INTRODUCTION
Endoscopic ultrasound (EUS) has largely been demonstrated to be a highly accurate technique for locoregional gastrointestinal cancer staging as well as for evaluation of large gastric folds and pancreatobiliary disturbances1-6. The addition of EUS-guided real-time fine-needle aspiration (EUS-FNA) has improved the performance characteristics of EUS alone, although, surprisingly, few studies have prospectively and blindly compared EUS and EUS-FNA in large series of homogeneously studied lesions. For example, this kind of study has been performed in esophageal and rectal cancer but with different results7,8. In the present large series of patients homogeneously studied by EUS and EUS-FNA, we aimed to assess what EUS-FNA adds to the diagnosis of lesions that cannot be classified as benign or malignant by EUS alone.
Some strategies, such as increasing the number of passes or having an attendant cytopathologist, have been demonstrated to be useful in improving the diagnostic yield of EUS-FNA9,10. The type of lesion and its location (lymph node, pancreatic or other extramural masses, or intramural lesion) could also be related to the accuracy of the technique11,12. However, the predictive factors of an accurate EUS-FNA diagnosis are still unknown.
Several studies have demonstrated that EUS-FNA is a cost-effective approach for the preoperative staging of esophageal carcinoma13, pancreatic tumors14,15 and rectal cancer16 but the cost-effectiveness of having an on-site cytopathologist has not yet been evaluated.
The present study aimed to investigate: a) the clinical impact of EUS-FNA in terms of new diagnoses with respect to EUS alone in relation to the type and location of the lesion; b) the independent predictive factors for an accurate EUS-FNA diagnosis, and c) the cost-effectiveness of an on-site cytopathologist.
PATIENTS AND METHODS
Patients
Between January 2002 and February 2004, all consecutive patients referred to our unit for EUS-FNA were prospectively evaluated following the protocol described below. Patients were referred for EUS FNA of mediastinal, perigastric, periduodenal or perirectal lesions of unknown origin or for staging of gastrointestinal or pulmonary malignancies.
The study was approved by the ethical research committee of Hospital Clinic and a written informed consent was obtained from all patients.
Methods
Technique
EUS-FNA was performed under conscious sedation by two fully trained endoscopists (AG, MP). Prophylactic antibiotics were administered in patients with cystic lesions as well as for endocarditis prophylaxis when appropriate. Evaluation of the target lesion and tumoral staging was initially performed with a radial scanning echoendoscope (GF UM20 and GF UM160, Olympus Europe, Hamburg, Germany). EUS-FNA was then carried out by using a curved linear array echoendoscope (GF UMC30P, Olympus Europe) with Doppler capability and a scanning plane in the long axis of the instrument. EUS and EUS-FNA were performed following previously described standards17.
Cytological material was considered adequate if the attendant cytopathologist reported that there were malignant cells or a sufficient number of representative cells for diagnosis. In putative cytological benign lesions, the decision to cease making needle passes was established by taking into account meaningful clinical factors such as the degree of clinical suspicious for underlying malignancy, the clinical impact of a non-diagnostic aspirate, cytological appearance of the aspirated material, and the total number of passes. More than one lesion could be targeted by EUS-FNA in the same patient.
The final diagnosis was made after reviewing all the material in the laboratory.
Definitions
For analysis purposes, EUS without FNA diagnosis was categorized into malignant or benign. Lesions that could not be classified into either or these two categories were considered as «indeterminate». Cytopathological diagnoses were classified as benign, malignant, or indeterminate if only «atypical cells» were found or the sample was inadequate. «Suspicious» samples were considered malignant.
Pathological examination of resected specimens or clinical follow-up of patients not undergoing surgery were used as gold standard. In this latter group, lesions were considered malignant if there was clinical progression of the disease or response to chemotherapy or radiation therapy. Lesions were considered benign when spontaneous resolution or lack of progression was observed on imaging studies after a minimum follow-up of 12 months. In pancreatic cysts, it is now well known that mucinous cystadenomas and intraductal mucinous papillary tumors are premalignant lesions that should be treated surgically. For this reason, the clinical criterion was considered the most important, and these lesions were considered malignant.
Variables recorded
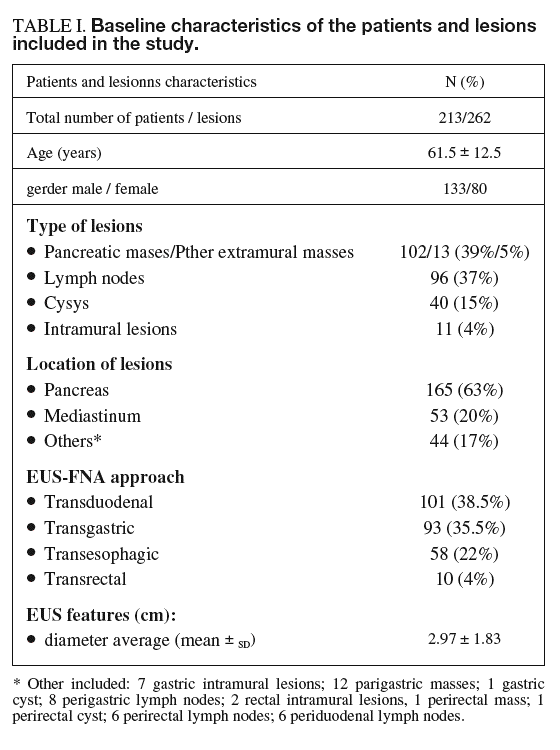
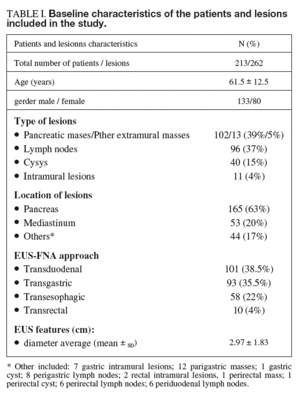
The following variables were recorded from each procedure according to the standard protocol for EUS in our unit and were collected in a database: demographic variables (gender, age), variables related to the lesion such as location (pancreas, mediastinum, other), type of lesion (lymph node, extramural or intramural mass, cyst) and size, EUS diagnosis (malignant, benign or indeterminate), variables related to EUS-FNA such as approach (transesophagic, transgastric, transduodenal or transrectal), the number of passes needed to reach a cytological diagnosis, and EUS-FNA diagnosis (benign, malignant or non-diagnostic). To ensure blindness to the results of EUS-FNA, the EUS diagnosis was made before any information on cytologic assessment was registered.
Statistical analysis
Operating characteristics of EUS FNA. Accuracy, sensitivity, specificity, and positive and negative predictive values of conventional cytology were calculated with respect to the gold standard. Calculations were performed for the whole series and for each type of lesion (lymph node, extramural mass, intramural lesion and cyst). In addition, to establish the accuracy of EUS-FNA in the clinical setting, this parameter was also calculated by considering inadequate samples as misdiagnosed.
Clinical impact of EUS-FNA in terms of new definitive diagnoses. The clinical impact of EUS-FNA in terms of new definitive diagnoses was calculated by comparing the percentage of lesions diagnosed by EUS-FNA and its performance characteristics with the same figures for EUS alone. The calculations were made for the whole series and for each specific type of lesion.
Predictive factors for an accurate EUS-FNA diagnosis. Evaluation of the factors influencing the results of EUS-FNA was performed for the whole series and for each type of lesion using all the above-mentioned variables. Comparisons between qualitative variables were performed by the *2 test, with application of Yates' correction when needed. Continuous variables were expressed as mean ± standard deviation and analyzed by Student's t-test. A stepwise logistic regression model was used to assess the independent predictive factors for correct diagnosis. A p-value of less than 0.05 was considered statistically significant.
Accuracy of EUS-FNA according to the number of passes. To establish the effectiveness of the presence of a cytopathologist during EUS-FNA, the results achieved in the presence of a pathologist were compared with those that theoretically would have been obtained if a particular number of passes had been performed without on-site evaluation. For this calculation, accuracy was determined after each particular number of passes, assuming that when a lesion was actually diagnosed in a specific pass, any additional subsequent pass would produce an identical result. This analysis was performed for the whole series and for each type of lesion. The relationship between the type of lesion and the number of passes was analyzed by analysis of variance (ANOVA) procedures using the F test statistic.
Cost-minimization analysis of on-site evaluation. The outcome measure for the cost-minimization analysis was correct diagnosis. Costs included the salaries of the endoscopist, pathologist, anesthesiologist, nurse and technician, as well as sedation and material for conventional cytology. Physician, nurse and technician fees were calculated assuming a 1-year full salary of 47,000 USD, 30,000 USD and 18,000 USD, respectively, according to Spanish national health system rates, and an average time for obtaining the specimen and performing on-site examination of 15 min per sample. The general costs of EUS imaging and FNA procedures (material, overnight admission, etc.) were not considered since they were identically imputed to both strategies. Similarly, the costs derived from minor procedure-related complications that did not require hospital admission or therapeutic measures were not considered either.
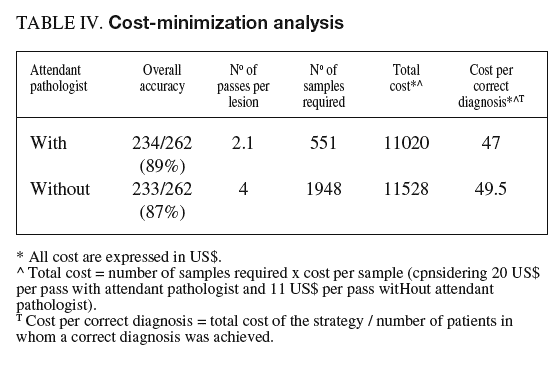
Taking into account all these considerations, the cost of cytological evaluation without on-site examination and cytological evaluation with an attendant cytopathologist were estimated to be 11 USD and 20 USD per sample, respectively. The number of samples required to achieve a correct diagnosis was multiplied by these costs.
RESULTS
A total of 213 consecutive patients underwent EUS-FNA. According to the definitive diagnosis, lesions were benign in 54 (25%) patients and malignant in 159 (75%). The definitive diagnosis was established by clinical follow-up in 137 patients (64%) and by surgical specimen in the remaining 76 (36%). Demographic characteristics and the number and characteristics of the 262 targeted lesions are detailed in table I. A total of 551 samples were obtained from these lesions, representing 2.1 ± 1.1 passes per patient on average (range, 1-6). The average number of passes required to reach a cytological diagnosis was higher for intramural lesions (3.4 ± 1.4) and masses (2.3 ± 1.1) than for lymph nodes (1.8 ± 1.0) and cysts (1.9 ± 1.0) (F = 8.811; p < 0.001).
No major complications resulting in hospital admission or significant therapeutic measures were registered.
Operating characteristics of EUS-FNA
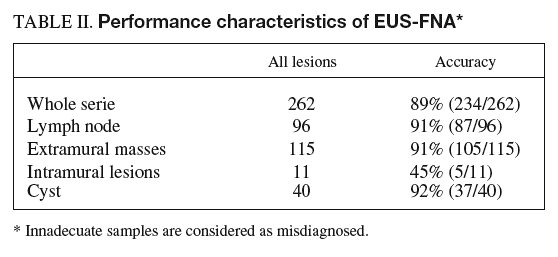
Adequate cytological specimens were obtained in 250 of the 262 lesions (95%). In lesions in which the material was adequate for diagnosis, EUS-FNA revealed malignancy in 179 of the 193 malignant lesions (sensitivity, 93%; CI, 90-96) and in 2 of the 57 benign lesions (specificity, 96%; CI, 94-98). Both false-positive results occurred in pancreatic lesions. One corresponded to a patient with a pancreatic tumor diagnosed by helical CT in whom EUS findings were not conclusive for malignancy but those of EUS-FNA were consistent with adenocarcinoma. The pathologic diagnosis after surgery was of focal «non-specific» pancreatitis. Three years later the patient is alive and has no signs of pancreatic cancer. The other false-positive result occurred in a patient with a pancreatic cyst in which EUS-FNA was consistent with mucinous cystoadenoma whereas the surgical specimen showed a serous cystoadenoma. There were no false-positive results in lymph nodes or intramural lesions (specificity, 100%). The sensitivity of EUS-FNA for the diagnosis of malignancy in each different type of lesion was as follows: 89% for lymph nodes (CI, 83-95), 95% for extramural tumors (CI, 91-99), 67% for intramural lesions (CI, 26-100) and 100% for cysts; therefore, diagnosis was correct in 250 of the 262 lesions evaluated (accuracy 94%; CI, 91-97).
To establish the actual performance characteristics of EUS-FNA in a clinical setting, the analysis was repeated considering inadequate samples as misdiagnosed.
The overall accuracy of EUS-FNA was 89% (CI, 85-93) (234/262 lesions). When this figure was calculated for each specific type of lesion, the overall accuracy for intramural lesions (45%; CI, 16-74) was much lower than that for other types of lesion (lymph node, 91% [CI, 85-97], masses, 91% [CI, 86-96], and cysts, 92% [CI, 83-100]) (table II).
Clinical impact of EUS-FNA with respect to EUS alone
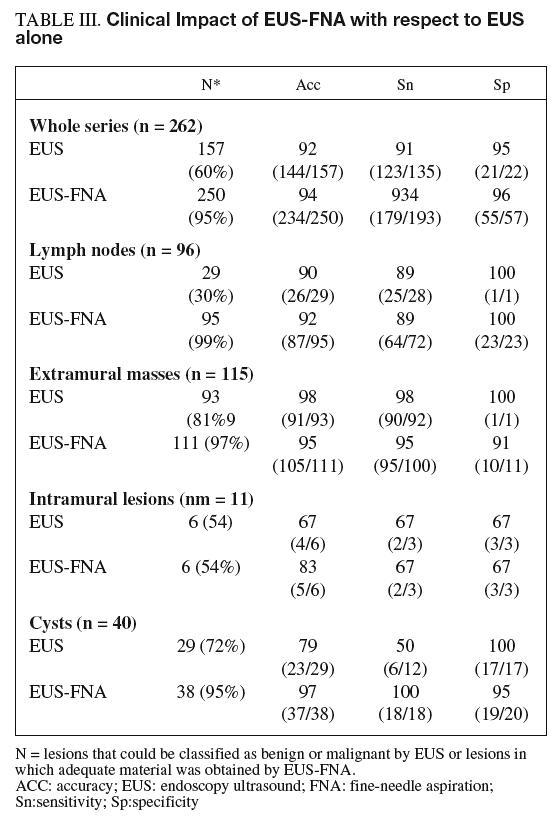
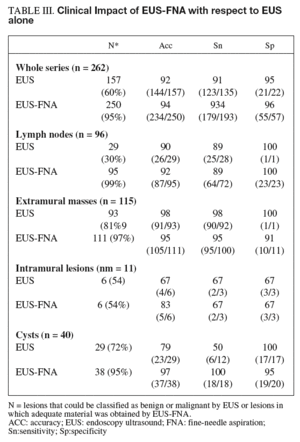
EUS alone was able to discriminate between malignant and benign disease in 60% of lesions with 92% accuracy. The remaining 105 lesions (40% of the total) were classified as indeterminate. The addition of FNA to EUS allowed diagnosis of almost all lesions (89%) with an accuracy of 90%.
As shown in table III, the addition of FNA in lymph nodes increased the number of lesions with a definitive diagnosis (from 29 to 95) but did not increase accuracy (90 vs 92%). For cysts, the addition of FNA doubled sensitivity in the detection of malignancy (50 vs 100%). Importantly, for lymph nodes and intramural lesions, the addition of FNA produced no false-positive or false-negative results in the subgroup of patients with a correct EUS diagnosis. However, FNA led to a mistaken diagnosis of benign disease in 3 of the 87 malignant extramural lesions with a correct diagnosis by EUS alone, the three lesions being pancreatic cancers.
Predictive factors of accurate diagnosis
Among the analyzed variables (table I), the only factors associated with a correct diagnosis by EUS-FNA were the type and diameter of the lesion (p < 0.05). Lesions located in the gastrointestinal wall or those with a larger diameter were associated with a higher proportion of incorrect diagnoses than the remaining lesions. After the multivariate analysis, the only variable independently associated with an incorrect diagnosis was intramural location of the target lesion.
Accuracy of EUS-FNA according to the number of passes
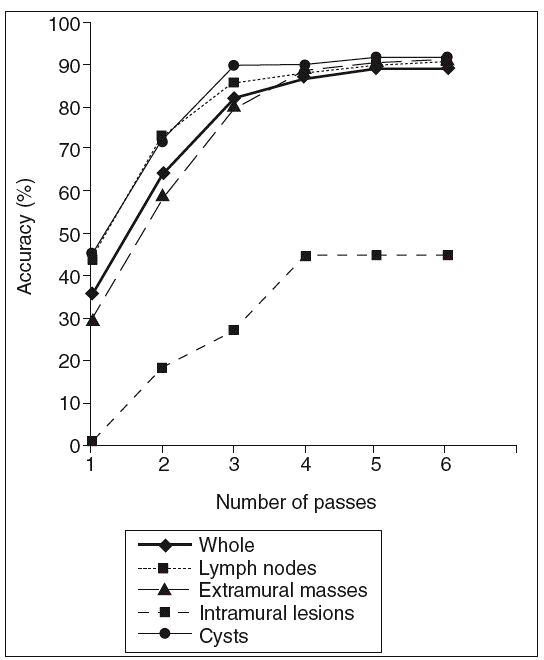
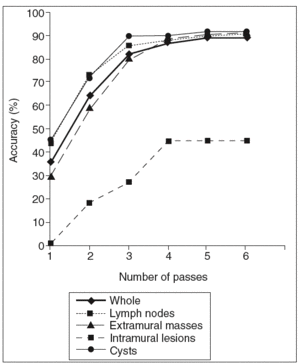
The overall accuracy of EUS-FNA was calculated after a particular number of passes (fig. 1). The effectiveness of EUS-FNA in the whole series progressively increased from 36% to 89%, reaching a plateau in the fourth pass. This curve was similar for extramural masses, lymph nodes and cysts but the plateau appeared in the third pass in lymph nodes and cysts. The accuracy of EUS-FNA for intramural lesions increased from 0.9% to 45% and reached a plateau in the fourth pass.
Fig. 1. Accuracy of EUS-FNA according tohe number of passes
Cost-minimization analysis of on-site evaluation
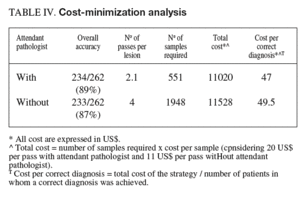
To establish the cost-effectiveness of the presence of an attendant cytopathologist, the results obtained with this strategy were compared with those that would have been obtained with a particular number of passes without on-site evaluation (table IV). The overall accuracy obtained with 4 passes (87%) was similar to that obtained under pathologist guidance (89%) with a mean of 2.1 passes per lesion. Considering 20 US$ per pass with an attendant pathologist and 11 US$ per pass without on-site examination, the presence of an attendant cytopathologist was cost-effective with respect to the corresponding strategy without on-site examination.
DISCUSSION
This large single-center experience confirms that EUS-FNA is an accurate modality for cytological diagnosis of malignancies adjacent to the gastrointestinal wall but is less efficient for the diagnosis of intramural lesions. According to our results, the efficacy of EUS-FNA mainly depends on the location of the lesion. Indeed, EUS-FNA is a highly specific and sensitive technique for the diagnosis of lesions located in the posterior mediastinum and the pancreatic, perirectal and perigastric areas. However, the overall accuracy of this technique in the diagnosis of intramural lesions, especially when inadequate samples are considered as misdiagnosed, is much lower. The results of the multivariate analysis showed that intramural location was the only independent factor related to low accuracy of EUS-FNA and this location was also probably the reason why an on-site cytopathologist was not cost-effective in this group of lesions. Nevertheless, in our series, 8 of the 11 intramural lesions were submucosal tumors, in which obtaining adequate material for cytological diagnosis appears to be more difficult. Moreover, even with an adequate sample, the possibility of establishing a diagnosis of malignancy or benign disease by cytology alone is low, since diagnosis is based on mitotic count and tumoral size. Ando et al18 recently demonstrated that immunohistochemical analysis (c-KIT and Ki-67) is feasible from EUS-FNA samples but is limited to wide samples of adequate material. Therefore, the use of larger diameter needles or trucut devices could be an alternative approach for the diagnosis of intramural lesions through immunohistochemical analysis.
The clinical impact of EUS-FNA in patients with malignancies of the gastrointestinal tract has previously been studied19,20. However, there is little information on the role of EUS-FNA versus EUS alone in the diagnosis of malignancy according to the type and location of the lesion in large series of patients homogeneously studied with both techniques. As a whole, EUS was able to classify only 60% of the lesions whereas the addition of FNA allowed the majority of the lesions to be diagnosed without decreasing accuracy. The impact of EUS-FNA is obvious in lymph nodes. EUS-based criteria for malignant lymph nodes are highly specific but only 25% of these nodes present these features21,22. For this reason, and because cytological diagnosis is crucial for therapeutic decision-making and prognosis, EUS-FNA is of prime importance in a high percentage of oncologic patients with lymph node lesions. The clinical impact of EUS-FNA in patients with pancreatic masses who are candidates for surgery is less important since a cytological result negative for malignancy does not usually change the management. In contrast, EUS-FNA is mandatory in non-surgical lesions to confirm or preclude malignancy before any treatment is decided23. Finally, the impact of EUS-FNA in cystic lesions has not been specifically evaluated. However, we demonstrate that the addition of FNA increases the sensitivity of EUS in the diagnosis of malignancy from 50 to 100% and allows diagnosis of 100% of cysts classified as indeterminate by EUS alone.
The most important predictive factor for obtaining an adequate sample for pathological diagnosis is the number of passes performed. However, the availability of an on-site cytopathologist may greatly influence this parameter. Klapman et al10 have recently analyzed the importance of having an attendant cytopathologist. This study compared the EUS-FNA cytological results obtained by the same endosonographer in a center with an on-site cytopathologist and in another center without a cytopathologist. The chances of obtaining a diagnosis at the former were approximately twice those at the latter. However in the center where the cytopathologist was not in the operating room, the low number of passes performed (from 2 to 3 passes in pancreatic lesions) could have biased the results. Erickson et al analyzed the number of needle passes required to diagnose pancreatic malignancies using EUS-FNA and concluded that without a cytopathologist in attendance, 5 to 6 passes should be made for pancreatic masses and 2 to 3 for liver or node metastases9. In our series, the accuracy of EUS-FNA was directly related to the number of passes performed. Accuracy progressively increased with the number of passes but reached a steady value at the third or fourth pass, depending on the type of lesion. These findings suggest that if an attendant pathologist is not available, the number of passes performed should be at least 3 to 4, depending on the type of lesion. However, data in the literature indicate a higher number of passes are required to obtain such results and consequently certain factors related to the patient, clinical aspects or the lesion may influence the decision on how many biopsies to perform.
The cost-effectiveness of an attendant cytopathologist has not previously been analyzed. Prior data from percutaneous FNA suggest that on-site evaluation is cost-effective because it avoids repeating FNA procedures due to non-diagnostic samples24. Our results demonstrate that an attending cytopathologist can minimize the number of passes required for diagnosis and that this strategy is cost-effective.
This study presents certain limitations. Firstly, there was no control group with respect to the availability of an attendant cytopathologist. Nevertheless, since the lesions were systematically sampled until adequate material was obtained, the results that would have been obtained with the same number of passes in the absence of an attendant pathologist can be inferred. Secondly, the final diagnosis depended on surgical pathology in only 36% of patients. However, most of the patients that lacked a surgical specimen had pancreatic cancer, in which short survival allowed us to confirm the diagnosis. Thirdly, cost-effectiveness analysis can show noteworthy deviations depending on the country and health system considered, mainly due to differences in salaries. Accordingly, extrapolation of our results to other centers or medical organizations would require these figures to be recalculated.
In conclusion, this study demonstrates that EUS-FNA allows diagnosis of most lesions classified as indeterminate by EUS alone. The only factor independently associated with low accuracy is intramural location of the lesion. The availability of an attendant pathologist seems to increase the diagnostic yield of FNA, minimizes the number of passes and is a cost-effective strategy.