Angiodysplasias, the most common malformation of the gastrointestinal tract, are distributed diffusely in the intestinal mucosa and submucosa. They cause chronic anaemia or gastrointestinal bleeding in 10% of patients. This disease affects the elderly in particular, with a 50% likelihood of recurrence following the first bleeding episode. The large number and location of the lesions often makes it difficult to access and treat them.1 Endoscopic argon plasma coagulation (APC) is considered the treatment of choice, with arteriography and/or surgery reserved for cases of severe haemorrhaging.2
Several pharmacological alternatives have been tried, both with, or when, endoscopic APC fails. These include combined oestrogen–progesterone therapy, thalidomide and somatostatin analogues.3 Hormone treatment has not demonstrated any long-term benefits.4 As for the somatostatin analogues, octreotide is the most widely used active substance.2,5,6 Another of the analogues used is lanreotide.
The first study on the use of octreotide concluded that it was safe and effective in controlling recurrent gastrointestinal bleeding due to angiodysplasia in elderly patients unsuitable for endoscopic or surgical treatment. However, recurrent bleeding occurs in up to 20% of patients treated with octreotide, with an adverse event occurring in up to 53% of cases.
Lanreotide is a synthetic analogue of somatostatin, which, among other actions, inhibits meal-induced increases in superior mesenteric artery and portal venous blood flow. Lanreotide autogel has an aqueous base that facilitates subcutaneous administration. Its safety profile is very similar to that of subcutaneous octreotide; adverse reactions, which occur in approximately 30% of patients, are basically mild and transient and mainly gastrointestinal in nature.
Four patients in our department diagnosed with gastrointestinal angiodysplasia are currently on treatment with lanreotide autogel.
We describe two cases that have been followed up for the longest time after initiation of lanreotide treatment.
Case 1A 94-year-old woman with gastric and jejunal angiodysplasias was started on treatment with octreotide LAR and underwent argon fulguration of the gastric lesions. She was admitted to hospital twice for severe gastrointestinal bleeding (haemoglobin 5g/dL). Between the admissions and outpatient follow-up, she required intravenous iron therapy on one occasion and transfusion of 12 units of packed red blood cells. Since the treatment was not effective, she was switched to lanreotide autogel. In the subsequent 10 months, she presented no further episodes of melaena, nor did she require blood transfusions or endoscopic fulguration. Her haemoglobin values are currently normal.
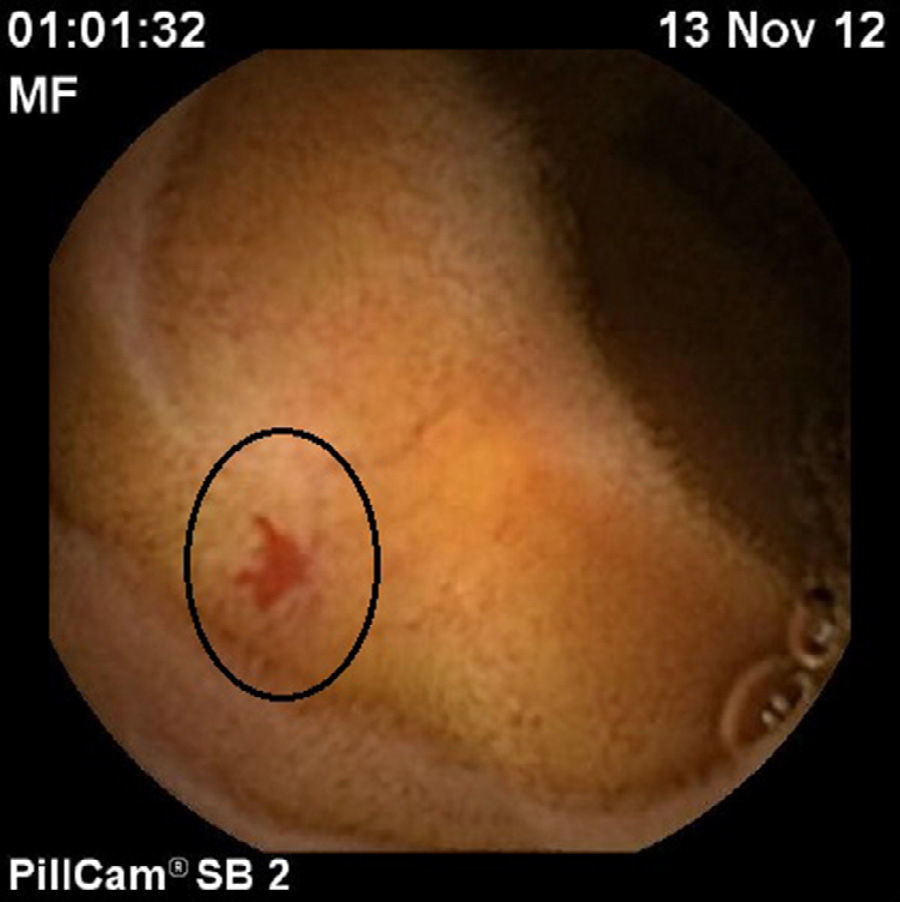
Case 2An 84-year-old woman diagnosed with jejunoileal angiodysplasias (Fig. 1) was prescribed treatment with octreotide LAR. She was admitted on 2 occasions for melaena and received transfusion of 3 units of packed red blood cells and intravenous iron infusion on several occasions. However, in the 10 months after being switched to lanreotide, the patient has shown no further signs of gastrointestinal bleeding, nor has she required blood transfusion or iron therapy. Her haemoglobin levels are now normal.
The drug doses used were 20mg of octreotide monthly and 120mg of lanreotide autogel every 6 weeks.
The other two patients with gastrointestinal angiodysplasia have been on lanreotide for 5 and 6 months and maintain normal haemoglobin levels.
Scientific evidence on the usefulness of treating angiodysplasias with somatostatin analogues is based on studies consisting of few patients. Octreotide was mainly administered, and when octreotide or lanreotide were used indiscriminately, there was no indication of any difference in response, nor were dose and administration regimen clearly defined. No studies exist that assess the usefulness of switching from one analogue to another.
The cases reported above support the efficacy of switching from octreotide to lanreotide in patients for whom the former is not effective or has lost its efficacy. These findings need to be demonstrated in studies with a larger number of cases and supported by greater scientific evidence. It should also be stressed that in patients on anticoagulant treatment, the risk of haematomas is higher for intramuscular octreotide than for subcutaneous lanreotide.
This series—like most of those published—has limitations, namely the absence of a control group and the small sample size. Moreover, since it is likely that drug efficacy depends on bleeding severity and on concurrent treatments that predispose the patient to bleeding (especially anticoagulants), equivalent studies on the efficacy of different doses are therefore warranted.
Please cite this article as: Ramos-Rosario HA, Badia Aranda E, Martín Lorente JL, Arias García L, Sicilia Aladrén B, Sáez-Royuela F. Eficacia de lanreótido en pacientes con angiodisplasias gastrointestinales refractarias al tratamiento con octreótido. Gastroenterol Hepatol. 2016;39:213–214.