To analyze the scientific evidence regarding to the effect of the Mediterranean diet on the imaging tests and biopsy characteristics in subjects with non-alcoholic fatty liver disease (NAFLD).
MethodsA bibliographic search was carried out in this narrative review in Pubmed and Web of Science databases, considering studies published between 2011 and 2020, in English or Spanish, randomized clinical trials and observational studies in patients with a diagnosis of NAFLD, in subjects over 18 years of age of both sexes.
ResultsIn the observational studies found, an inverse association between adherence to a Mediterranean diet and liver damage stands out, while in the intervention studies with measurement of liver biopsy a 4.4% reduction in intrahepatic lipids and with magnetic resonance a reduction between 4.2 and 10.2% was found. In experimental studies with ultrasound measurement, the proportion of people with moderate (6–16%) and severe (25%) degree decreased and the studies with transient elastography showed a decrease in liver stiffness between 0.5 and 2.1 kPa.
ConclusionsThe Mediterranean diet contributes to the treatment of NAFLD, which is manifested in the liver and histological imaging tests characteristics.
Analizar la evidencia científica respecto al efecto de la dieta mediterránea sobre las características de las pruebas de imagen y biopsia en sujetos con enfermedad de hígado graso no alcohólico (EHGNA).
MétodosSe realizó la búsqueda bibliográfica en esta revisión narrativa en las bases de datos Pubmed y Web of Science, considerando los estudios publicados entre los años 2011−2020, en idioma inglés o español, ensayos clínicos aleatorizados y estudios observacionales en pacientes con diagnóstico de EHGNA, en sujetos mayores de 18 años de ambos sexos.
ResultadosEn los trabajos observacionales encontrados destaca una asociación inversa entre la adherencia a dieta mediterránea y el daño hepático, mientras que en los estudios de intervención con medición de biopsia hepática se encontró una reducción del 4,4% en lípidos intrahepáticos, y con resonancia magnética, entre el 4,2 y el 10,2%. En estudios experimentales con medición de ultrasonido disminuyó la proporción de personas con grado moderado (6–16%) y severo (25%) y los estudios con elastografía transitoria presentaron disminución de la rigidez hepática entre 0,5−2,1 kPa.
ConclusionesLa dieta mediterránea contribuye al tratamiento de la EHGNA, lo cual se manifiesta en las características de las pruebas de imagen hepáticas e histológicas.
Non-alcoholic fatty liver disease (NAFLD) encompasses a clinical and pathological spectrum from simple steatosis (SS) through steatohepatitis (SH) and liver cirrhosis. Its prevalence is estimated to be 20–30% in Western countries.1
Its aetiopathogenesis is explained by the multiple impact theory. Lower sensitivity to peripheral insulin increases the release of free fatty acids from the adipose tissue. Those are taken up by the liver, stimulating greater de novo lipogenesis with subsequent hepatic triglyceride accumulation. If this stimulus persists over time, this mechanisms promotes lipotoxicity, thereby stimulating the release of proinflammatory cytokines and leading to progression to SH and activating factors that promote fibrosis and cirrhosis.1
NAFLD has no clear symptoms in the initial stages, therefore timely detection is essential for decreasing progression.1 It can be diagnosed through histology tests via liver biopsy, imaging tests, or NAFLD risk estimation scores.2
The baseline treatment is an appropriate diet and physical activity. The effectiveness of several types of diets on NAFLD has been studied, and the Mediterranean diet (MedD) is a novel alternative recommended by several organisations.3
This diet is considered a “set of skills, knowledge, rituals, symbols and traditions concerning crops, harvesting, fishing, animal husbandry, conservation, processing, cooking, and particularly the sharing and consumption of food.”4
The MedD has been shown to have benefits on lipid metabolism, oxidative stress, insulin sensitivity, glycaemic control, liver transaminases, and cardiovascular events.3
Several studies recommend the MedD to people with NAFLD as a therapeutic approach for preventing its onset or halting progression;5 however, this study is relevant because there is scant evidence that analyses the effect of the Mediterranean diet on the histological characteristics and imaging tests of liver damage.
The objective of this review is to analyse the scientific evidence regarding the MedD’s effect on the imaging test and biopsy characteristics of subjects with NAFLD.
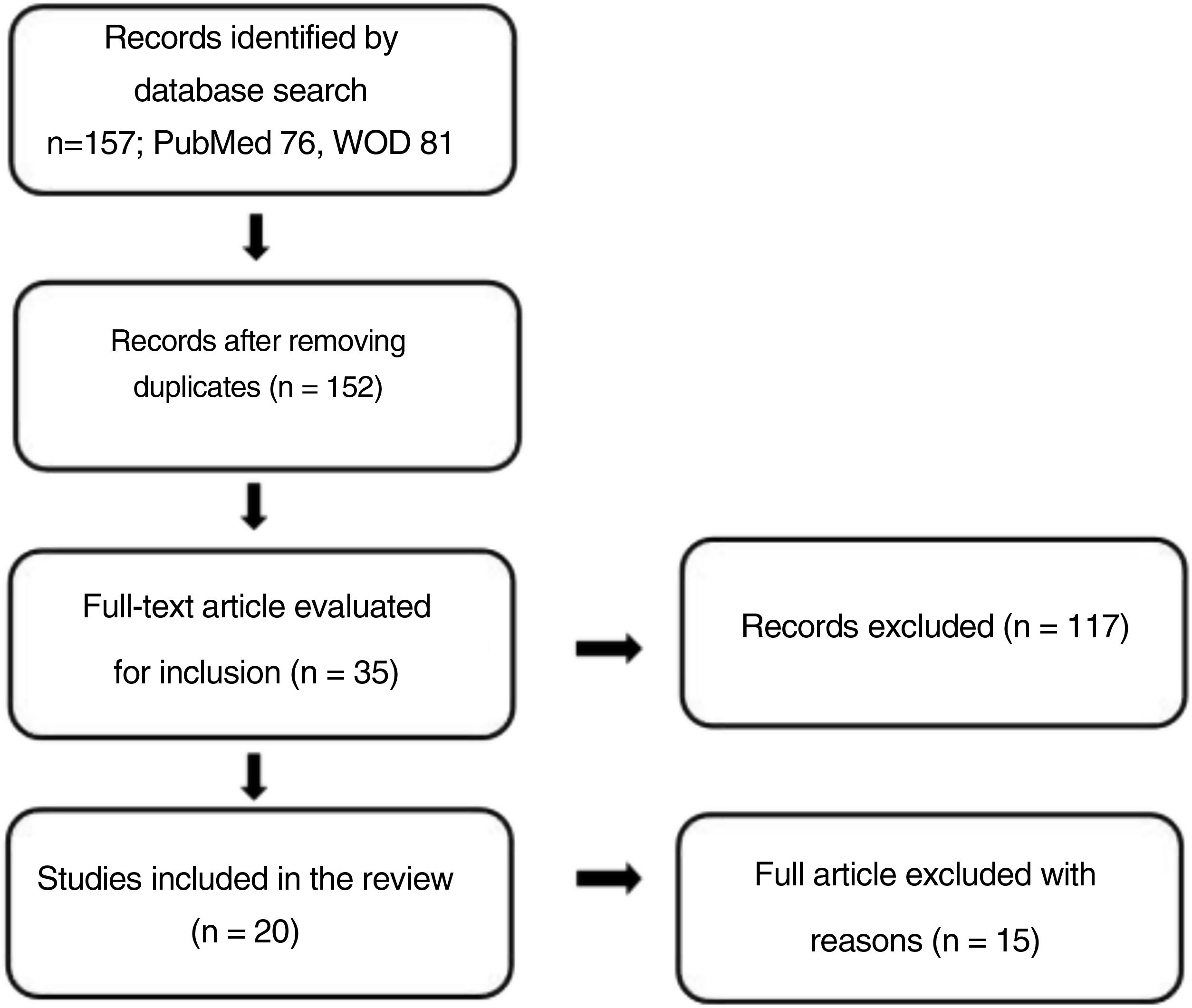
MethodologyA literature search was performed from April to June 2020 in the PubMed and Web of Science databases. The PubMed search strategy was (("Diet, Mediterranean"[Mesh] OR "Mediterranean diet") AND ("Non-alcoholic Fatty Liver Disease"[Mesh])) and for Web of Science it was (("Mediterranean diet") AND (NAFLD)) with the study type filter set to "Article". Both searches included studies published between 2011 and 2020. The review process is shown in Fig. 1.
The selection criteria were articles published in English or Spanish, randomised clinical trials and observational studies in patients diagnosed with NAFLD via biopsy, imaging tests, or steatosis and/or fibrosis score in subjects over 18 years of age of both sexes.
The studies must consider the MedD as a pattern of eating instead of separate foods. Both open access and subscription articles were included.
Studies with fatty liver disease caused by alcohol were excluded as the metabolic alterations and progression of damage are different from those caused in NAFLD.
Each study was analysed critically using an adapted questionnaire that included questions about methodology and results from “Guía práctica de lectura crítica de artículos científicos originales en ciencias de la salud” [Practical guide on the critical reading of original health science articles],6 with a maximum score of 100 points and agreement that this work includes all studies that reach at least 80 points.
ResultsTables 1 and 2 summarise the methodology and results of the included experimental and observational studies, respectively.
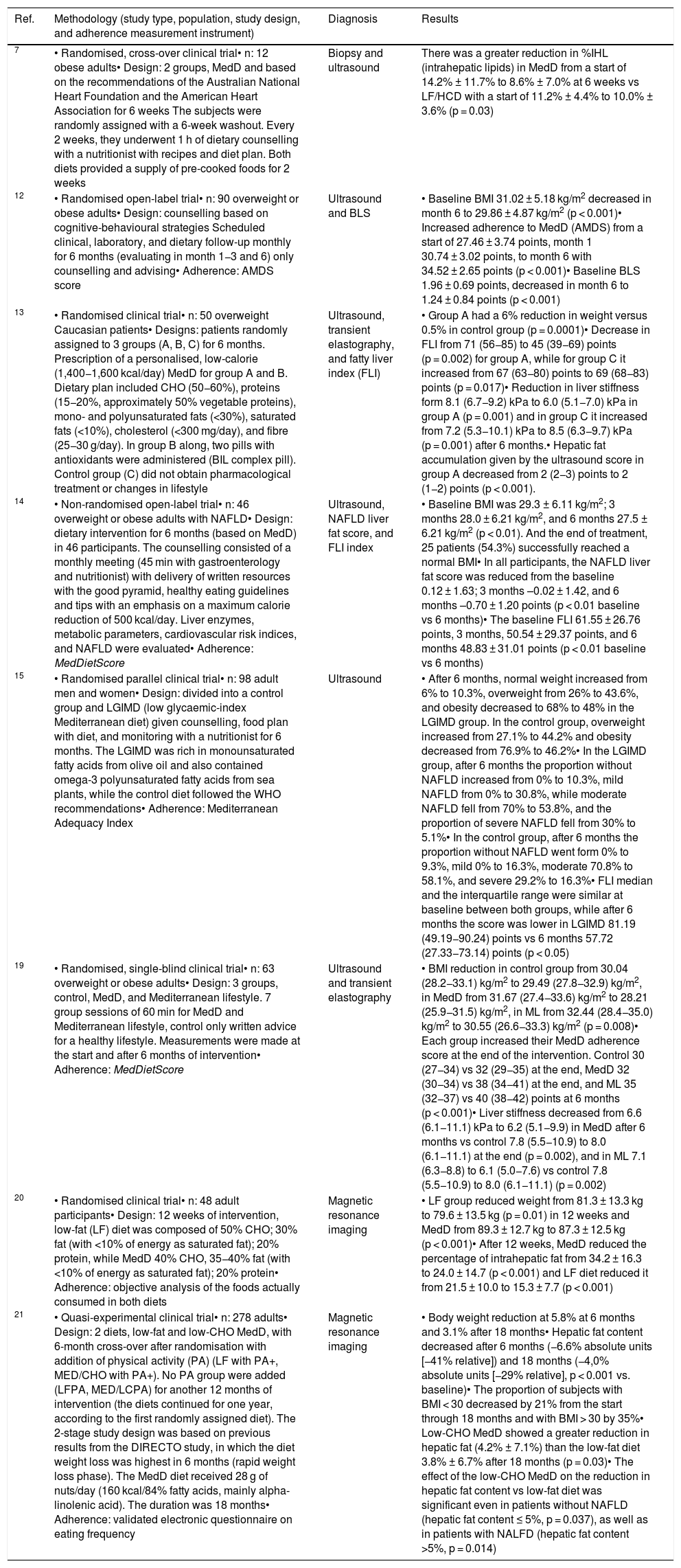
Experimental studies on Mediterranean diet intervention on the histological and imaging test characteristics.
| Ref. | Methodology (study type, population, study design, and adherence measurement instrument) | Diagnosis | Results |
|---|---|---|---|
| 7 | • Randomised, cross-over clinical trial• n: 12 obese adults• Design: 2 groups, MedD and based on the recommendations of the Australian National Heart Foundation and the American Heart Association for 6 weeks The subjects were randomly assigned with a 6-week washout. Every 2 weeks, they underwent 1 h of dietary counselling with a nutritionist with recipes and diet plan. Both diets provided a supply of pre-cooked foods for 2 weeks | Biopsy and ultrasound | There was a greater reduction in %IHL (intrahepatic lipids) in MedD from a start of 14.2% ± 11.7% to 8.6% ± 7.0% at 6 weeks vs LF/HCD with a start of 11.2% ± 4.4% to 10.0% ± 3.6% (p = 0.03) |
| 12 | • Randomised open-label trial• n: 90 overweight or obese adults• Design: counselling based on cognitive-behavioural strategies Scheduled clinical, laboratory, and dietary follow-up monthly for 6 months (evaluating in month 1−3 and 6) only counselling and advising• Adherence: AMDS score | Ultrasound and BLS | • Baseline BMI 31.02 ± 5.18 kg/m2 decreased in month 6 to 29.86 ± 4.87 kg/m2 (p < 0.001)• Increased adherence to MedD (AMDS) from a start of 27.46 ± 3.74 points, month 1 30.74 ± 3.02 points, to month 6 with 34.52 ± 2.65 points (p < 0.001)• Baseline BLS 1.96 ± 0.69 points, decreased in month 6 to 1.24 ± 0.84 points (p < 0.001) |
| 13 | • Randomised clinical trial• n: 50 overweight Caucasian patients• Designs: patients randomly assigned to 3 groups (A, B, C) for 6 months. Prescription of a personalised, low-calorie (1,400−1,600 kcal/day) MedD for group A and B. Dietary plan included CHO (50−60%), proteins (15−20%, approximately 50% vegetable proteins), mono- and polyunsaturated fats (<30%), saturated fats (<10%), cholesterol (<300 mg/day), and fibre (25−30 g/day). In group B along, two pills with antioxidants were administered (BIL complex pill). Control group (C) did not obtain pharmacological treatment or changes in lifestyle | Ultrasound, transient elastography, and fatty liver index (FLI) | • Group A had a 6% reduction in weight versus 0.5% in control group (p = 0.0001)• Decrease in FLI from 71 (56−85) to 45 (39−69) points (p = 0.002) for group A, while for group C it increased from 67 (63−80) points to 69 (68−83) points (p = 0.017)• Reduction in liver stiffness form 8.1 (6.7−9.2) kPa to 6.0 (5.1−7.0) kPa in group A (p = 0.001) and in group C it increased from 7.2 (5.3−10.1) kPa to 8.5 (6.3−9.7) kPa (p = 0.001) after 6 months.• Hepatic fat accumulation given by the ultrasound score in group A decreased from 2 (2−3) points to 2 (1−2) points (p < 0.001). |
| 14 | • Non-randomised open-label trial• n: 46 overweight or obese adults with NAFLD• Design: dietary intervention for 6 months (based on MedD) in 46 participants. The counselling consisted of a monthly meeting (45 min with gastroenterology and nutritionist) with delivery of written resources with the good pyramid, healthy eating guidelines and tips with an emphasis on a maximum calorie reduction of 500 kcal/day. Liver enzymes, metabolic parameters, cardiovascular risk indices, and NAFLD were evaluated• Adherence: MedDietScore | Ultrasound, NAFLD liver fat score, and FLI index | • Baseline BMI was 29.3 ± 6.11 kg/m2; 3 months 28.0 ± 6.21 kg/m2, and 6 months 27.5 ± 6.21 kg/m2 (p < 0.01). And the end of treatment, 25 patients (54.3%) successfully reached a normal BMI• In all participants, the NAFLD liver fat score was reduced from the baseline 0.12 ± 1.63; 3 months –0.02 ± 1.42, and 6 months –0.70 ± 1.20 points (p < 0.01 baseline vs 6 months)• The baseline FLI 61.55 ± 26.76 points, 3 months, 50.54 ± 29.37 points, and 6 months 48.83 ± 31.01 points (p < 0.01 baseline vs 6 months) |
| 15 | • Randomised parallel clinical trial• n: 98 adult men and women• Design: divided into a control group and LGIMD (low glycaemic-index Mediterranean diet) given counselling, food plan with diet, and monitoring with a nutritionist for 6 months. The LGIMD was rich in monounsaturated fatty acids from olive oil and also contained omega-3 polyunsaturated fatty acids from sea plants, while the control diet followed the WHO recommendations• Adherence: Mediterranean Adequacy Index | Ultrasound | • After 6 months, normal weight increased from 6% to 10.3%, overweight from 26% to 43.6%, and obesity decreased to 68% to 48% in the LGIMD group. In the control group, overweight increased from 27.1% to 44.2% and obesity decreased from 76.9% to 46.2%• In the LGIMD group, after 6 months the proportion without NAFLD increased from 0% to 10.3%, mild NAFLD from 0% to 30.8%, while moderate NAFLD fell from 70% to 53.8%, and the proportion of severe NAFLD fell from 30% to 5.1%• In the control group, after 6 months the proportion without NAFLD went form 0% to 9.3%, mild 0% to 16.3%, moderate 70.8% to 58.1%, and severe 29.2% to 16.3%• FLI median and the interquartile range were similar at baseline between both groups, while after 6 months the score was lower in LGIMD 81.19 (49.19−90.24) points vs 6 months 57.72 (27.33−73.14) points (p < 0.05) |
| 19 | • Randomised, single-blind clinical trial• n: 63 overweight or obese adults• Design: 3 groups, control, MedD, and Mediterranean lifestyle. 7 group sessions of 60 min for MedD and Mediterranean lifestyle, control only written advice for a healthy lifestyle. Measurements were made at the start and after 6 months of intervention• Adherence: MedDietScore | Ultrasound and transient elastography | • BMI reduction in control group from 30.04 (28.2−33.1) kg/m2 to 29.49 (27.8−32.9) kg/m2, in MedD from 31.67 (27.4−33.6) kg/m2 to 28.21 (25.9−31.5) kg/m2, in ML from 32.44 (28.4−35.0) kg/m2 to 30.55 (26.6−33.3) kg/m2 (p = 0.008)• Each group increased their MedD adherence score at the end of the intervention. Control 30 (27−34) vs 32 (29−35) at the end, MedD 32 (30−34) vs 38 (34−41) at the end, and ML 35 (32−37) vs 40 (38−42) points at 6 months (p < 0.001)• Liver stiffness decreased from 6.6 (6.1−11.1) kPa to 6.2 (5.1−9.9) in MedD after 6 months vs control 7.8 (5.5−10.9) to 8.0 (6.1−11.1) at the end (p = 0.002), and in ML 7.1 (6.3−8.8) to 6.1 (5.0−7.6) vs control 7.8 (5.5−10.9) to 8.0 (6.1−11.1) (p = 0.002) |
| 20 | • Randomised clinical trial• n: 48 adult participants• Design: 12 weeks of intervention, low-fat (LF) diet was composed of 50% CHO; 30% fat (with <10% of energy as saturated fat); 20% protein, while MedD 40% CHO, 35−40% fat (with <10% of energy as saturated fat); 20% protein• Adherence: objective analysis of the foods actually consumed in both diets | Magnetic resonance imaging | • LF group reduced weight from 81.3 ± 13.3 kg to 79.6 ± 13.5 kg (p = 0.01) in 12 weeks and MedD from 89.3 ± 12.7 kg to 87.3 ± 12.5 kg (p < 0.001)• After 12 weeks, MedD reduced the percentage of intrahepatic fat from 34.2 ± 16.3 to 24.0 ± 14.7 (p < 0.001) and LF diet reduced it from 21.5 ± 10.0 to 15.3 ± 7.7 (p < 0.001) |
| 21 | • Quasi-experimental clinical trial• n: 278 adults• Design: 2 diets, low-fat and low-CHO MedD, with 6-month cross-over after randomisation with addition of physical activity (PA) (LF with PA+, MED/CHO with PA+). No PA group were added (LFPA, MED/LCPA) for another 12 months of intervention (the diets continued for one year, according to the first randomly assigned diet). The 2-stage study design was based on previous results from the DIRECTO study, in which the diet weight loss was highest in 6 months (rapid weight loss phase). The MedD diet received 28 g of nuts/day (160 kcal/84% fatty acids, mainly alpha-linolenic acid). The duration was 18 months• Adherence: validated electronic questionnaire on eating frequency | Magnetic resonance imaging | • Body weight reduction at 5.8% at 6 months and 3.1% after 18 months• Hepatic fat content decreased after 6 months (−6.6% absolute units [−41% relative]) and 18 months (−4,0% absolute units [−29% relative], p < 0.001 vs. baseline)• The proportion of subjects with BMI < 30 decreased by 21% from the start through 18 months and with BMI > 30 by 35%• Low-CHO MedD showed a greater reduction in hepatic fat (4.2% ± 7.1%) than the low-fat diet 3.8% ± 6.7% after 18 months (p = 0.03)• The effect of the low-CHO MedD on the reduction in hepatic fat content vs low-fat diet was significant even in patients without NAFLD (hepatic fat content ≤ 5%, p = 0.037), as well as in patients with NALFD (hepatic fat content >5%, p = 0.014) |
AMDS: Adherence Mediterranean Diet Score; BLS: Bright Liver Score; BMI: body mass index; CHO: carbohydrates; FLI: Fatty Liver Index; IHL: intrahepatic lipids; LF: low-fat diet; LF/HCD: low-fat/high-carbohydrate diet; LGIMD: low glycaemic index Mediterranean diet; MedD: Mediterranean diet; ML: Mediterranean lifestyle; NAFLD: non-alcoholic fatty liver disease; PA: physical activity; SS: simple steatosis; WHO: World Health Organisation.
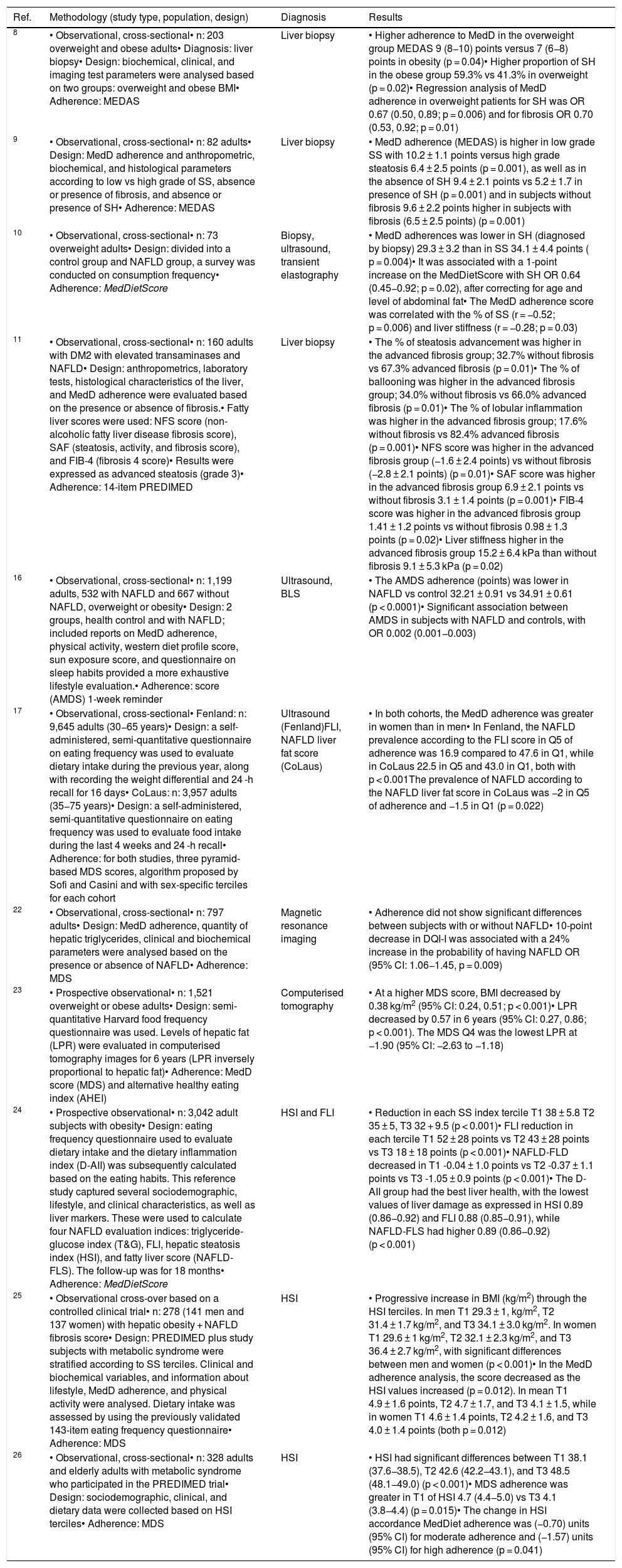
Observational studies on Mediterranean diet of the association with the histological and imaging test characteristics.
| Ref. | Methodology (study type, population, design) | Diagnosis | Results |
|---|---|---|---|
| 8 | • Observational, cross-sectional• n: 203 overweight and obese adults• Diagnosis: liver biopsy• Design: biochemical, clinical, and imaging test parameters were analysed based on two groups: overweight and obese BMI• Adherence: MEDAS | Liver biopsy | • Higher adherence to MedD in the overweight group MEDAS 9 (8−10) points versus 7 (6−8) points in obesity (p = 0.04)• Higher proportion of SH in the obese group 59.3% vs 41.3% in overweight (p = 0.02)• Regression analysis of MedD adherence in overweight patients for SH was OR 0.67 (0.50, 0.89; p = 0.006) and for fibrosis OR 0.70 (0.53, 0.92; p = 0.01) |
| 9 | • Observational, cross-sectional• n: 82 adults• Design: MedD adherence and anthropometric, biochemical, and histological parameters according to low vs high grade of SS, absence or presence of fibrosis, and absence or presence of SH• Adherence: MEDAS | Liver biopsy | • MedD adherence (MEDAS) is higher in low grade SS with 10.2 ± 1.1 points versus high grade steatosis 6.4 ± 2.5 points (p = 0.001), as well as in the absence of SH 9.4 ± 2.1 points vs 5.2 ± 1.7 in presence of SH (p = 0.001) and in subjects without fibrosis 9.6 ± 2.2 points higher in subjects with fibrosis (6.5 ± 2.5 points) (p = 0.001) |
| 10 | • Observational, cross-sectional• n: 73 overweight adults• Design: divided into a control group and NAFLD group, a survey was conducted on consumption frequency• Adherence: MedDietScore | Biopsy, ultrasound, transient elastography | • MedD adherences was lower in SH (diagnosed by biopsy) 29.3 ± 3.2 than in SS 34.1 ± 4.4 points ( p = 0.004)• It was associated with a 1-point increase on the MedDietScore with SH OR 0.64 (0.45−0.92; p = 0.02), after correcting for age and level of abdominal fat• The MedD adherence score was correlated with the % of SS (r = −0.52; p = 0.006) and liver stiffness (r = −0.28; p = 0.03) |
| 11 | • Observational, cross-sectional• n: 160 adults with DM2 with elevated transaminases and NAFLD• Design: anthropometrics, laboratory tests, histological characteristics of the liver, and MedD adherence were evaluated based on the presence or absence of fibrosis.• Fatty liver scores were used: NFS score (non-alcoholic fatty liver disease fibrosis score), SAF (steatosis, activity, and fibrosis score), and FIB-4 (fibrosis 4 score)• Results were expressed as advanced steatosis (grade 3)• Adherence: 14-item PREDIMED | Liver biopsy | • The % of steatosis advancement was higher in the advanced fibrosis group; 32.7% without fibrosis vs 67.3% advanced fibrosis (p = 0.01)• The % of ballooning was higher in the advanced fibrosis group; 34.0% without fibrosis vs 66.0% advanced fibrosis (p = 0.01)• The % of lobular inflammation was higher in the advanced fibrosis group; 17.6% without fibrosis vs 82.4% advanced fibrosis (p = 0.001)• NFS score was higher in the advanced fibrosis group (−1.6 ± 2.4 points) vs without fibrosis (−2.8 ± 2.1 points) (p = 0.01)• SAF score was higher in the advanced fibrosis group 6.9 ± 2.1 points vs without fibrosis 3.1 ± 1.4 points (p = 0.001)• FIB-4 score was higher in the advanced fibrosis group 1.41 ± 1.2 points vs without fibrosis 0.98 ± 1.3 points (p = 0.02)• Liver stiffness higher in the advanced fibrosis group 15.2 ± 6.4 kPa than without fibrosis 9.1 ± 5.3 kPa (p = 0.02) |
| 16 | • Observational, cross-sectional• n: 1,199 adults, 532 with NAFLD and 667 without NAFLD, overweight or obesity• Design: 2 groups, health control and with NAFLD; included reports on MedD adherence, physical activity, western diet profile score, sun exposure score, and questionnaire on sleep habits provided a more exhaustive lifestyle evaluation.• Adherence: score (AMDS) 1-week reminder | Ultrasound, BLS | • The AMDS adherence (points) was lower in NAFLD vs control 32.21 ± 0.91 vs 34.91 ± 0.61 (p < 0.0001)• Significant association between AMDS in subjects with NAFLD and controls, with OR 0.002 (0.001−0.003) |
| 17 | • Observational, cross-sectional• Fenland: n: 9,645 adults (30−65 years)• Design: a self-administered, semi-quantitative questionnaire on eating frequency was used to evaluate dietary intake during the previous year, along with recording the weight differential and 24 -h recall for 16 days• CoLaus: n: 3,957 adults (35−75 years)• Design: a self-administered, semi-quantitative questionnaire on eating frequency was used to evaluate food intake during the last 4 weeks and 24 -h recall• Adherence: for both studies, three pyramid-based MDS scores, algorithm proposed by Sofi and Casini and with sex-specific terciles for each cohort | Ultrasound (Fenland)FLI, NAFLD liver fat score (CoLaus) | • In both cohorts, the MedD adherence was greater in women than in men• In Fenland, the NAFLD prevalence according to the FLI score in Q5 of adherence was 16.9 compared to 47.6 in Q1, while in CoLaus 22.5 in Q5 and 43.0 in Q1, both with p < 0.001The prevalence of NAFLD according to the NAFLD liver fat score in CoLaus was −2 in Q5 of adherence and −1.5 in Q1 (p = 0.022) |
| 22 | • Observational, cross-sectional• n: 797 adults• Design: MedD adherence, quantity of hepatic triglycerides, clinical and biochemical parameters were analysed based on the presence or absence of NAFLD• Adherence: MDS | Magnetic resonance imaging | • Adherence did not show significant differences between subjects with or without NAFLD• 10-point decrease in DQI-I was associated with a 24% increase in the probability of having NAFLD OR (95% CI: 1.06−1.45, p = 0.009) |
| 23 | • Prospective observational• n: 1,521 overweight or obese adults• Design: semi-quantitative Harvard food frequency questionnaire was used. Levels of hepatic fat (LPR) were evaluated in computerised tomography images for 6 years (LPR inversely proportional to hepatic fat)• Adherence: MedD score (MDS) and alternative healthy eating index (AHEI) | Computerised tomography | • At a higher MDS score, BMI decreased by 0.38 kg/m2 (95% CI: 0.24, 0.51; p < 0.001)• LPR decreased by 0.57 in 6 years (95% CI: 0.27, 0.86; p < 0.001). The MDS Q4 was the lowest LPR at −1.90 (95% CI: −2.63 to −1.18) |
| 24 | • Prospective observational• n: 3,042 adult subjects with obesity• Design: eating frequency questionnaire used to evaluate dietary intake and the dietary inflammation index (D-AII) was subsequently calculated based on the eating habits. This reference study captured several sociodemographic, lifestyle, and clinical characteristics, as well as liver markers. These were used to calculate four NAFLD evaluation indices: triglyceride-glucose index (T&G), FLI, hepatic steatosis index (HSI), and fatty liver score (NAFLD-FLS). The follow-up was for 18 months• Adherence: MedDietScore | HSI and FLI | • Reduction in each SS index tercile T1 38 ± 5.8 T2 35 ± 5, T3 32 + 9.5 (p < 0.001)• FLI reduction in each tercile T1 52 ± 28 points vs T2 43 ± 28 points vs T3 18 ± 18 points (p < 0.001)• NAFLD-FLD decreased in T1 -0.04 ± 1.0 points vs T2 -0.37 ± 1.1 points vs T3 -1.05 ± 0.9 points (p < 0.001)• The D-AII group had the best liver health, with the lowest values of liver damage as expressed in HSI 0.89 (0.86−0.92) and FLI 0.88 (0.85−0.91), while NAFLD-FLS had higher 0.89 (0.86−0.92) (p < 0.001) |
| 25 | • Observational cross-over based on a controlled clinical trial• n: 278 (141 men and 137 women) with hepatic obesity + NAFLD fibrosis score• Design: PREDIMED plus study subjects with metabolic syndrome were stratified according to SS terciles. Clinical and biochemical variables, and information about lifestyle, MedD adherence, and physical activity were analysed. Dietary intake was assessed by using the previously validated 143-item eating frequency questionnaire• Adherence: MDS | HSI | • Progressive increase in BMI (kg/m2) through the HSI terciles. In men T1 29.3 ± 1, kg/m2, T2 31.4 ± 1.7 kg/m2, and T3 34.1 ± 3.0 kg/m2. In women T1 29.6 ± 1 kg/m2, T2 32.1 ± 2.3 kg/m2, and T3 36.4 ± 2.7 kg/m2, with significant differences between men and women (p < 0.001)• In the MedD adherence analysis, the score decreased as the HSI values increased (p = 0.012). In mean T1 4.9 ± 1.6 points, T2 4.7 ± 1.7, and T3 4.1 ± 1.5, while in women T1 4.6 ± 1.4 points, T2 4.2 ± 1.6, and T3 4.0 ± 1.4 points (both p = 0.012) |
| 26 | • Observational, cross-sectional• n: 328 adults and elderly adults with metabolic syndrome who participated in the PREDIMED trial• Design: sociodemographic, clinical, and dietary data were collected based on HSI terciles• Adherence: MDS | HSI | • HSI had significant differences between T1 38.1 (37.6−38.5), T2 42.6 (42.2−43.1), and T3 48.5 (48.1−49.0) (p < 0.001)• MDS adherence was greater in T1 of HSI 4.7 (4.4−5.0) vs T3 4.1 (3.8−4.4) (p = 0.015)• The change in HSI accordance MedDiet adherence was (−0.70) units (95% CI) for moderate adherence and (−1.57) units (95% CI) for high adherence (p = 0.041) |
AMDS: Adherence Mediterranean Diet Score; BLS: Bright Liver Score; BMI: body mass index; D-AII: dietary inflammation index; Dg: diagnosis; FLI: Fatty Liver Index; HEI: Healthy Eating Index; HEI: Healthy Eating Index; NAFLD: non-alcoholic fatty liver disease; SH: steatohepatitis; SS: simple steatosis.
Only one experimental study was found, which compared a low-fat high-carbohydrate diet with a MedD for 6 weeks. It found a 4.4% decrease in the percentage of intrahepatic lipids in the MedD subjects.7
As for observational studies, four of them determined adherence to the MedD and found that a lower degree of liver damager and a better nutritional status were associated with greater adherence to the MedD, regardless of the tool used to assess adherence to the MedD.8–11
Studies with ultrasound measurementA total of four experimental studies were found with ultrasound measurement.12–15 One study demonstrated a decrease of 0.72 points on the Bright Liver Score (BLS).12 A second presented a decrease in the ultrasound score after 6 months in MedD subjects going from 2 (2−3) points at the start to 2 (1−2) points.13 The other two showed that after 6 months of intervention with MedD, the proportion without steatosis (20% and 10%, respectively) and a mild grade of steatosis (26% and 31%) increased, and the proportion of people with a moderate (6% and 16%) and severe (25% only in one study) degree decreased.14,15
Two observational studies were found where the MedD adherence score was inversely associated with the prevalence of NAFLD.16,17
Studies with transient elastography measurementLiver stiffness was determined using transient elastography in three experimental studies. Subjects who underwent MedD intervention presented a 0.5 and 0.2 kPa reduction in liver stiffness18,19, while in another study a 2.1 kPa decrease was found in comparison with the control group, which increased 1.3 kPa, respectively.13
Studies with measurement using other imaging tests and indicesTwo experimental studies using magnetic resonance imaging were analysed. They demonstrated a decrease in the hepatic fat content in the MedD subjects. One demonstrated a 10.2% decrease in intrahepatic lipids in the patients after 12 weeks,20 and the other showed a 6.6% decrease when evaluated after 6 months and 4% after 18 months.21
Furthermore, an observational study was analysed with this imaging test, finding that a 10-point decrease on the Diet Quality Index-International (DQI-I) had a 24% likelihood of having NAFLD, without find any association with the Mediterranean Diet Scale (MDS).22
Only one observational study with computerised tomography was included, which found a greater reduction in hepatic fat in the highest quartile of MedD adherence.23
Regarding the studies with diagnostic indices, three observational studies were included using the Hepatic Steatosis Index (HSI), establishing an association between the degree of MedD adherence and a decreased risk of disease progression.24–26 Similar results to those observed in the studies that determined the Fatty Liver Index (FLI).13,15
DiscussionAccording to the presented results, the MedD demonstrated beneficial results on the liver characteristics on imaging and histology tests.
It was found in the experimental studies that the liver biopsy determination showed a similar reduction in intrahepatic lipids to that observed in studies in magnetic resonance imaging measurement. The steatosis score decreased and the proportion of moderate and severe grades of steatosis were lower in the works with ultrasound measurement, and the studies with transient elastography determination demonstrated a decrease in liver stiffness.
Moreover, the observational studies demonstrated an inverse association between MedD adherence and the severity of the liver damage.
The mechanisms of the compounds present in the foods belonging to the MedD that could explain the mentioned results in the liver are primarily antioxidant and anti-inflammatory mechanisms, which would contribute to reducing the oxidative damage, inflammation, steatosis, and liver fibrosis.27
The phenolic compounds and lycopene (carotenoid) from fruits, greens, vegetables, and whole grains, along with the monounsaturated fatty acids and antioxidants from olive oil have been demonstrated to posses antioxidant activity in the hepatocytes, reducing lipid peroxidation and liver inflammation from SH, thus improving insulin sensitivity.27,28
Dietary fibre regulates cholesterol and glucose absorption, in addition to contributing to creating satiety to promote weight control.29
For these results, it is important to analyse the magnitude of the changes and the heterogeneity of the methodologies, both in terms of the characteristics of the subjects as well as the wide range of MedD adherence scoring tools.30
There are significant differences inherent to each diagnostic method. Most of the studies determined NAFLD using ultrasound, one of the most commonly used imaging tests to diagnose liver steatosis due to its accessibility, safety, low invasiveness and low cost; however, it has a lower sensitivity when the liver presents fewer than 33% hepatocytes, while fewer studies evaluated liver biopsy or magnetic resonance imaging, techniques that are limited due to a high cost.2
Other factors to consider are weight change, level of physical activity, and dietary calorie restriction, variables that influence energy use and, therefore, changes at the hepatic level;3,5 the latter was not explicit in the methodology of some works, therefore we cannot assume it was done.
The strengths of this narrative review include the quantity of studies included and using a systematised methodology for searching in two databases, with previously established search and selection criteria, and applying a critical analysis tool.
On the other hand, it also has several limitations, such as excluding grey literature or external registries from the searches and not having incorporated metabolic variables in the analysis of the results, which could have complemented the conclusions.
The studies presented a heterogeneous methodology, which, on the one hand, expanded the comparisons between liver characteristic evaluation methods for NAFLD, but made it harder to draw uniform conclusions from them.
All the studies, independently of their design, demonstrated positive results regarding the MedD intervention. In addition, an inverse association was also observed between the MedD adherence score with the severity of the different liver damage characteristics.
Interventional studies lasting at least 6 months that evaluate the changes in the liver imaging test characteristics are required, considering maintaining body weight and controlled isocaloric intake as part of the diet of volunteers with NAFLD.
ConclusionThe MedD demonstrated a reduced accumulation of liver fat and decreased liver stiffness. The MedD could be considered a good dietary strategy for both the prevention and treatment of NAFLD; however, there are no interventional studies that evaluate the changes in histological parameters after a long-term intervention in comparison to a control group.
Conflicts of interestNone.
We thank the contribution of Gonzalo Cruz Neculpán, Centre of Integrative Neurobiology and Physiopathology, Institute of Physiology, Faculty of Sciences, University of Valparaiso, and Claudia Vega Soto, School of Nutrition and Diet, Faculty of Pharmacy, University of Valparaiso, for their detailed review during the initial phase of preparing the manuscript.
Please cite this article as: Saavedra Y, Mena V, Priken K. Efecto de la dieta mediterránea sobre indicadores histológicos y pruebas de imagen en enfermedad de hígado graso no alcohólico. Gastroenterol Hepatol. 2022;45:350–360.