Endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) have much in common, including their main indications (biliopancreatic disorders), powerful therapeutic capacities and a steep learning curve. Over the years they have evolved from novel diagnostic procedures to interventional therapeutic techniques, but along different paths (different scopes or devices and endoscopists specializing exclusively in one or the other technique). However, EUS has gradually developed into a therapeutic technique that requires skills in the use of ERCP devices and stents, leading some ERCP specialists to explore the therapeutic potential of EUS. The corresponding literature, which has grown exponentially, includes recent experiments on combining the two techniques, which have gradually come to be used in routine care in a number of centers, with positive technical, clinical and financial outcomes. We review EUS and ERCP as individual or combined procedures for managing biliopancreatic disorders.
La ecografía endoscópica (EE) y la colangiopancreatografía retrógrada endoscópica (CPRE) comparten muchascosas, tales como sus indicaciones principales (las enfermedades biliopancreáticas) y potentes capacidades terapéuticas, y una empinada curva de aprendizaje. Con el paso del tiempo, estos procedimientos diagnósticos novedosos han evolucionado por diferentes vías (distintos ámbitos o dispositivos y endoscopistas especializados exclusivamente en una u otra técnica), hasta convertirse en técnicas terapéuticas intervencionistas. Sin embargo, de manera gradual, la EE ha llegado a ser una técnica terapéutica que requiere habilidades en el manejo de los instrumentos y stents que se emplean en la CPRE, lo que ha conducido a algunos especialistas en CPRE a explorar el potencial terapéutico de la EE. Las publicaciones relativas a este tema, que han crecido de forma exponencial, incluyen experimentos recientes de combinación de estas técnicas, que diversos centros han introducido progresivamente en sus protocolos de atención rutinaria, con resultados técnicos, clínicos y económicos positivos. Hemos revisado la EE y la CPRE como procedimientos individuales o combinados en el tratamiento de enfermedades biliopancreáticas.
Endoscopic Ultrasound (EUS) and Endoscopic Retrograde Cholangiopancreatography (ERCP) are advanced endoscopic procedures. The two techniques have similarities and differences. ERCP is older, indications are purely focused on biliopancreatic disorders, and it is mainly a therapeutic intervention procedure. On the other hand, EUS is a more modern technique, indications are wider and more varied (mediastinal and rectal lesions, staging of GI lesions, subepithelial lesions), and it is mainly a diagnostic procedure, including interventional diagnostic procedures such as EUS-guided puncture with fine needle aspiration. EUS and ERCP have many similarities: a demanding learning curve, a common main indication (biliopancreatic disorders), and powerful therapeutic capacity.1
In recent years, the literature has included experiments that combine the two techniques, and in some centers they have been introduced naturally in routine care. The positive effects of this combination are technical, clinical, logistical, and financial.2
This review focuses on the relationship between the two techniques, firstly considering them individually, and secondly as competing procedures, with a final look at combined procedures.
Endoscopic ultrasoundThe learning curve for EUS and EUS-guided fine needle aspiration (FNA)As the applications for EUS have become increasingly recognized by other clinical practitioners, the demand for well-trained endosonographers has increased.3 The limited availability of EUS is largely the result of a lack of skilled endosonographers. A relative lack of training centers combined with the extensive commitment required by the trainee has limited the growth of EUS and its availability in community practice. For most trainees, the amount of EUS exposure and training is highly variable and often program-dependent. Many fellowship programs do not provide the opportunity to learn EUS.
EUS is operator-dependent. The American Society for Gastrointestinal Endoscopy (ASGE) recommends a minimum of 150 total supervised procedures, 75 of which have a pancreatobiliary indication and 50 cases of FNA (25 of which are pancreatic FNA) before competency can be determined.4 Other studies by experts claim that the number of procedures must be twice the ASGE recommendations.5–7
The learning process of EUS-FNA has been studied for solid pancreatic lesions and has shown a learning curve with increasing sensitivity in the cytopathological diagnosis of cancer (reaching 80% after 20–30 EUS-FNA), with a decreasing number of passes needed to obtain adequate results (reaching a median of 3 after 150 EUS-FNA) but without variation in severe morbidity.8,9
Trainees should demonstrate competence in linear EUS before undertaking EUS-FNA.10,11 The Technical Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) recommend the use of a combination of different simulators and, if available, live pigs during training in EUS-FNA. They recommend that a minimum of 20–30 supervised non-pancreatic and pancreatic lesions, respectively, be performed with rapid on-site cytopathological examination (ROSE).12 The clinical effectiveness of EUS and EUS-FNA depends on the judicious use of these techniques and the skill of the endosonographer, but also on the indicators established by the ASGE to aid in the recognition of high quality EUS examinations.13
General review (overview) of EUS indications: diagnostic, interventional, and therapeuticEUS continues to evolve as a diagnostic and therapeutic technique. EUS should be performed when it has the potential to affect patient management, such as when establishing a diagnosis, performing locoregional tumor staging, and providing therapeutic interventions. Since the introduction of EUS in 1980, its indications and role have continued to expand.
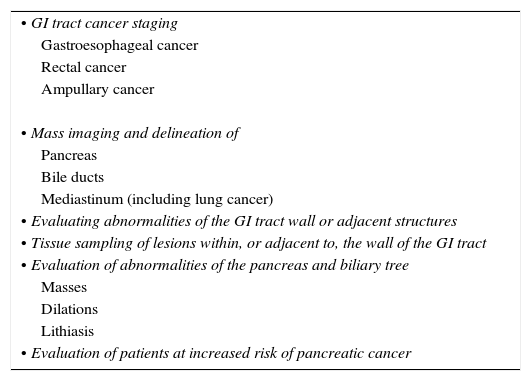
We must differentiate between diagnostic and therapeutic indications. The indications for EUS are determined by the anatomic conditions and the technical capabilities of the equipment. The primary role of EUS is to delineate gastrointestinal tract (GIT) and immediate surroundings to a depth of 4–5cm.14Table 1 summarizes diagnostic indications. These indications can be divided between GIT cancer staging, mass imaging and delineation, evaluation of abnormalities of the pancreas and biliary tree, and study and evaluation of the mediastinum of patients at increased risk of pancreatic cancer.
Diagnostic indications for EUS.
| • GI tract cancer staging |
| Gastroesophageal cancer |
| Rectal cancer |
| Ampullary cancer |
| • Mass imaging and delineation of |
| Pancreas |
| Bile ducts |
| Mediastinum (including lung cancer) |
| • Evaluating abnormalities of the GI tract wall or adjacent structures |
| • Tissue sampling of lesions within, or adjacent to, the wall of the GI tract |
| • Evaluation of abnormalities of the pancreas and biliary tree |
| Masses |
| Dilations |
| Lithiasis |
| • Evaluation of patients at increased risk of pancreatic cancer |
The ability to pass a hollow needle under ultrasound guidance has expanded the applications of EUS. The needle is essentially a conduit that allows for the passage or placement of materials with therapeutic intent. The EUS therapeutic interventions have continued evolving since the beginning of the EUS-guided celiac plexus neurolysis or block, followed by the EUS-guided pseudocyst drainage. Table 2 summarizes the therapeutic indications at present. But perhaps in the near future some other indications that are now on evaluation will be established. New indications are evolving and now only expert centers are using them, or they are on evaluation in animal labs. Some of these indications have been published with small groups of patients included.
Therapeutic indications for EUS on evaluation.
| • EUS-guided FNI into tumors |
| Chemotherapy |
| Antitumor agents |
| • EUS-guided FNI vascular interventions |
| Sclerosant agents |
| Coil application |
| Intrahepatic portosystemic shunts |
| • Drainage of the gallbladder |
| • EUS-guided anastomosis |
| • EUS-guided photodynamic therapy |
| • EUS-guided brachytherapy |
Implementing a standardized protocol for an ERCP training program is very difficult, and might not be feasible in practice. Procedure volume, indications, and technical approaches vary widely among different countries, institutions, and even endoscopists in a same institution.
Competence has been classically evaluated according to a minimum number of procedures performed. Based on a first prospective study,15 the ASGE recommended a minimum of 180–200 ERCPs. The number of procedures recommended in most training programs ranges from 100 to 200. Current research supports establishing a standard of 80–90% technical success before trainees are deemed competent in a specific skill.16,17 Nevertheless, individual trainees may differ in the acquisition of technical skills. Therefore these threshold numbers might be inadequate.
Recently, a study reported the use of a standardized form for continuous self-assessment.18 The form included previously proposed quality indicators for ERCP such as procedural indication, degree of technical difficulty, previous ERCP failure, and success or failure options (such as cannulation of the CBD or pancreatic duct, stent placement, sphincterotomy, and stone extraction). It also comprised an improvement plan for self-evaluation every 10 procedures. This method allowed not only for the quantitative evaluation of trainees, but also determined the learning curve of each individual and of the average group progression. As such, current training should probably be based on learning curves, although more studies are needed to validate these resources.
General overview of ERCP indicationsERCP was introduced more than four decades ago as a diagnostic procedure, but only evolved into therapeutic endoscopy once sphincterotomy was introduced. Nowadays, due to the technological evolution of non-invasive tests (i.e. imaging tests or EUS), and taking into account potentials complications, this technique should be reserved almost exclusively for therapeutic indications.
ERCP should be performed for appropriate indications as defined in previously published guidelines for >90% of the procedures.16 When there is a nonstandard indication, the reasons for this should be made sufficiently clear. Therefore, the indication has to be carefully evaluated, and radiological studies reviewed to anticipate the therapeutic strategy.16 The main indications have been previously described in detail, and they are summarized in Table 3.19–22 We will briefly review the most common indications.
ERCP indications.
| Biliary diseases | Pancreatic diseases |
|---|---|
| • Choledocholithiasis | • Recurrent idiopathic acute pancreatitis |
| • Benign biliary stricture | • Chronic pancreatitis |
| Inflammatory | Stricture |
| Congenital | Lithiasis |
| Postoperative | • Pancreatic duct leak |
| • Malignant biliary stricture | • Pancreatic collections (pseudocysts, disconnected pancreatic duct syndrome) |
| Pancreatic cancer | |
| Cholangiocarcinoma | |
| Ampullary lesions | |
| Infiltration by other neoplasias, lymph nodes or metastatic disease | |
| • Bile leak | |
| • Sphincter of Oddi dysfunction | |
| • Rare indications: hemobilia, infectious (hydatidosis or others) |
Choledocholithiasis is the most common cause of obstruction of the bile duct and ERCP has sensitivity greater than 95% for diagnosis. ERCP can be performed after cholecystectomy. However, in patients with jaundice, elevated liver enzymes, or worsening or persistent pancreatitis or cholangitis, it should be considered preoperatively, especially if a magnetic resonance cholangiopancreatography (MRCP) or EUS demonstrates choledocholithiasis. In addition, it should be performed urgently in patients with severe acute cholangitis, and those with severe acute biliary pancreatitis with suspected persistence of impacted lithiasis.
Biliary stricturesFor malignant strictures, drainage with plastic and metal stents is effective in up to 90% of cases of the stenosis in the middle or distal choledochus. Nevertheless, ERCP is not generally indicated to relieve a biliary obstruction in patients with potentially resectable malignant distal bile duct obstruction, in whom surgical resection will not be delayed by neoadjuvant therapy or other preoperative assessments or treatments. Despite this, in current clinical practice preoperative biliary decompression is widely performed. Drainage of more proximal lesions, such as Klatskin tumors, is usually less successful due to incomplete biliary drainage.
For benign biliary strictures different dilation or stenting can be effective, although treatment success varies widely depending on the indication.
A special indication is primary sclerosing cholangitis. There is usually a good response to dilatation. However, there is also an increased risk of developing acute cholangitis. For this reason, indications need to be individualized and considered carefully. Additionally, cytology of the dominant stricture should always be performed.
Bile leakSphincterotomy alone may be sufficient. Combination with a plastic biliary stent is recommended for large or highly productive leaks. The treatment is successful 80–100% of cases, depending on size and location.
Sphincter of Oddi dysfunctionPatients with sphincter of Oddi type I dysfunction respond in as many as 90% of cases to sphincterotomy. For type II dysfunction, clinical response is usually associated with abnormal manometry. However, many groups perform this treatment empirically. ERCP is not recommended for type III dysfunction.
ERCP for pancreatic diseases23,24Acute recurrent pancreatitisEUS and MRCP allow non-invasive diagnosis of anatomical pancreatic abnormalities and other etiologies of pancreatitis such as microlithiasis. As such, ERCP is generally reserved for treatment procedures. Occasionally, ERCP may be required to obtain the pancreatic duct anatomy or to collect bile for the study of microlithiasis. For selected patients with pancreas divisum, minor papilla sphincterotomy might be effective.
Chronic pancreatitisERCP is useful for the diagnosis and treatment of pancreatic duct lithiasis, strictures, and pseudocysts. Fragmentation and extraction of pancreatic duct lithiasis is often difficult or even impossible, and it seems less effective than surgical treatment. New approaches with direct cholangioscopy and laser or electrohydraulic lithotripsy might improve the endoscopic management of this situation.
Pancreatic leaksTranspapillary pancreatic stents can treat pancreatic leaks. However, it is advisable to place larger stents to bridge the leak when feasible, as this is associated with better outcomes.
EUS or ERCPFor patients with suspected choledocholithiasis (or with intermediate probability of bile duct stones)Cholelithiasis is quite prevalent in most western countries. Prevalence ranges between 10% and 20% while incidence increases with age.25 However cholelithiasis is asymptomatic in 80% of patients. Among these patients, passage of gallstones into the common bile duct (CBD) stones or choledocholitiasis occurs in 15–20%.26
When CBD stones are demonstrated prior to cholecystectomy, patients should undergo stone extraction, either by ERCP or by intraoperative bile duct examination during cholecystectomy.27
The diagnosis of choledocholithiasis is usually made by cholangiography, either preoperatively with ERCP, MRCP, or EUS, or intraoperatively (IOC) at the time of cholecystectomy.27–30
A combination of clinical, biochemical, and morphological criteria is used to risk-stratify individuals with suspected choledocholithiasis and an intact gallbladder into low, intermediate and high risk of choledocholithiasis groups.29 In patients with high risk of choledocholithiasis, ERCP remains the gold standard given its dual diagnostic and therapeutic role, even though it carries a risk of complications. At least two studies have analyzed the performance of ERCP as a diagnostic test and compared the performance of ERCP to that of EUS, along with ERCP vs intraductal ultrasound for CBD stone detection in patients at high risk of choledocholithiasis. ERCP showed 89–93% sensitivity, 100% specificity, and 94% accuracy for choledocholithiasis.31,32 Patients with intermediate risk of choledocholithiasis benefit the most from a non-invasive diagnostic evaluation of their CBD by EUS or MRCP, based on which a decision is then made to perform a therapeutic ERCP.28,29 EUS is an accurate test for diagnosis of choledocholithiasis in patients who are at intermediate risk of having a CBD stone based on clinical predictors.33
The performance of EUS for the evaluation for choledocholithiasis has been extensively studied. Two extense meta-analyses reported 89–94% sensitivity and 94–95% specificity of EUS in detecting choledocholithiasis, with ERCP, IOC, or surgical exploration used as criterion standards.30,34
It has been proposed that EUS could be used in selecting patients for therapeutic ERCP, avoiding diagnostic ERCP, given the higher morbidity of ERCP compared with EUS. For this reason, it has been suggested that EUS should replace diagnostic ERCP to assess patients with intermediate probability of choledocholithiasis.35
EUS appears comparable to ERCP as a diagnostic test for CBD stones, is superior to other techniques for detecting biliary stones, and can be used to select patients who will need therapeutic ERCP. This results in a significantly lower risk of complications in comparison with the use of ERCP for both diagnosis and treatment of choledocholithiasis.30,36,40,41
By performing EUS first, ERCP may be safely avoided in patients with common bile duct stones. Application of EUS in the selection of patients for therapeutic ERCP significantly reduces the complication rate.35
However, there are only a few randomized studies this setting.35–39 These 4 trials randomized patients at intermediate to high risk for choledocholithiasis to an EUS-first strategy versus an ERCP-first strategy. Patients found to have CBD stones at EUS underwent subsequent therapeutic ERCP, which was performed in the same setting in 3 of the 4 trials. Taken together, these studies provide evidence that the EUS-first strategy with selective therapeutic ERCP can reduce the number of diagnostic ERCPs by 60–75% in patients who are at moderate risk for choledocholithiasis.
It was also found that EUS first is more successful in evaluating the bile duct for stones because of possible unsuccessful biliary cannulation with diagnostic ERCP. Finally, the studies in question suggest that there was either less morbidity or a trend toward less morbidity and fewer complications using EUS first to screen for CBD stones, rather than starting with ERCP.
For tissue diagnosis of biliary strictures (or tissue sampling in suspected malignant biliary obstruction)It is difficult to differentiate between benign and malignant biliary strictures. In this regard, endoscopic tissue acquisition may obviate the need for further invasive testing, thus allowing for optimal intervention without delay.42
Biliary brush cytology has been a mainstay diagnostic method for suspected extrahepatic biliary tree malignancies because it is technically easy and generally is safe. However, its sensitivity for cancer is modest, ranging from 30 to 57% in most published studies.42,43 The addition of endobiliary forceps and endoscopic needle aspiration to brush cytology during ERCP increases the sensitivity of tissue sampling, but these procedures are time-consuming, increase the procedural costs, and may not be technically feasible for some biliary strictures.44
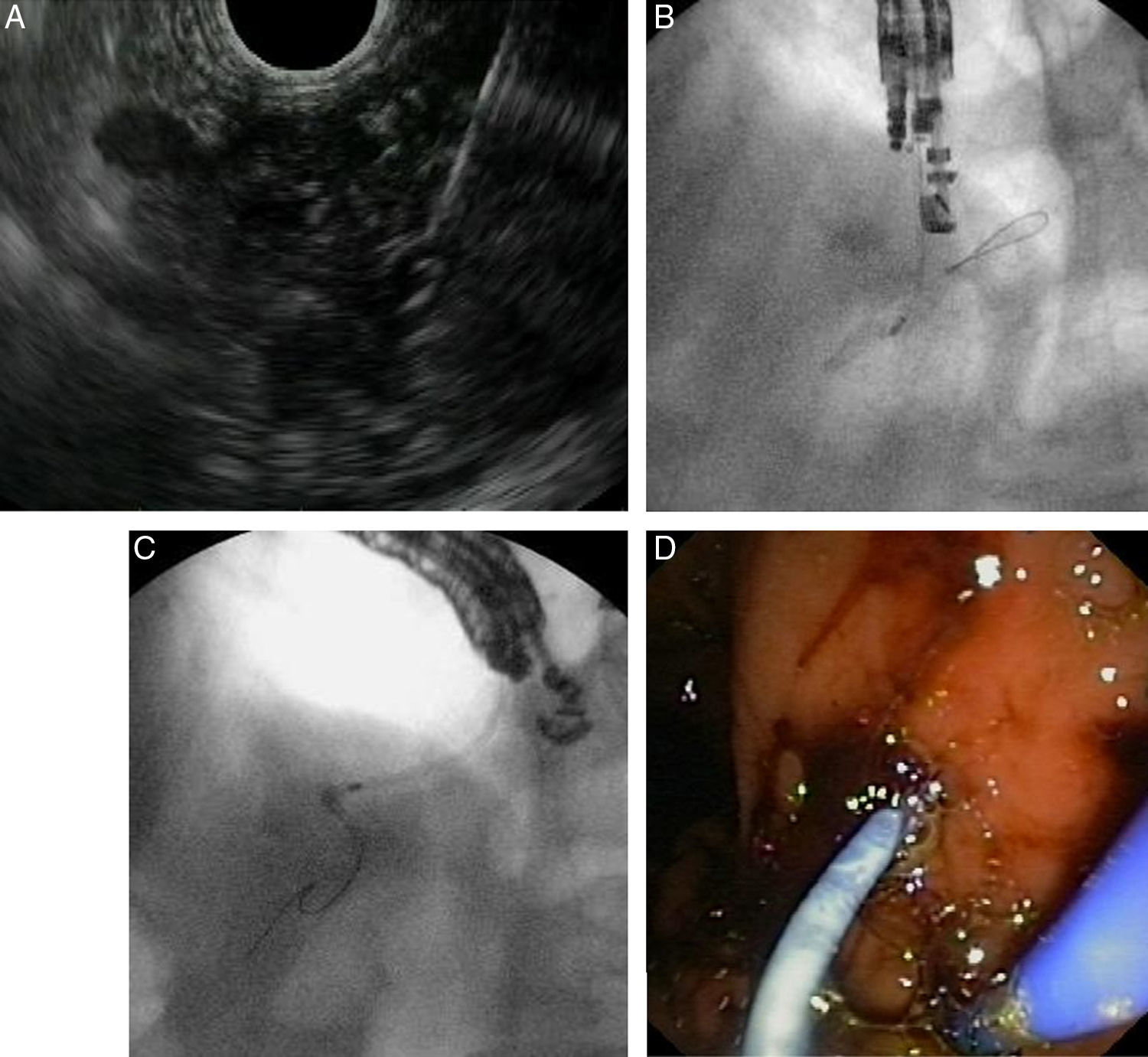
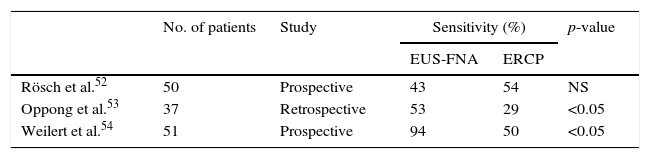
Some studies have documented EUS-FNA as a useful technique for the diagnosis of bile duct strictures, with a reported sensitivity of 43–86%45–48 (Fig. 1). In addition, three studies have attempted to use EUS-FNA on biliary strictures following negative or unsuccessful ERCP sampling. These studies showed that EUS-FNA was technically feasible, without significant risks, and with sensitivities after negative ERCP sampling of 68–89%.49–51
Three studies have directly compared the diagnostic yield of EUS-FNA and ERCP-based tissue sampling in patients with suspected malignant biliary obstruction.52–54 They found that EUS-FNA had greater sensitivity compared with ERCP (Table 4). In these studies, the sensitivity and accuracy of EUS-FNA were significantly superior to ERCP sampling among patients with pancreatic masses; EUS-FNA directly samples pancreatic mass, whereas ERCP samples are usually obtained from the area where a mass is causing compression on the bile duct. However, the sensitivity and accuracy of EUS-FNA seemed comparable to or lower than those of ERCP tissue sampling among patients with biliary masses and strictures.52–54 Therefore, it appears reasonable, when a tissue diagnosis is required, to start with EUS-FNA when a biliopancreatic mass is suspected, and with ERCP in biliary strictures in the absence of a biliopancreatic mass.
One potential additional risk in EUS-FNA with malignant biliary structures is peritoneal tumor seeding.55 Although a published study suggested there was no adverse effect on overall or progression-free survival from preoperative EUS-FNA, the theoretical risk of tumor seeding must be considered.56 Bile leakage during EUS-FNA may raise the risk of cancer-cell seeding in EUS-FNA performed for biliary lesions.51 Therefore, care must be taken not to puncture the lesion through the bile duct lumen, if possible, in order to avoid bile leakage.
EUS and ERCPSingle or separate sessionsEUS with or without FNA and ERCP are very often required for the management of pancreaticobiliary disorders, and they are usually performed in separate sessions.57 The experience of combining both procedures in a single session is limited and it is not common in routine care. EUS is a safe and useful technique in the evaluation of pancreaticobiliary diseases, especially in the evaluation of small choledocholitiasis and small pancreatic tumors, allowing a locoregional staging for neoplastic lesions with the possibility of performing FNA for cytological diagnosis.58,59 Currently, ERCP should only be performed as a therapeutic technique for biliary drainage with or without stent insertion.60,61 In cases of failed transpapillary ERCP cannulation, EUS-guided interventions could provide access for direct drainage, as the procedure was first described in 1996.62 Therefore, the combination of the two techniques in a single session offers the advantage of adding the potential of both techniques, allowing accurate diagnosis with endoscopic drainage.1,63 Despite the numerous potential advantages, implementation in clinical practice has encountered important obstacles regarding safety, diagnostic accuracy, and cost.
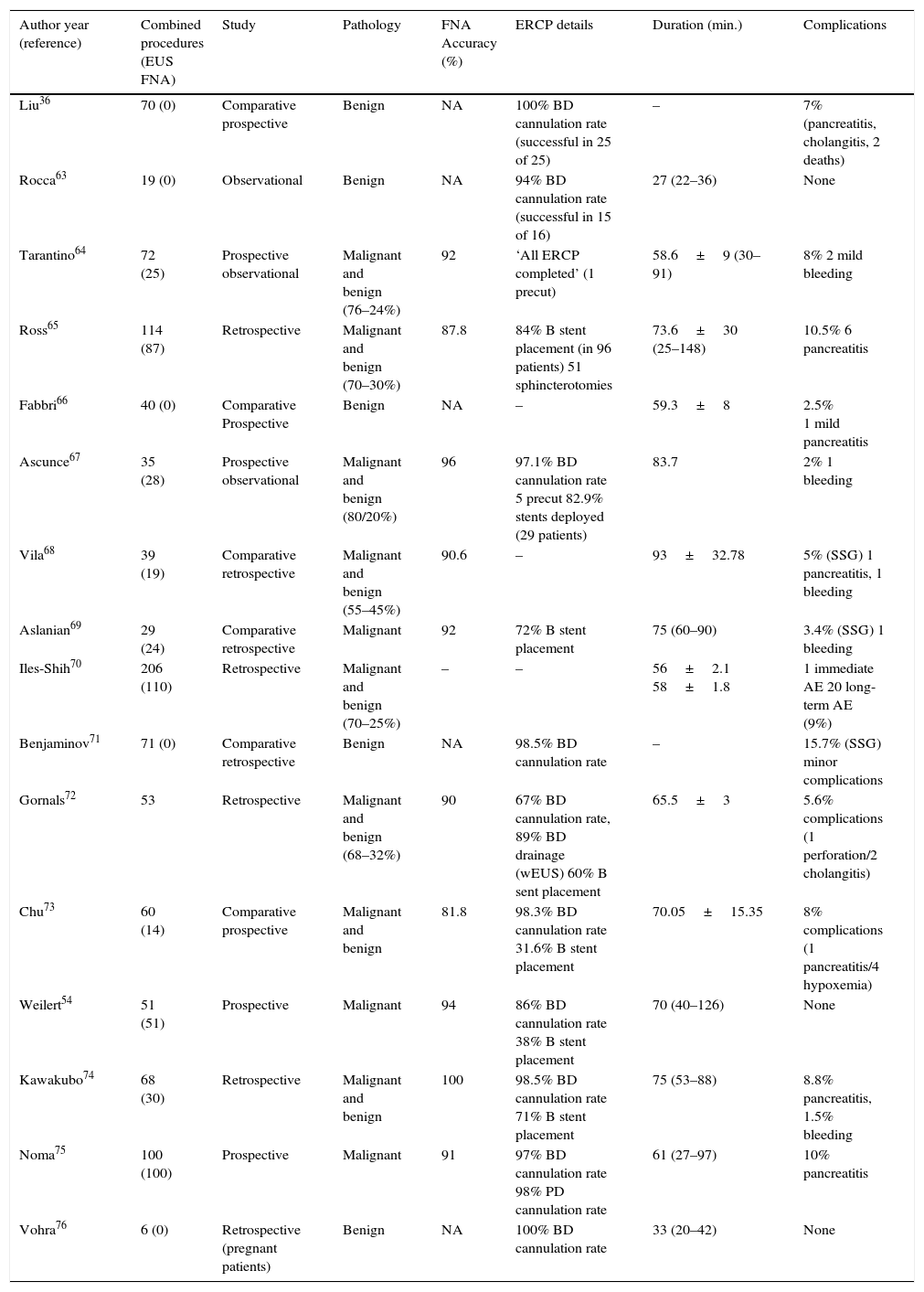
Table 5 provides a summary of the various papers published in relation to single-session EUS-ERCP in pancreatobiliary diseases.36,63–76
Summary of single-session EUS and ERCP procedures reported in the literature.
| Author year (reference) | Combined procedures (EUS FNA) | Study | Pathology | FNA Accuracy (%) | ERCP details | Duration (min.) | Complications |
|---|---|---|---|---|---|---|---|
| Liu36 | 70 (0) | Comparative prospective | Benign | NA | 100% BD cannulation rate (successful in 25 of 25) | – | 7% (pancreatitis, cholangitis, 2 deaths) |
| Rocca63 | 19 (0) | Observational | Benign | NA | 94% BD cannulation rate (successful in 15 of 16) | 27 (22–36) | None |
| Tarantino64 | 72 (25) | Prospective observational | Malignant and benign (76–24%) | 92 | ‘All ERCP completed’ (1 precut) | 58.6±9 (30–91) | 8% 2 mild bleeding |
| Ross65 | 114 (87) | Retrospective | Malignant and benign (70–30%) | 87.8 | 84% B stent placement (in 96 patients) 51 sphincterotomies | 73.6±30 (25–148) | 10.5% 6 pancreatitis |
| Fabbri66 | 40 (0) | Comparative Prospective | Benign | NA | – | 59.3±8 | 2.5% 1 mild pancreatitis |
| Ascunce67 | 35 (28) | Prospective observational | Malignant and benign (80/20%) | 96 | 97.1% BD cannulation rate 5 precut 82.9% stents deployed (29 patients) | 83.7 | 2% 1 bleeding |
| Vila68 | 39 (19) | Comparative retrospective | Malignant and benign (55–45%) | 90.6 | – | 93±32.78 | 5% (SSG) 1 pancreatitis, 1 bleeding |
| Aslanian69 | 29 (24) | Comparative retrospective | Malignant | 92 | 72% B stent placement | 75 (60–90) | 3.4% (SSG) 1 bleeding |
| Iles-Shih70 | 206 (110) | Retrospective | Malignant and benign (70–25%) | – | – | 56±2.1 58±1.8 | 1 immediate AE 20 long-term AE (9%) |
| Benjaminov71 | 71 (0) | Comparative retrospective | Benign | NA | 98.5% BD cannulation rate | – | 15.7% (SSG) minor complications |
| Gornals72 | 53 | Retrospective | Malignant and benign (68–32%) | 90 | 67% BD cannulation rate, 89% BD drainage (wEUS) 60% B sent placement | 65.5±3 | 5.6% complications (1 perforation/2 cholangitis) |
| Chu73 | 60 (14) | Comparative prospective | Malignant and benign | 81.8 | 98.3% BD cannulation rate 31.6% B stent placement | 70.05±15.35 | 8% complications (1 pancreatitis/4 hypoxemia) |
| Weilert54 | 51 (51) | Prospective | Malignant | 94 | 86% BD cannulation rate 38% B stent placement | 70 (40–126) | None |
| Kawakubo74 | 68 (30) | Retrospective | Malignant and benign | 100 | 98.5% BD cannulation rate 71% B stent placement | 75 (53–88) | 8.8% pancreatitis, 1.5% bleeding |
| Noma75 | 100 (100) | Prospective | Malignant | 91 | 97% BD cannulation rate 98% PD cannulation rate | 61 (27–97) | 10% pancreatitis |
| Vohra76 | 6 (0) | Retrospective (pregnant patients) | Benign | NA | 100% BD cannulation rate | 33 (20–42) | None |
AE: adverse event; B: biliary; BD: biliary duct; PD: pancreatic duct; NA: not applicable; SSG: single session group; wEUS: with EUS helping.
Performing EUS before ERCP can prevent two thirds of unnecessary ERCPs.36,59 Although MRCP is as powerful as EUS in choledocholithiasis detection, we have to take into account that EUS is more accurate than MRCP in the detection of small CBD stones, and it is preferred in cases where MRCP is not possible, such as with patients with claustrophobia and patients with metal devices.36,77 As previously reported, it seems that performing EUS-ERCP in a single session for choledocholithiasis is a safe, feasible and effective strategy, with no increase in procedure- or sedation-related complications (Table 5). Moreover, in one study an increased risk of complications was noted when ERCP was delayed, resulting in significant biliary complications in 14% of patients.71 It also seems that a single session reduces hospital stay, costs and repeat sedation compared to separate sessions.36,66,72 A negative point of this strategy is the long procedure duration that may be especially problematic in elderly patients. As reported by Iles-Shih, a single session in elderly patients was not related with more adverse events than in nonelderly patients.70 An important element in establishing this approach is having technology that allows the procedure scope without the need to change the scope. Although there is a study that did the whole procedure with the linear echoendoscopy, in real practice the cannulation of the papilla is not possible in the majority of the cases; improvement in the endoscopic devices is necessary.63 Another situation in which a single-session EUS and ERCP would be useful is the case of pregnant patients with suspected choledocholithiasis. As reported by Vohra et al., a single-session procedure was done without fluoroscopy in 10 pregnant women. The whole procedure was done with two different tubes and EUS helped the study of the number, size and location of common bile stones.76
Combined EUS-guided FNA and ERCP for obstructive jaundice from presumed biliopancreatic malignancyEUS is highly sensitive for the detection of pancreatic tumors with a very high negative predictive value and with the ability to provide FNA.78 Establishing a tissue diagnosis of malignancy is mandatory prior to surgical or oncologic treatment in patients with suspected malignant biliary obstruction. ERCP is a well-established procedure for evaluating and managing biliary obstructions. These two procedures are often required for the diagnosis and treatment of biliopancreatic malignancy. Therefore it is reasonable to perform these two techniques during a single session under the same anesthesia. Based on the published data, it seems that combined EUS-FNA and ERCP for obstructive jaundice is a safe, feasible procedure (Table 5).
Regarding which technique is better to perform first, some authors advocate beginning with ERCP and stent placement, to improve the EUS study of biliary system using the stent as a guide.57 In contrast, other evidence supports performing EUS prior to ERCP, as it is more accurate for cancer staging72,79,80 and because EUS-FNA has greater sensitivity than ERCP for detecting malignancy, especially in primary pancreatic cancer.54 This strategy could avoid unnecessary ERCP, brushings and stents, and decrease the possibility of inconclusive cytology in the case of stent placement. Moreover, as reported in several papers, the sensitivity and accuracy of FNA are not affected when the two procedures are done in the same session (Table 5).
Regarding safety, although initial experiences reported severe complications related to EUS-FNA prior to ERCP, with the consequence of contrast leakage and pneumoperitoneum,81,82 this has not been observed in later studies with series of patients ranging from 19 to 206 (Table 5).
Regarding procedure duration, as previously noted it does not seem to be the case that a longer procedure is associated with more cardiopulmonary adverse events.65,70,72 Moreover in those studies that have compared the procedure time of the two techniques in a separate session versus in a single sessions, it seems that there are no differences or even a timesaving in the single session strategy.66,68,69,73 In these previous comparative studies similar results were obtained, with the dose of propofol administered being better for the single session group.
Another important issue to be considered is the financial. As we mentioned above, for benign disease there have been few cost-analysis studies performed, and these show a clear benefit in single-session EUS-ERCP.36,66,72
In conclusion, it seems that a single-session EUS-ERCP for pancreaticobiliary diseases provides benefits in clinical and financial terms, by simplifying patient management, shortening hospital stay, reducing cost, and avoiding repeat sedation. The procedure time and the logistical possibility of performing the two techniques at the same time are the principal limiting factors for applying this strategy in real practice.
Biliary and pancreatic drainage guided by EUS after failed ERCPERCP is the preferred procedure for biliary and pancreatic drainage. However failure occurs in 3–10% of cases due to anatomic variation, prior surgery, operator inexperience, tumor extension, or periampullary pathology. In these situations, the rescue options include precut, percutaneous transhepatic drainage (PTBD), surgical intervention, EUS-guided biliary drainage (BD), and pancreatic duct (PD) drainage.68,78,83–85
Biliary drainage guided by EUSEUS-guided biliary drainage (EUS-BD) has been described as an alternative method to achieve internal biliary drainage in those patients in whom ERCP has failed. This is a promising technique which offers some advantages over the other options (radiological or surgery). Multiple retrospective and some prospective studies have shown it to be safe and effective. But it is important to note that all these data come from highly skilled advanced endoscopists at tertiary centers with expertise in both EUS and therapeutic endoscopy.
EUS-guided BD has been performed in more than 1000 published cases, with mean technical and clinical success rates of 91% and 88%, respectively. However, the mean overall complication rate was 26% (16–35%) with mortality of 1–5%, especially in the early stage of the learning-curve (Table 6).68,72,85–110
EUS-guided biliary drainage in the literature.
| Study | Design | Cases (n) | Technical success (%) | Clinical success (%) | Adverse events (%) |
|---|---|---|---|---|---|
| Bories et al.86 | R | 11 | 91 | 80 | 72 |
| Maranki et al.87 | R | 49 | 84 | 80 | 18 |
| Brauer et al.88 | P | 12 | 92 | 72 | 16 |
| Horaguchi et al.89 | P | 16 | 100 | 94 | 37 |
| Kim et al.90 | R | 15 | 80 | 80 | None |
| Fabbri et al.91 | P | 16 | 75 | 75 | 8 |
| Park et al.92 | R | 57 | 96 | 89 | 47 |
| Hara et al.93 | P | 18 | 94 | 94 | 77 |
| Komaki et al.94 | R | 15 | 100 | 100 | 46 |
| Ramírez-Luna et al.95 | P | 11 | 91 | 82 | 18 |
| Shah et al.96 | R | 68 | 85 | 85 | 9 |
| Iwashita et al.97 | R | 40 | 73 | 73 | 12 |
| Dhir et al.98 | R | 58 | 98 | 98 | 3 |
| Artifon et al.85 | RCT | 13 | 100 | 100 | 15 |
| Song et al.99 | P | 15 | 87 | 87 | 47 |
| Kim et al.100 | P | 13 | 92 | 84 | 38 |
| Vila et al.101 | R | 106 | 70 | 70 | 23 |
| Horaguchi et al.102 | R | 21 | 100 | 100 | 10 |
| Hara et al.103 | P | 18 | 94 | 89 | 27 |
| Park et al.104 | P | 45 | 91 | 87 | 11 |
| Kawakubo et al.105 | R | 14 | 100 | 100 | 14 |
| Dhir et al.106 | R | 35 | 97 | 97 | 23 |
| Khashab et al.107 | R | 35 | 94 | 91 | 14 |
| Gornals et al.72 | R | 15 | 87 | 73 | 40 |
| Gupta et al.108 | R | 240 | 99 | 87 | 35 |
| Dhir et al.109 | R | 68 | 97 | 97 | 21 |
| Kawakubo et al.110 | R | 64 | 95 | 95 | 42 |
| Total 27 studies | 27 | 1088 | 91 (70–100) | 87 (70–100) | 29 (3–77) |
RCT: randomized controlled trial; P: prospective study; R: retrospective study.
The nomenclature of the different variations of this technique can be confusing and repetitive. In short, there are two different routes: intrahepatic and extrahepatic. After bile duct access, drainage can be accomplished via (A) direct transgastric transluminal stenting (i.e., hepaticogastrostomy) or transduodenally (i.e., choledochoduodenostomy); (B) rendezvous technique passing a guidewire through to the papilla; and (C) antegrade stent placement, similar to the PTBD technique.
When these different methods are compared, EUS-guided BD shows similar technical and clinical success rates to both intrahepatic (usually transgastric) and extrahepatic (usually transduodenal) access. However, the extrahepatic route seems to be safer than the intrahepatic.84,109
This technique has been compared with the percutaneous BD, and when both techniques are performed by skilled interventional operators, the technical and clinical success rates are similar, with no difference in the complication rate.83
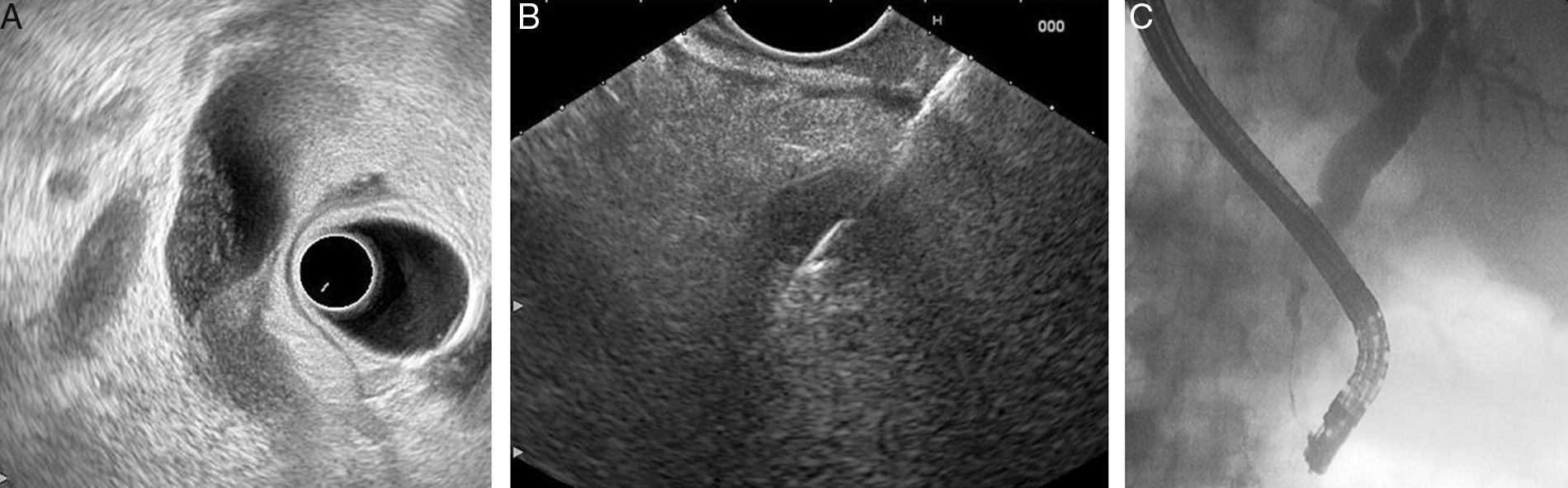
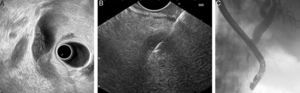
Fig. 2 shows a case of EUS-guided BD after a failed ERCP in a malignant biliary stricture with signs of tumor invasion in the papilla. In this case extrahepatic access (transduodenal route) with transmural drainage (choledochoduodenostomy) was accomplished with a novel device designed for use with an echoendoscope. It creates an internal ostomy and delivers a biliary lumen-apposing metal stent at the same time (6-mm diameter, 8-mm saddle length, HotAXIOS, Xlumena Inc., Mountain View, CA, USA).
EUS-guided biliary drainage. (A) Failed ERCP in a duodenal papilla with tumor invasion signs; (B) common bile duct (CBD) access by EUS-guided puncture; (C) cholangiography shows a dilated CBD with a malignant distal stenosis; (D) EUS-guided choledochoduodenostomy using a cautery-tipped stent delivery system (HotAXIOS, Xlumena Inc.); (E) EUS image of a lumen-apposing metal stent delivered inside the CBD; (F) biliary AXIOS stent (6×8mm) delivered and well positioned in the duodenum.
EUS-guided PD is indicated after failed ERCP, usually in patients with benign disorders such as stenosis, lithiasis, or post-interventional strictures. At present, there are more than 300 descriptions of this technique in the literature.
This technique is more challenging technically than EUS-guided BD. For this reason, general clinical and technical outcomes (78%) are poorer.83,84
As with the previous technique, there is a nomenclature of the different variations. Briefly, there are 2 different approaches: transmural, usually transgastric (i.e., pancreaticogastrostomy), and transpapillary. Transpapillary guidewire placement allows retrograde access via rendezvous ERCP and anterograde stent placement for PD drainage.
The mean overall complication rate is 20% (7–55%). The transmural route is associated with a higher complication rate than the rendezvous route. This is clearly due to the fact that EUS-guided transmural PD stenting requires more aggressive dilation of the tract than does the rendezvous technique.84
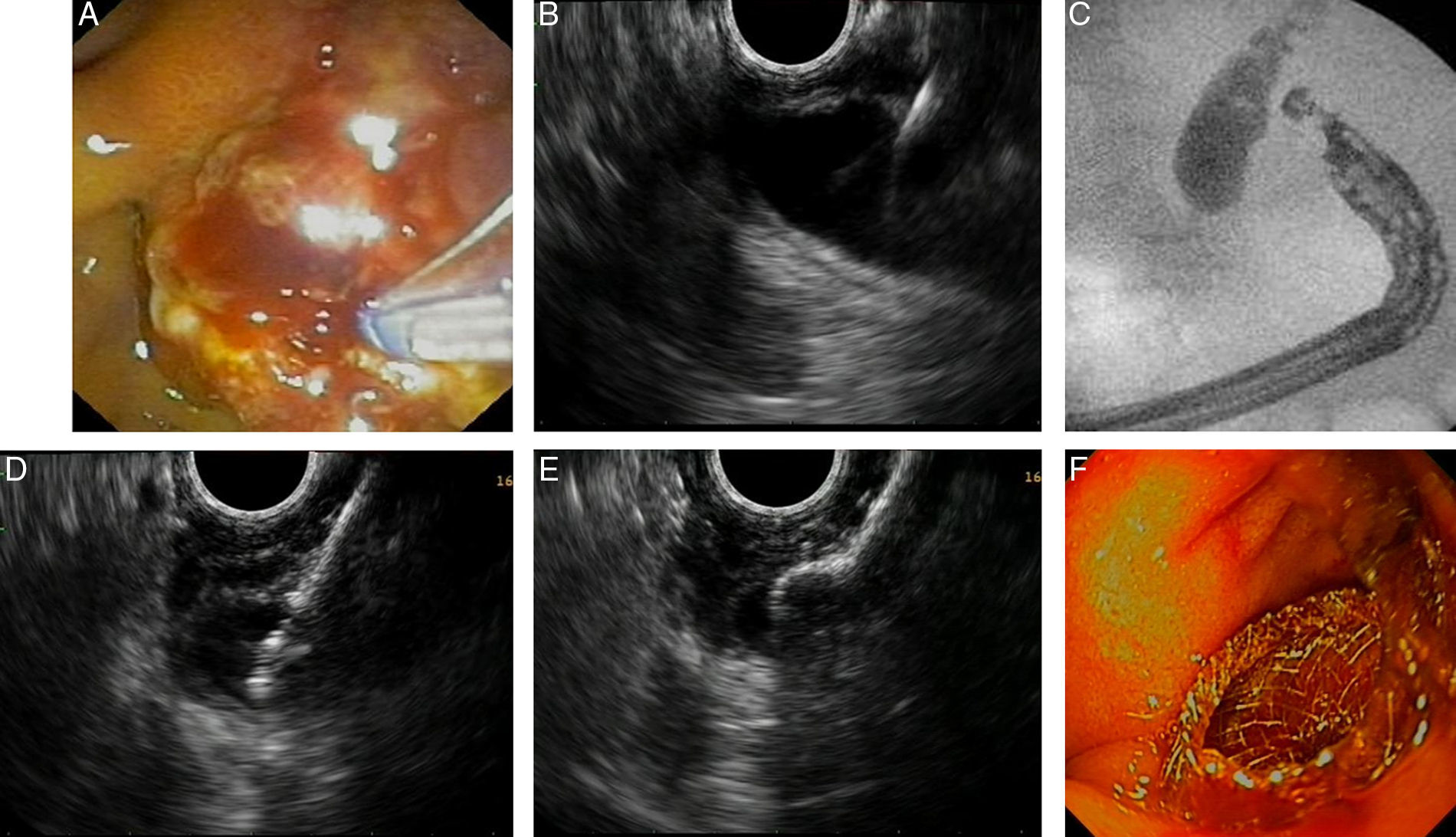
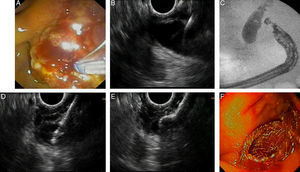
Fig. 3 illustrates EUS-guided PD drainage in a case of benign stenosis in chronic pancreatitis. The internal ostomy is performed using a 6F cystotome, and a 7F transgastric plastic stent is delivered.
EUS-guided pancreatic duct drainage after failed ERCP. (A) Pancreatic duct (PD) access by EUS-guided puncture using a 19G needle; (B) pancreatography and creation of a pancreaticogastrostomy using a cystotome over a guidewire; (C) plastic stent delivered inside the PD. (D) A 7Fr transgastric plastic stent, well positioned in the gastric cavity.
EUS is useful for the differential diagnosis of a prominent ampulla, with the majority of these lesions (>95%) being either adenomas or adenocarcinomas.
The universal incorporation of the EUS into the diagnostic evaluation of an ampullary adenoma is still controversial. If clinical suspicion of invasive carcinoma is low, and the lesion appears amenable to endoscopic resection, then EUS may not impact the decision to stage the lesion via papillectomy.111
However, EUS is recommended to assess the endoscopic resectability of ampullary adenomas because it is the best technique for T staging of these lesions and it is highly accurate in evaluating intraductal extension and infiltration of the periampullary wall layers.112,113
Although the accuracy of ERCP compared with EUS for delineating ductal extension of tumor requires further study, the performance of a cholangiopancreatogram at the time of resection is helpful unless EUS has previously confirmed ductal involvement or irresectability.
In addition, ERCP has a crucial role in palliation of obstructive jaundice in the setting of ampullary adenocarcinoma and in minimizing the risk of post-papillectomy pancreatitis by placement of a pancreatic stent.114
Endoscopic drainage for pancreatic collections with disrupted pancreatic ductEndoscopic transmural drainage of pancreatic collections (pseudocysts or walled-off pancreatic necrosis), with or without EUS-guidance, is safe, effective, has lower morbidity and mortality than surgery, and has been adopted as the first therapeutic option for drainage of pancreatic collections, when indicated, in many centers.115
ERCP allows the endoscopic transpapillary drainage by placement of an endoprosthesis through the papilla into the PD and is highly effective in the presence of a partial PD disruption that can be successfully bridged.
Data on the role of combined transmural and transpapillary drainage of pancreatic collections are limited and inconsistent. Based on available evidence, it seems reasonable to combine the two therapies in patients with pseudocysts and underlying chronic pancreatitis or known ductal abnormalities.116,117
In the event of complete PD disruption, transpapillary drainage is hardly effective, and the placement of a permanent transmural stent is usually recommended.
The future of the relationship: discussionWith all the above in mind, it is easy to think that the relationship between EUS and ERCP will be close and strong, in the management of biliopancreatic disorders. The two techniques have experienced similar growth over the years, as new diagnostic procedures that have become interventional therapeutic techniques. Until recently, the two techniques were treated as different scopes or devices, and two different groups of endoscopists applied themselves to either ERCP or EUS.
However, in the last 5 or 10 years, EUS has undergone a transformation into a therapeutic technique, demanding skills in devices and stents from the ERCP. For this reason, some endoscopists dedicated to ERCP have taken up EUS, exploiting its therapeutic potential, which, to judge by the literature, has grown exponentially.
Following on from the era in which EUS and ERCP were in different worlds, some young endoscopists have been able to bridge this gap between the two advanced endoscopy techniques, and in so doing have created a single specialization.
The belief that the upcoming endoscopist should have to choose between EUS and ERCP is negative and detrimental to the progress of advanced endoscopy.118
The learning-curve of the two techniques will be demanding and challenging because a high level of skill is required for these pancreaticobiliary endoscopy procedures. Modern training must view the two techniques as one in order to facilitate training and motivate the learner.
ConclusionsEUS and ERCP in good hands are advanced endoscopic procedures, and if they are used in combination, they offer a powerful positive effect on the management of biliopancreatic disorders. Nowadays, the relationship between them depends on the historic context of each center where they are used. But have no doubt that both techniques share a promising future together. More than friends, they are brothers.
Author contributionsJoan B. Gornals was involved in all the stages and in the conception and design of the article, and distributed each author with each section. J. M. Esteban, C. Guarner-Argente, C. Marra-López, A. Repiso, O. Sendino, and C. Loras were involved with all stages of the manuscript development: analysis and interpretation of the data, drafting of the article, critical revision of the article, and final approval of the article.
Conflict of interestAuthors declare no conflict of interests for this article.