retrosternal pain
- •
Dyspepsia unresponsive to clinical management in patients over 45 years of age
- •
Atrophic gastritis
- •
Persistent nausea and vomiting
Stomach - •
Suspected neoplasm
- •
Control of digestive bleeding
- •
Polypectomy
- •
Percutaneous gastrostomy
Duodenum - •
Polypectomy
- •
Malabsorption syndrome (biopsies)
- •
Control of bleeding lesion
- •
Duodenal ulcer and duodenitis
- •
- •
Removal of an impacted foreign body, particularly in megaoesophagus stages III–IV.
- •
Fitting of a nasogastric feeding tube, in cases of advanced oesophageal disease.
It is estimated that each year between 2–5% of all patients with the indeterminate form of Chagas disease evolve towards chronic cardiac or digestive forms of the illness10.
Asymptomatic patientAlthough the patient shows no digestive symptoms, a guided medical history will be carried out on each of the yearly visits. Body weight will be taken and nutritional condition evaluated.
Symptomatic patientA.With normal complementary test findingsOnce all the test results have been obtained and are found to be within normal limits, the following is recommended even if the patient continues to present symptoms:
- 1.
Evaluation of some other possible underlying diseases (anxiety, digestive disorders of some other aetiology).
- 2.
Start symptomatic treatment.
Megaoesophagus. The management of megaoesophagus depends on the degree of oesophageal disease (Table 4), the nutritional condition of the patient and the existing comorbidities. Clinical or surgical options are available, as well as tube and/or balloon dilatation measures.
Classification of chagasic oesophageal disease72
| Group 0: Asymptomatic cases without radiological alterations, showing some degree of denervation as established by pharmacological methods. Air in gastric fundus. |
| Group I: Oesophagus with apparently normal calibre in radiological study. Slow transit, with minor retention on X-rays one minute after ingestion of contrast. The contrast medium forms a small residual column, levelled at the upper extremity and perpendicular to the walls of the oesophagus. Air in gastric fundus. |
| Group II: Oesophagus with small-moderate increase in calibre. Appreciable contrast retention. Presence of residual column of variable height. Frequent presence of tertiary waves, with or without lower oesophageal hypotonus. Absence of air in gastric fundus. |
| Group III: Oesophagus with large increase in diameter. Reduced motor activity. Lower oesophageal hypotonus. Important contrast retention. Absence of air in gastric fundus. |
| Group IV: Dolichomegaoesophagus. Oesophagus with great retention capacity, atonic, elongated. Absence of air in gastric fundus |
There are no specific treatments capable of restoring oesophageal function, though partial recovery of oesophageal peristalsis can be observed following clinical, endoscopic or surgical management. The therapeutic measures are the same as those applied in cases of idiopathic achalasia, and are basically destined to reduce lower oesophageal sphincter (LES) pressure (cardia). The management option should be based on the general patient characteristics, the symptoms, and the degree of radiological and manometric involvement.
Nitrates (isosorbide dinitrate, 2.5-5 mg po, 5 minutes before meals) and nifedipine (10°mg po 45min before meals),58 induce LES relaxation and can be used in some cases. However, these measures are not always effective, undesirable effects are frequent, and their action tends to subside over time (tachyphylaxis); such drugs are therefore only recommended as temporary treatment whilst waiting for definitive management.
The two most widely used treatment options are pneumatic balloon dilatation (presently performed via endoscopy),59 and laparoscopic myotomy (Heller technique). In advanced cases, or in the presence of relapse, the Serra-Dória surgical technique or oesophagectomy is performed. Cardiomyectomy with Pinotti funduplicature may be used as a surgical alternative.60,61
Although all of these procedures have advantages and inconveniences, their overall efficacy is similar. It is therefore advisable to use the technique with which the centre has most experience.
Endoscopic botulin toxin injection in the sphincter region is able to reduce the symptoms in patients with idiopathic achalasia and Chagas megaoesophagus. The main inconvenience of this treatment is the limited duration of the effect obtained (months). As a result, it would be indicated in patients with oesophageal involvement and a high surgical risk or serious concomitant diseases.
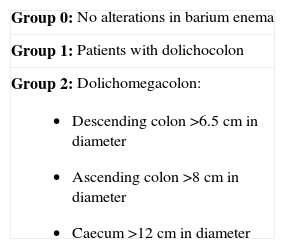
Megacolon. The management of megacolon basically depends on the degree of constipation (defined according to the Rome III criteria), the degree of colon dilatation/lengthening, the nutritional condition of the patient and the existing comorbidities. Clinical (symptomatic) or surgical treatment options are available. It is important to mention that patients with megacolon can present without constipation, and that the first manifestation of the disease may be a complication (e.g., volvulus).
The literature offers no established classification of the degrees of colon involvement. A sigmoid colon or descending colon diameter of over 6.5 cm is considered pathological from the radiological perspective, and defines megacolon. However, a range of colonic changes have been described in patients with Chagas disease and constipation (Table 5).
The literature likewise offers no consensus regarding the surgical management of choice for Chagas megacolon. At present, some specialized centres advocate technical variants of the classical procedure of Duhamel-Haddad62 (rectosigmoidectomy with retrocecal interpositioning) or rectosigmoidectomy with end-to-side low colorectal anastomosis. Recently a new procedure has been described consisting of rectosigmoidectomy with ileal loop interpositioning.63 To date, this technique has been used via the laparoscopic approach in a single published experience in Spain12.
Treatment and management of colon involvement according to the degree of involvement.
Groups 0–1
- •
Initial hygiene – dietary measures for all patients.
- ○
Abundant fluid intake: at least two litres of water a day.
- ○
Limitation of astringent foods, and provision of a fibre-rich diet.
- ○
Definition of times for bowel movement, taking care not to delay defecation in case of need.
- ○
Increased gentle daily physical exercise: favour walking and minimize sedentary lifestyle.
- ○
- •
Clinical treatment: in patients with constipation who do not respond to hygiene – dietary measures.
- ○
Laxatives, particularly osmotic agents: mineral oil, milk of magnesia, lactulose, macrogol.
- –
Attempts should be made to minimize their use.
- –
- ○
Cleansing enemas if the mentioned laxatives prove ineffective. The use of glycerine is suggested.
- ○
Group 2
- •
Hygiene – dietary measures and clinical treatment with the same indications as in patients belonging to groups 0-1.
- •
Surgery: in patients of this group presenting symptoms refractory to the above management measures, in the absence of surgical contraindications.
- ○
Sigmoidectomy and low colorectal anastomosis.
- ○
Rectosigmoidectomy with ileal loop interpositioning.
- ○
Rectosigmoidectomy with retrorectal colon descent (Duhamel-Haddad surgery).63
- ○
Annex B addresses management of the complications of megacolon.
Chagas enteropathy. In megaduodenum, surgery is only indicated if the patient shows unequivocal evidence of duodenal stasis. The most common procedure is duodenojejunal anastomosis close to the duodenojejunal flexure.64 In cases of megajejunum or megaileum, partial enterectomy is only indicated if the affected sections are not very extensive.65 In situations of extensive involvement, clinical management is indicated with continuous gastric aspiration, correction of dehydration and electrolyte imbalances, and the provision of parenteral nutrition.
The diagnostic technique of choice for establishing bacteriological overgrowth is the exhaled hydrogen test following the administration of glucose.66 If testing proves positive, non-absorbable antibiotic use (rifaximin) for two weeks is recommended.
H. pylori infection. If the breath test proves positive, eradicating treatment is indicated, with control testing starting four weeks after the end of treatment47,48.
If the symptoms persist despite efficacy of the mentioned treatment, symptomatic management with proton pump inhibitors is indicated, together with fibrogastroscopy to discard underlying pathology.
Parasites in stools. Specific treatment should be provided, depending on the diagnosed parasite, in accordance with the national and international guidelines.67,68
Etiological treatment of patients with chronic Chagas diseaseAt present, two drugs have been approved for the treatment of Chagas disease. The dose of benznidazole (5mg/kg/day) and nifurtimox (10mg/kg/day), and the recommended treatment duration (60 days) have experienced no changes since publication of the first consensus document in Spain. Benznidazole is recommended as first choice treatment, since it offers a lesser incidence of side effects.69
The healing rate in the chronic phase of Chagas disease is estimated to be 8–25% (in observations of up to 8 years after the end of treatment),70 though recent studies in patients in the chronic indeterminate phase (group 0 of the Kuschnir classification, 64% of the included patients) or with Chagas myocardiopathy and no signs of heart failure (groups I and II of the classification of Kuschnir) report reduction in disease progression and even regression to earlier stages of heart disease after treatment with benznidazole.71 No similar studies in patients with digestive involvement are available.
According to the consensus documents of 20055 and 200721, specific treatment of patients with T. cruzi infection is recommended. Treatment is also advised in subjects with early onset digestive disease, establishing patient–physician agreement with due information on the potential appearance of side effects and on the effectiveness of therapy as established by previous experience.
Final considerationsChagas disease is to be suspected in all patients with a compatible epidemiological history and symptoms suggestive of digestive involvement. The low specificity of the digestive symptoms and the scant knowledge of the latter in the Spanish setting make it necessary to increase awareness of the diagnostic and management protocols for these patients, with a view to improving knowledge of the disease among the healthcare professionals in charge of detecting and managing the illness.
Most patients with digestive involvement can be diagnosed and treated in non-specialized centres, though in situations of first line treatment failure, diagnostic uncertainties, or the need for complex complementary techniques, referral is advised to a specialist in gastrointestinal diseases and to a centre specialized in imported diseases.
In any case, patient referral does not imply obviation on the part of the primary care physician (i.e., the professional who initially sees the patient) of the need to compile a good medical history, conduct a clinical exploration with correct cardiological and digestive evaluation, and obtain a conventional ECG tracing and chest X-ray study before referring the patient.
Conflict of interestAll the authors declare that there are no conflicts of interest.
The authors thank Dr. Pere Albajar (Control of Neglected Tropical Diseases, WHO) for critical review of the manuscript.
Study sources of funding/support:
The consensus document was started by the SEMTSI Work Group on Chagas disease, as an initiative of the SEMTSI and CRESIB (Centre de Recerca en Salut Internacional de Barcelona). The final meeting was set in the context of the workshop sessions on imported Chagas disease, organized by the CRESIB and RIVEMTI (Red Catalana de Vigilancia Epidemiológica de Enfermedades Tropicales e Importadas), and held on February 2-3, 2009. The workshop sessions were financed by the Fundación Mundo Sano (España), the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR; 2008ARCS200050) of the Generalitat de Catalunya; the SEMTSI and the Agencia Española de Cooperación Internacional (AECI).
Oesophagogram (Rezende technique73)
- •
In evolved cases, prior tube cleansing of the oesophagus is indicated to remove food remains.
- •
Patient in the standing position, in right anterior oblique projection.
- •
Administer contrast until a column of sufficient height is obtained to ensure passage to the stomach, allowing visualization of the shape of the oesophagus, its diameter, wall contours and kinetic activity.
- •
X-rays of this area are to be obtained at the time of contrast administration and after 60 seconds.
Barium enema (Ximenes technique73)
- •
The study is to be made without prior preparation.
- ○
Initial plain abdominal X-rays.
- ○
Introduction of 300 ml of barium contrast diluted in 1,200 ml of water to form 1,500 ml of suspension.
- ○
The patient is to remain in the right lateral position for 5 minutes.
- ○
Three radiological projections are indicated after contrast administration: dorsal decubitus, ventral decubitus and lateral. The focus-film distance should be one meter.
- ○
- a)
Fecaloma
- •
Proctolysis with liquid vaseline through a rectal tube: 300 ml in slow drip, followed by saline cleansing enemas.
- •
Manual removal.
- •
- b)
Sigmoid volvulus
- •
Endoscopic reduction.
- •
Surgery, if endoscopic resolution is not possible.
- •
- c)
Perforation
- •
Early surgical intervention.
- •