The aim of this systematic review is to summarize epidemiological data and areas of future acute pancreatitis research in Spain.
MethodsWe conduct an independent search in PubMed and Web of Science and analyse articles by Spanish researchers from 2008 to 2018.
ResultsWe identified an overall incidence of 72/100,000 person-years, with biliary pancreatitis as the most common etiology. BISAP was useful but suboptimal for predicting severity and some biomarkers such as Oleic acid chlorohydrin have shown promising results. The modified determinant-based classification can help to classify patients admitted to intensive care units. Ringer's lactate solution is currently the fluid of choice and classic surgery has been surpassed by minimally-invasive approaches. Starting a full-caloric diet is safe when bowel sounds are present.
DiscussionThere are numerous well-defined research fields in Spain. Future multicentre studies should focus on management, predicting severity and cost-effectiveness.
El objetivo de esta revisión sistemática es sintetizar datos epidemiológicos y campos de investigación en pancreatitis aguda en España.
MétodosRealizamos una búsqueda independiente en PubMed y Web of Science, analizando artículos de investigadores españoles desde 2008 hasta 2018.
ResultadosLa incidencia global fue de 72/100.000 personas/año, siendo la etiología biliar la más común. BISAP resultó útil, aunque subóptimo en predicción de gravedad y ciertos biomarcadores como el ácido oleico clorhídrico han mostrado resultados prometedores. La clasificación basada en determinantes modificada puede ayudar en la clasificación de pacientes ingresados en UCI. La sueroterapia basada en Ringer lactato es actualmente de elección y la cirugía tradicional ha sido sustituida por abordajes mínimamente invasivos. La dieta amplia de inicio, cuando los ruidos intestinales están presentes es segura.
DiscusiónExisten múltiples áreas de investigación bien definidas en España. Futuros estudios multicéntricos deberían centrarse en manejo, predicción de la gravedad y el coste/efectividad.
Acute pancreatitis (AP) is the third cause of hospitalization in the United States1 and constitutes a heterogeneous disease with various causes, morbidity and potential mortality.2 For these reasons, it is a major problem in gastroenterology departments worldwide and its management has been well described in several recent guidelines.2,3 In addition, there is significant heterogeneity not only related to individual patient factors but with the variety of incidence rates,4 etiology,5 scoring systems for severity6 and management,7 which can be diverse among countries. Thus, efforts to understand these differences while unifying areas of similarity between countries are important. In this setting, we have reviewed data from Spanish centers with the main aim of describing Spanish areas of research in AP and guide future research. We also have searched for epidemiological data to better understanding the current situation of AP in our country.
MethodsReview protocolsThe Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist8 was used for the performance of this systematic review.
EthicsThe authors declare no conflict of personal interests and no funding has been received for the present study.
DefinitionsAP was diagnosed according to current guidelines2,3 with patients meeting 2 of the 3 of the following criteria: abdominal pain consistent with the disease, serum amylase and/or lipase greater than 3 times the upper limit of normal, and/or characteristic findings on abdominal imaging.
Eligibility criteriaIdentification of relevant studiesA systematic search was independently conducted in PubMed and Web Of Science by two authors (F.V.L., E.R.C.) with the aims of assessing data regarding epidemiology and etiology about AP from Spain, but also to classification, severity prediction and general management. In order to analyze current advances, only articles published from 2008 until 2018 were included. The following search terms were used in PubMed: “epidemiology acute pancreatitis Spain” [All fields] OR “severe acute pancreatitis Spain” [All fields] OR “management acute pancreatitis Spain” [All fields]. When searching in Web of Science, the term “Acute Pancreatitis Spain” was used.
Study selectionThe studies enrolled could be either prospective or retrospective but they had to include data about epidemiology of AP (at least age, gender, sex and etiology), diagnosis, severity prediction and classification or data about management focusing general aspects of AP such as nutrition, fluid therapy or severe acute pancreatitis (SAP) management but not those which were related to specific situations such as hypertriglyceridemia. All of them had to be performed in a Spanish Hospital or directed by a Spanish group but we also included studies in which there were collaboration between a Spanish Hospital and an international center. We excluded studies that were case series; case reports or studies with less than 5 patients; abstracts or letters to the editor; studies in the pediatric population or animal studies.
Data extraction and analysisAbstracted data included authors, year of publication, country(s) in which the study was performed, number of patients included, age and etiology of AP. When assessing diagnosis, data about diagnostic yield, sensitivity, specificity, positive predictive value and negative predictive value were analyzed. In the studies in which prediction of SAP was assessed, analysis were focused on factors associated with severity (preferably independent risk factors) or the area under the curve (AUC) of parameters analyzed in terms of prediction of SAP or mortality. The conclusions and results of each study were evaluated to assess their overall contribution.
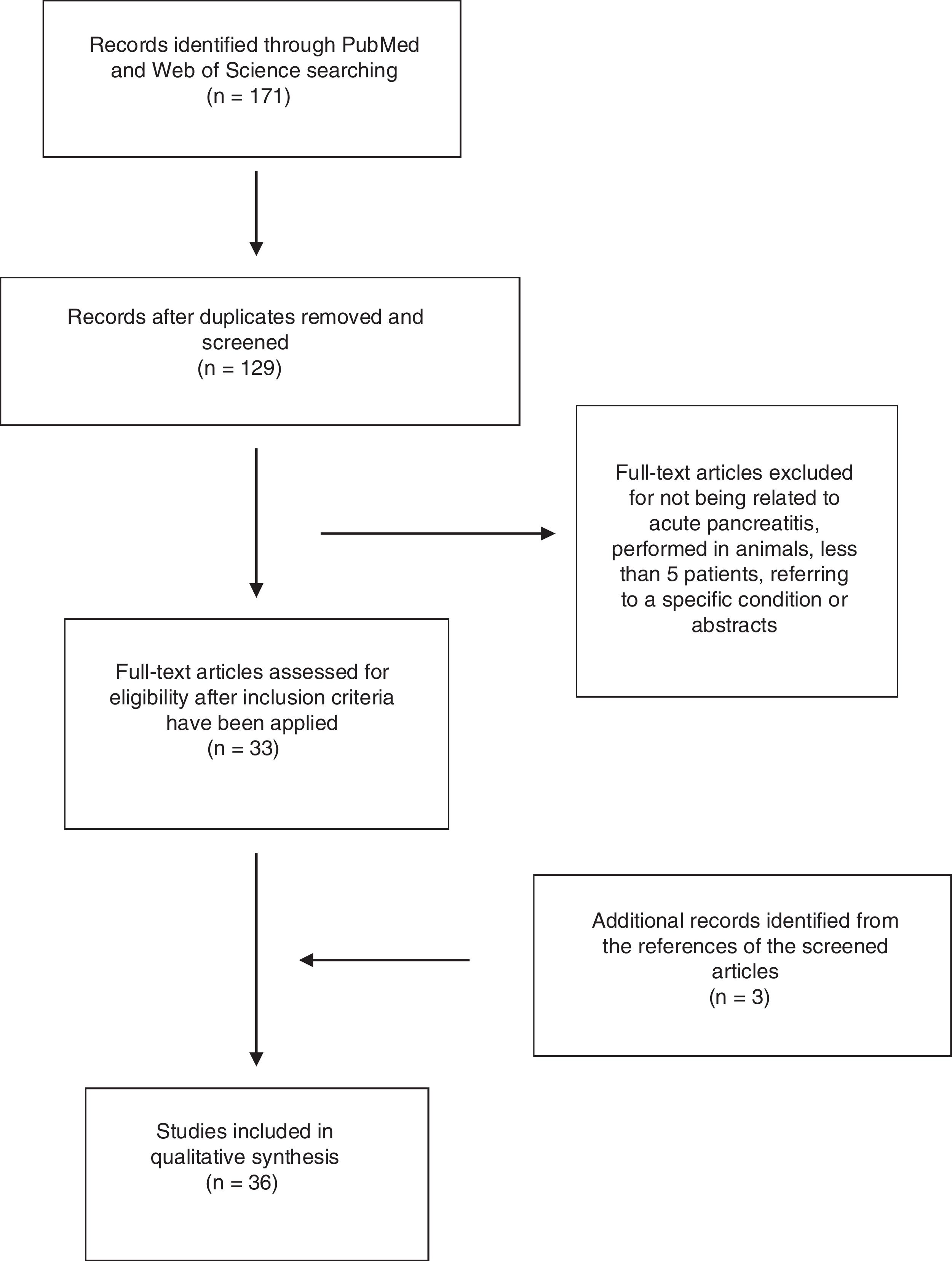
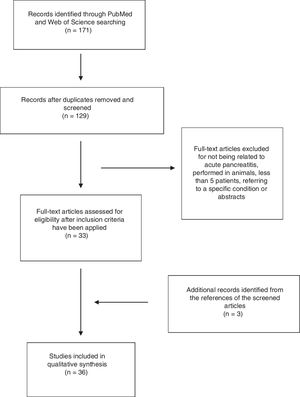
ResultsA total of 171 abstracts were initially identified in the literature search (Fig. 1), 137 of them in PubMed and 44 in Web Of Science. 25 articles were identified when using the term “epidemiology Acute pancreatitis Spain”, 74 when using the term “severe acute pancreatitis Spain” and 28 articles when using the term “management acute pancreatitis Spain”. 44 articles were identified by using the term “Acute Pancreatitis Spain” in Web of Science. After removing the duplicate, 129 articles remained and were screened by title and abstract. After a careful review, 33 articles were included following the previously mentioned criteria (14 by epidemiology, 16 by severe acute pancreatitis, none extra articles by management and 3 in the search in Web of Science). Another 3 articles were included from the references of selected articles. Finally, 36 articles were included in the study.
EpidemiologyIn Spain, the overall incidence was 72 patients per 100,000 inhabitants-year in a population-based retrospective study using the Spanish National Hospital Database. This study also reports a higher incidence rate especially in patients with type 2 diabetes, but these patients had lower in hospital mortality than non-diabetics.9 Another population-based case-control study found a slight increase in risk of AP in patients with type 2 diabetes although apparently less in those using insulin.10 Regarding mortality, a multicenter Spanish prospective study which included 1655 patients, found an overall mortality of 4.2%, of which 30% died by an sterile organ failure and 24.3% due to septic organ failure in the setting of infected pancreatic necrosis. Interestingly, 14.3% cases died from sepsis not related with infected pancreatic necrosis.11 Epidemiology and outcomes of patients admitted to the intensive care units (ICU) have been assessed in a prospective observational multicenter international study directed by the Spanish Intensive Care Society (SEMICYUC),12 which included 374 patients, all of whom had AP and at least one organ failure. An overall mortality of 28.9% was found in this study, with the highest mortality rates in those with organ failure and infected necrosis. High morbidity (defined as long stay in ICU and need of surgery in patients who do not die) was also found in patients with infected necrosis and in patients with both infected necrosis and organ failure.12
Prior studies have found recurrence of biliary AP in patients to whom cholecystectomy is not performed during the first episode. Barreiro-Alonso et al. found, in a prospective observational study, 36 episodes of AP in a period of 4 months, 9 of them were recurrent episodes of biliary AP. The mean cost per patient and readmission was 143€/day due to hospitalization, 332€ due to emergency evaluation and 2381€ due to imaging tests and ERCP. The Reported median overall length of hospital stay was 10 days, and there were no deaths or severe episodes of acute pancreatitis.13 A prospective descriptive study of 296 patients admitted for biliary AP established an overall recurrence rate of 15.5% with a median time to recurrence of 82 days. At the end of follow up, 14.2% patients relapsed after a first episode of AP without cholecystectomy or ERCP.14
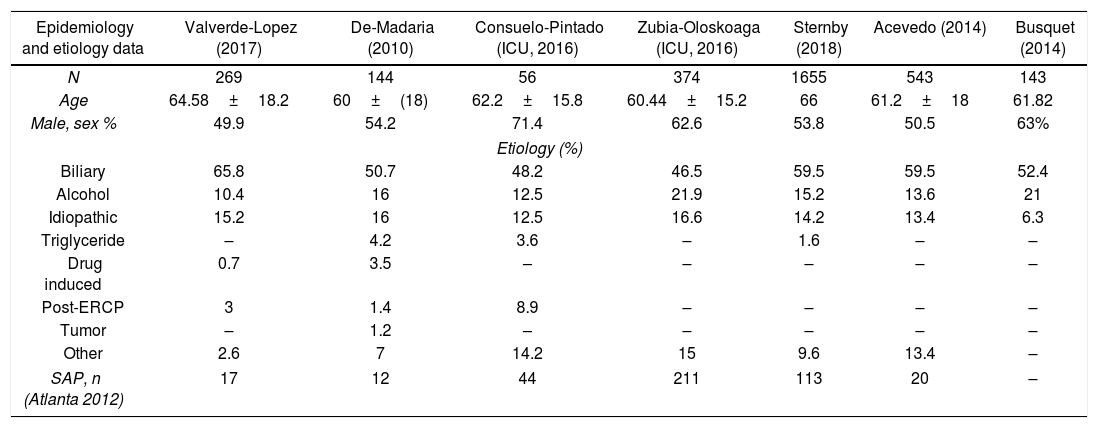
EtiologyData about etiologies is shown in Table 1 but only those articles in which, the most common etiologies were identified are listed.11,12,15–19 Regarding general cohorts, the articles included show that biliary etiology is the most common cause in patients with AP, ranging from 50.7% to 65.8%, slightly lower in ICU cohorts (46.5–48.2%), and followed by alcoholic (10.4–21%) and idiopathic (6.3–15.2%).
Patients characteristics in studies in which etiology is completely described.
| Epidemiology and etiology data | Valverde-Lopez (2017) | De-Madaria (2010) | Consuelo-Pintado (ICU, 2016) | Zubia-Oloskoaga (ICU, 2016) | Sternby (2018) | Acevedo (2014) | Busquet (2014) |
|---|---|---|---|---|---|---|---|
| N | 269 | 144 | 56 | 374 | 1655 | 543 | 143 |
| Age | 64.58±18.2 | 60±(18) | 62.2±15.8 | 60.44±15.2 | 66 | 61.2±18 | 61.82 |
| Male, sex % | 49.9 | 54.2 | 71.4 | 62.6 | 53.8 | 50.5 | 63% |
| Etiology (%) | |||||||
| Biliary | 65.8 | 50.7 | 48.2 | 46.5 | 59.5 | 59.5 | 52.4 |
| Alcohol | 10.4 | 16 | 12.5 | 21.9 | 15.2 | 13.6 | 21 |
| Idiopathic | 15.2 | 16 | 12.5 | 16.6 | 14.2 | 13.4 | 6.3 |
| Triglyceride | – | 4.2 | 3.6 | – | 1.6 | – | – |
| Drug induced | 0.7 | 3.5 | – | – | – | – | – |
| Post-ERCP | 3 | 1.4 | 8.9 | – | – | – | – |
| Tumor | – | 1.2 | – | – | – | – | – |
| Other | 2.6 | 7 | 14.2 | 15 | 9.6 | 13.4 | – |
| SAP, n (Atlanta 2012) | 17 | 12 | 44 | 211 | 113 | 20 | – |
ICU: intensive care unit; ERCP: endoscopic retrograde cholangiopancreatography; SAP: severe acute pancreatitis.
A quasi-experimental study from an emergency department has assessed the role of amylase and lipase in diagnosis of AP.20 This study shows higher sensitivity and specificity for lipase (0.85 and 0.96 respectively) in comparison with amylase (0.70 and 0.85). When adding amylase in patients with limits values of lipase, sensitivity and specificity did not change but they found higher rates of positive predictive values than using lipase alone (77% vs. 47%).20
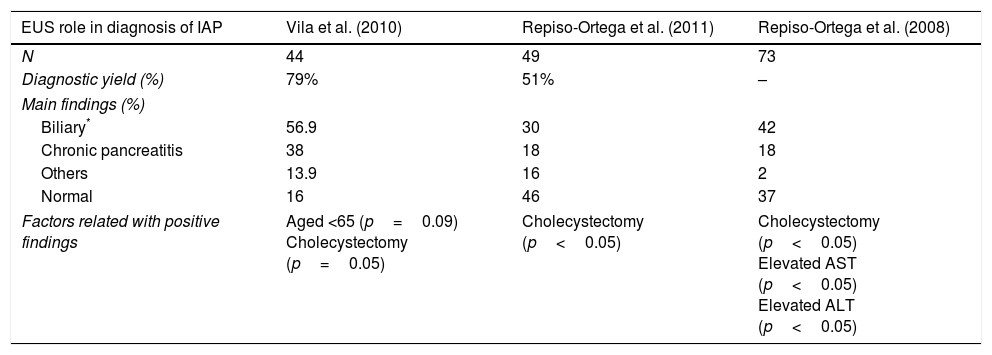
The diagnostic role of endoscopic ultrasound (EUS) is summarized in Table 2. In a prospective study of 44 patients, positive findings were identified in 79% patients, mainly biliary (52.3%), and the highest rates were from patients with gallbladder in situ in comparison with cholecystectomy (p=0.05) and age <65 years old (p=0.09).21 Another study found positive findings in 44% with biliary etiology as the most common (36%). Elevated AST or ALT on admission for AP (elevated AST 68% vs. 31%, p=0.002; elevated ALT 63 vs. 26%, p=0.001) and “in situ” gallbladder (49% vs. 16%, p=0.037) were factors that improved the diagnostic yield of EUS. There were no differences in diagnostic yield of EUS between the first episode of AP or those with recurrent AP (48% vs. 37% p=0.40).22 When comparing the role of EUS and magnetic resonance cholangiopancreatography (MRCP), another prospective study found a higher diagnostic yield with EUS (51% vs. 20%; p=0.001), especially in patients with “in situ” gallbladder. Cholelithiasis and biliary sludge were the main findings from EUS whereas pancreas divisum was the most frequent diagnosis at MRCP.23
Diagnostic yield of EUS in diagnosis of Idiopathic Acute Pancreatitis.
| EUS role in diagnosis of IAP | Vila et al. (2010) | Repiso-Ortega et al. (2011) | Repiso-Ortega et al. (2008) |
|---|---|---|---|
| N | 44 | 49 | 73 |
| Diagnostic yield (%) | 79% | 51% | – |
| Main findings (%) | |||
| Biliary* | 56.9 | 30 | 42 |
| Chronic pancreatitis | 38 | 18 | 18 |
| Others | 13.9 | 16 | 2 |
| Normal | 16 | 46 | 37 |
| Factors related with positive findings | Aged <65 (p=0.09) Cholecystectomy (p=0.05) | Cholecystectomy (p<0.05) | Cholecystectomy (p<0.05) Elevated AST (p<0.05) Elevated ALT (p<0.05) |
* Including gallbladder lithiasis, microlithiasis, biliary sludge and choledocholithiasis are included in biliary category.
The severity classifications in AP (Determinants Based Classification and Revised Atlanta) have been assessed by a study which performed a post hoc analysis of a prospective cohort in Spain, finding that the in hospital mortality was higher in patients with SAP (80%) in the Revised Atlanta classification and in severe and critical categories (67%) in the Determinant-Based Classification. The other categories in both classifications showed no deaths during the hospital stay.19 In a prospective multicenter study which compares Atlanta classification, Revised Atlanta classification and Determinant Based classification,12 mortality rates in severe patients were 14.4% following classic Atlanta classification whereas it raised to 52.2% according to the Revised Atlanta. The ‘severe’ category in Determinant Based Classification shows a mortality rate of 39.2% and it reaches 54.1% in the ‘critical’ category. Both Revised Atlanta and Determinant Based classification shows a better AUC in predicting mortality than Atlanta classification (0.951 and 0.953 vs. 0.863 with p<0.007 and p<0.008 respectively). This study also reports the effect of different determinants of morbidity and mortality, showing that persistent organ failure had an adjusted OR of 16 for mortality compared with transient organ failure, concluding that the former is the most significant determinant of severity. The role of infected pancreatic necrosis is also assessed, showing that persistent organ failure is more usual in this scenario than in patients with sterile necrosis (62.7% vs. 16.2%, p>0.001), but once persistent organ failure is established, mortality is not higher in infected pancreatic necrosis when it is compared with sterile necrosis. (54.1% vs. 51.3%; aOR 1.4: IC 95% 0.6–3.2).11
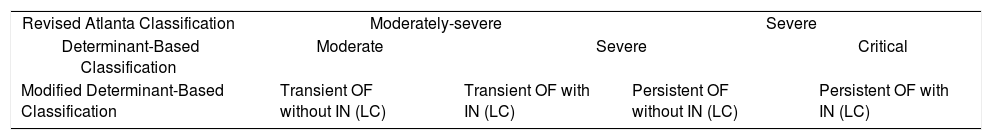
A Spanish group has developed a new classification called the modified determinant based classification,12 which only includes patients with organ failure. In this classification (Table 3), patients have been divided into four groups depending on the presence of transient organ failure with or without infected necrosis (Groups 1 and 2) and persistent organ failure with or without infected necrosis (Groups 3 and 4). It defines a group with low morbidity and low mortality (2.26%, group 1), a group with low mortality (6.67%) but high morbidity (Group 2), a group with high mortality (41.46%) but low need of intervention (group 3) and lastly a group with high mortality (59.09%) and high need or intervention (group 4).12
Comparison between the Revised Atlanta Classification, Determinant-Based Classification, and the Modified Determinant-Based Classification (Epidemiology of Acute Pancreatitis in Intensive Care Medicine Study Group, Zubia-Olaskoaga et al.).
| Revised Atlanta Classification | Moderately-severe | Severe | ||
| Determinant-Based Classification | Moderate | Severe | Critical | |
| Modified Determinant-Based Classification | Transient OF without IN (LC) | Transient OF with IN (LC) | Persistent OF without IN (LC) | Persistent OF with IN (LC) |
OF: organic failure; IN: infected necrosis LC = local complications
In terms of factors related to SAP, fluid sequestration was proven to be a risk factor for SAP (with a median of 7.5L in patients with organic failure and 3.1L in patient without organ failure), and the factors independently related to this event were ages <40 years old, hematocrit >44%, alcoholic etiology, serum glucose >150mg/dL and SIRS (more than 2 criteria).24 Obesity has been recognized as a risk factor for SAP in comparison with non-obese patients (37.1% vs. 18% p=0.047) and also for local complications (28.6% vs. 10% p=0.027) in a cohort of 85 patients.25 Another Spanish prospective study also found that ages >65 years, leukocytes >13,000/mm3, albumin <2.5mg/dL, calcium <8.5mg/dL and C-reactive protein >150mg/dL in the first 72h after admission were independent prognostic factors related with adverse outcomes in AP.26
Non routine laboratory parameters related to SAP assessed in the studies reviewed are inflammasome-forming receptor absent in melanoma 2 (AIM 2) which expression and activation is increased early in the course of the disease when compared to healthy subjects27 and Malondialdehyde, an early oxidative stress product, which showed higher values in patients with acute pancreatitis when compared with healthy subjects (0.347μM in the control group vs. 0.6 at 24h in acute pancreatitis), but it was not found to have a significant AUC when measured at 24h and 48 in terms of severity prediction.28 Also, some genetic polymorphisms in Toll-like receptors (TLR) were related to severity in AP; CC genotype patients in TLR3 rs3775291 had an increased risk for severe pancreatitis compared with T genotype carriers (CC OR 2.426 [CI 95% 1.171–5.027]). On the other hand, TLR6 rs5743795 with GG genotype showed a lower risk for SAP compared with A genotype carriers (GG OR 0.909 [CI 95% 0.831–0.995]).29 Also, GA TNF 238 polymorphism has been associated with more frequent development of organ failure than GG genotype (p<0.05).30
Regarding the relationship between etiology and outcome, first episode of alcoholic AP has been proven to be an independent risk factor in a multicenter prospective observational study with an American and a Spanish cohort in terms of development of organ failure (54% vs. 33%, p=0.03 in the American cohort and 24% vs. 8%, p=0.001 in the Spanish validation cohort) although mortality rates have not shown differences between alcoholic and non-alcoholic patients (7% vs. 7%, p=0.92 in the American cohort and 8% vs. 2%, p=0.08 in the Spanish cohort validation).31
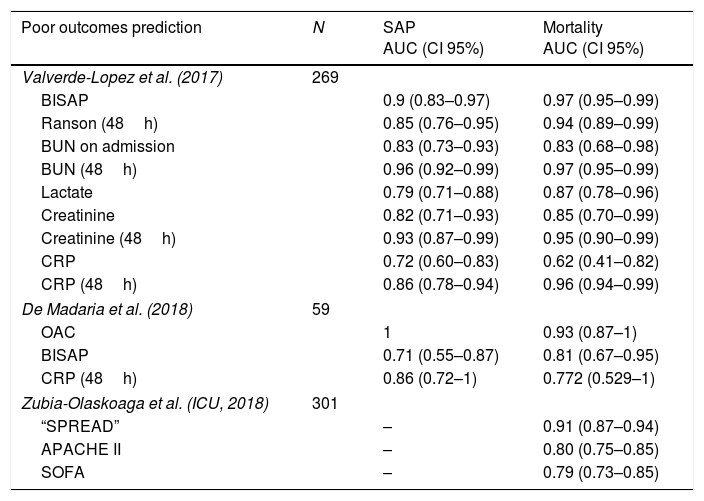
Beyond the studies about severity and mortality prediction, the results of the AUC of each parameter analyzed are shown in Table 4. Bedside Index for Severity in Acute Pancreatitis (BISAP) on admission showed an AUC of 0.9 (0.83–0.97) with a sensitivity of 70.6% and a specificity of 93.3% although the predictive positive value was only 41.4% in a cohort of 269 patients.17 In this prospective cohort, it was shown as the best predictor on admission in terms of SAP, ICU admission and mortality. Another prospective cohort analyzed an experimental biomarker (Oleic acid chlorohydrin) and observed an AUC of 1 for predicting severe acute pancreatitis and 0.93 for predicting mortality, surpassing CRP at 48h and BISAP score in both outcomes.32 A new prediction rule has been developed in a multicenter study in the setting of ICU patients, using a derivation cohort and a validation cohort. They found an AUC of 0.91, higher than APACHE II and SOFA in the first 24h (0.80 and 0.79, p=0.0002 and p=0.0001, respectively), and patients with a score >13 showed an in hospital mortality of 88.37%.33
Predictors of poor outcomes in acute pancreatitis.
| Poor outcomes prediction | N | SAP AUC (CI 95%) | Mortality AUC (CI 95%) |
|---|---|---|---|
| Valverde-Lopez et al. (2017) | 269 | ||
| BISAP | 0.9 (0.83–0.97) | 0.97 (0.95–0.99) | |
| Ranson (48h) | 0.85 (0.76–0.95) | 0.94 (0.89–0.99) | |
| BUN on admission | 0.83 (0.73–0.93) | 0.83 (0.68–0.98) | |
| BUN (48h) | 0.96 (0.92–0.99) | 0.97 (0.95–0.99) | |
| Lactate | 0.79 (0.71–0.88) | 0.87 (0.78–0.96) | |
| Creatinine | 0.82 (0.71–0.93) | 0.85 (0.70–0.99) | |
| Creatinine (48h) | 0.93 (0.87–0.99) | 0.95 (0.90–0.99) | |
| CRP | 0.72 (0.60–0.83) | 0.62 (0.41–0.82) | |
| CRP (48h) | 0.86 (0.78–0.94) | 0.96 (0.94–0.99) | |
| De Madaria et al. (2018) | 59 | ||
| OAC | 1 | 0.93 (0.87–1) | |
| BISAP | 0.71 (0.55–0.87) | 0.81 (0.67–0.95) | |
| CRP (48h) | 0.86 (0.72–1) | 0.772 (0.529–1) | |
| Zubia-Olaskoaga et al. (ICU, 2018) | 301 | ||
| “SPREAD” | – | 0.91 (0.87–0.94) | |
| APACHE II | – | 0.80 (0.75–0.85) | |
| SOFA | – | 0.79 (0.73–0.85) | |
BUN: blood urea nitrogen; OAC: Oleic acid chlorohydrin; CRP: C-reactive protein.
Developed in patient with at least one organic failure and admitted to ICU.
Imaging studies to predict severity have also been evaluated by a Spanish center and a correlation between contrast enhanced ultrasound (CEUS) and computed tomography (CT) scan has been observed for the CT severity index (r=0.926, p<0.01) as well as a correlation between CEUS and Ranson criteria (r=0.442, p<0.01) and CRP at 48h after admission (r=0.362, p<0.05).34
ManagementFluid therapyEarly aggressive intravenous hydration has been assessed by a multicenter retrospective study, enrolling 1010 patients. In this study, early aggressive hydration was defined as more than 1000ml from time of admission to the emergency room until more than 4h after diagnosis, moderate hydration as 500–1000ml and nonaggressive as less than 500ml. When comparing both moderate and aggressive with nonaggressive hydration, there was less need of invasive treatment compared to the moderate group (OR 0.37: CI 95% 0.14–0.98; p<0.025) and aggressive group (OR 0.21: CI 95% 0.05–0.84; p=0.03), but there was not a statistically significant difference when assessing death, persistent organ failure and local complication in adjusted analysis.35 The same Spanish group has found in a triple blinded randomized controlled trial, that the median number of SIRS criteria at 48h when using normal saline were 1 (1–2) whereas Ringer lactate showed 1 (0–1) and this difference was statistically significant (p=0.06). CRP values at 48 and 72h were also statistically higher in the normal saline group (166mg/L vs. 28mg/L; p=0.03 and 217mg/L vs. 25mg/L; p=0.04 respectively).36
Pancreatic necrosis and infected necrosisMinimally invasive surgery using a step-up approach has been compared with standard surgical treatment in a retrospective study of 164 patients37 divided in two groups collected in different periods (from 2006 to 2010 and from 2010 to 2014). The first group (A) was treated with traditional surgical management in SAP and group B was treated using minimally invasive surgery following a step-up approach strategy as the Dutch Pancreatitis Study Group proposed.38 Group A showed higher mortality in both sterile and infected necrosis patients (67% vs. 0% and 44% vs. 0% respectively; p<0.001) and overall mortality in infected necrosis patients was also higher in group A (39.1% vs. 4.8%; p=0.01).
The results of a Spanish cohort of 143 patients prospectively collected from 1999 until 2011 who underwent surgery for SAP15 showed a postoperative mortality of 25% and found that the only independent risk factor of post-operative mortality was time from symptom onset until surgery <7 days (RR 4.9; CI 95% 1.2–20; p=0.025). Other parameters such as age, organ failure or sterile intraoperative sample showed differences in univariate but no in multivariate analysis.15 Conversely, in another prospective cohort of 107 patients in which surgery was indicated for infected SAP or in cases of sterile necrosis with an unfavorable course,39 several factors were independently related to post-operative mortality including age >65 years old (RR 4.5; CI 95% 1.1–18; p=0.02), duration of onset of pain to time to surgery >12 days (RR 5.4; CI 95% 1.4–20.1; p=0.01) and sterile necrosis (RR 7.7; CI 95% 1.8–32.8; p=0.005).39 In terms of endoscopic management of local complication in AP, an irrigation technique through a lumen-apposing stent has been performed in a prospective cohort of 12 patients with clinical success in 100% of cases after a median of three sessions without adverse event described during the procedure. There was no need of surgery in any patient and there were no deaths during follow-up, although one recurrence was observed in a patient at 12 months after stent removal.40
Regarding the prophylactic use of antibiotics in pancreatic necrosis, a randomized, prospective, double-blinded, study assessed the role of intravenous ciprofloxacin in comparison with placebo in 41 patients (22 patients were treated with ciprofloxacin and 19 patients were treated with placebo). There were no differences between groups in terms of infected pancreatic necrosis (36% vs. 42% respectively; p=0.7) or mortality (18% vs. 11% respectively; p=0.6).41
NutritionA Spanish group has performed an open label trial comparing different feeding protocols. In this study, the groups in which feeding was started once bowel sounds returned had lower hospital stay (median 5 vs. 7 days; p=0.001) and there was no difference (p=1) in tolerance between patients initiating a full caloric diet (31/35, 89%) vs. stepwise increasing diet (33/37, 89%).42 A meta-analysis of individual patient data from 7 different countries attempted to assess the ideal time for starting enteral nutrition. In this study, patients who began enteral nutrition within 24h of admission had better outcomes (infected pancreatic necrosis, organ failure or mortality) than patients who started enteral nutrition after 24h of admission (45% vs. 16%; OR 0.42 CI 95% 0.19–0.94).43
Preventing post-ERCP APPost-ERCP pancreatitis was assessed by a Spanish group in collaboration with the University of Oxford (United Kingdom) in which 510 patients were included and randomized to either intravenous bolus of 250μg somatostatin slowly infused during 3min followed by a short continuous infusion of the drug at 250μg/h during 4h (total dose of 1250μg) to a placebo regimen. Post-ERCP pancreatitis developed in 19 patients in the somatostatin group vs. 17 patients in the placebo group (7.5% vs. 6.7% respectively; p=0.73) and the number of cases of moderate to SAP was also similar between groups (2.4% vs. 3.5%; p=0.43).44
DiscussionThis systematic review summarizes the most important findings in AP in the last decade from Spain in terms of epidemiology, etiology, diagnosis, classification, severity prediction and general management. Furthermore, we highlight important observations in our nation as well as from international collaboration, which can guide evaluation, management and future researches.
Regarding epidemiology, AP is an increasing problem with an overall mortality of 4.2%,11 but when persistent organ failure develops, mortality rates dramatically raises up to 30% according to some studies,45 although a multicenter Spanish cohort has found rates of 52.2% in this group of patients.11 In patients admitted to ICU overall mortality has been rated to 28.9% in another multicenter study.12
Recently, a European study found an incidence of AP between 4.6 and 100 cases per 100,000 inhabitants, showing that eastern and northern countries had the highest rates.4 A rising incidence as well as growing number of patients with SAP have also been observed in the last decades, although a reduction in mortality adjusted by organ failure has been found.4 In Spain, the incidence was found to be 72 patients per 100,000 inhabitants-year9 and biliary cause was the most frequent one, followed by alcohol.2,4,5,11,12,15–19 To our knowledge, there is a lack of studies in Spain assessing the costs of AP, although the cost of readmission after edematous biliary pancreatitis in patients in which early cholecystectomy was not been performed has been assessed, showing that not performing cholecystectomy within two weeks after the episode contributes to recurrence and avoidable costs.13 Furthermore, recurrence in biliary AP was found to be 15.5% in a cohort of 269 patients underscoring the importance of early cholecystectomy and reinforcing the fact of targeting resources to prevent new episodes by performing an early cholecystectomy.14 Studies with larger samples sizes and with the specific aim of defining the cost of recurrence in biliary AP when cholecystectomy is not performed should be done. Regarding post-ERCP AP, rectal NSAID has been proven to be the most cost effective approach, overcoming pancreatic stent, both rectal indomethacin and pancreatic stent or no prophylaxis in a cost effectiveness analysis.46 In Spain, few studies has been done in this term but Somatostatin intravenous continuous infusion after a bolus (total dose of 1250μg) has not proven to be better than placebo in terms of preventing post-ERCP AP in a randomized study performed by Spaniard and British researchers44 and similar findings have been found by Vila et al. in a study including 242 patients which compared using a bolus 250 mcg of somatostatin with placebo.47
In what concerns to diagnosis, lipase seems to be better than amylase when used alone, although amylase could help by increasing the positive predictive values when lipase levels are not diagnostic.20 When assessing a first episode of idiopathic AP, EUS plays an important role, especially in patients with “in situ” gallbladder, since it reveals biliary findings in 36–52.3% of patients.21,22 Some factors which can improve its diagnostic yield should be prospectively assessed. Ages <65 years21 or elevated ALT or AST22 have demonstrated in Spanish studies that could improve the diagnostic yield, although the results are heterogeneous and non-definitive compared to other studies. Prospective and multicenter studies with larger number of patients should analyze this setting.
In terms of classification, the Revised Atlanta Classification in 2012 established 3 grades of severity depending on the presence of organ failure and its persistence.48 Patients with organ failure, defined by Marshall modified score for more than 48h, are labeled as SAP with mortality rates up to 30% or even higher if infected necrosis developed,45 and they should undergo close monitoring in ICU for aggressive therapies. Although the Revised Atlanta classification is the most widely used, determinants based classification has also been proposed, including a new category (critical AP). Although this classification takes into account the presence of infected necrosis49 both severe patients in Atlanta and severe and critical in determinant based have high mortality (80% and 67% respectively)19 but patients with critical AP have the highest rates of mortality12,49 and differentiating between severe and critical may show a more accurate prognosis and can be useful in the setting of ICU. In another very recent prospective multicenter study in which 23 centers were involved, although infected necrosis was initially correlated with higher morbidity and higher mortality than sterile necrosis, when multivariate analysis was performed and persistent organ failure was added, infected pancreatic necrosis showed higher morbidity but no higher mortality than sterile necrosis. This suggests than persistent organ failure is more likely to develop in patients with infected pancreatic necrosis but once it develops, mortality is similar in both infected and sterile pancreatic necrosis. Of note, this study also shows that sepsis non-related to infected pancreatic necrosis was responsible of 14.3% of deaths. A multicenter study of Spanish hospitals has created a new classification called “modified determinant based classification” which only include patients with organ failure and establishes four groups of patients with different grades of mortality and morbidity or need of intervention.12 Although this classification needs prospective validation, it describes meticulously the mortality and need of intervention of the patients with organ failure, which can help in prognostication in the ICU setting.
When managing a case of SAP, early treatment must be administered to improve outcome,50 so early predictors of severity can be provided. Guidelines on AP management recommend an initial clinical risk stratification and close monitoring with SIRS, but this has also been proved to be a suboptimal approach for the initial triaging of patients.2,3 Many studies in Spain have assessed prediction severity in AP. Fluid sequestration,24 obesity,25 age >65 years and several laboratory parameters such as low albumin or calcium concentrations and high C-reactive protein26 have been associated with poor outcomes in AP. Also, some non-routine laboratory parameters have been studied, such as AIM2 activation and Malondialdehyde, Platelet-derived growth factor (PDGF) and hepatocyte growth factor (HGF),26,27,51 but especially Oleic acid chlorohydrin has shown excellent results in a small cohort of patients in terms of SAP and mortality prediction, surpassing BISAP and CRP at 48h in the cohort in which it has been tested.32 This observation requires confirmation. Genetics are also important in severity prediction, since several polymorphism in TLR29 and TNF30 have been related to more severe episodes, as well as the etiology, as it has been shown in a dual center study in Spain and United States in which a first episode of alcoholic etiology was shown to be an independent risk factor for organ failure but not higher mortality than other etiologies.31
Scoring systems have shown suboptimal results in terms of predicting severity52; they are cumbersome to use and have high false positive rates.53 Recent approaches seek to stratify risk by a dynamic assessment throughout the first days after the onset of the disease. For example, Koutroumpakis et al.54 found that a hematocrit value higher than 44% on admission, as well as an increased blood urea nitrogen (BUN) within the first 24h comprise a risk of persistent organ failure higher than 50%, showing a better and easier predictive ability than APACHE II. This finding constitutes a very interesting approach based on simple parameters and their modifications over the first 24h.54 Our group has found that BISAP on admission is a better predictor than hematocrit for SAP,17 so it could be used in a similar way as an initial assessment in combination with a dynamic assessment and universally available parameter such as BUN or lactate, as Koutroumpakis et al.54 has shown, in future Spanish multicenter studies. Of note, BISAP score can be easily calculated on admission in every patient, since it comprises clinical evaluation, routine blood testing and chest X-ray, but can be more difficult after admission. Conversely, the Ranson score is cumbersome, needs 48h to be completed and has shown lower AUC than several routine laboratory markers such as BUN or creatinine at 48h in a Spanish study of 269 patients, so it should not be used for severity prediction.17 A new prediction rule has also been developed in a Spanish cohort in patients with organ failure. This score assesses age, etiology, the presence of shock or respiratory failure, the need for continuous renal replacement therapy and intrabdominal pressure. This score termed SPREAD, has shown higher AUC when predicting mortality than APACHE II and it is easier to use so it could be used throughout the ICU stay.33
In terms of management, early aggressive hydration is probably the most important intervention in the early phase of AP.2 A Spanish group has found that Lactated Ringer solutions are better than other solutions, given that lower levels of C-reactive protein (CRP) after 48 and 72h and less SIRS criteria are found when using these solutions. The same study demonstrated that Lactate Ringer solutions inhibits nuclear factor kappa B (NF-kB) activation, which is an important mediator of the systemic inflammatory response in AP, but this effect was not proven in Ringers solution without lactate suggesting a direct anti-inflammatory effect of lactate.35,36 Therefore, Ringer solutions with Lactate could be used as fluid of choice. When assessing local complications and surgery in AP, it is currently well known that open surgery has higher rates of mortality than minimally invasive surgical and endoscopic therapy one.37,38,55,56 It has been proven that the delay in the intervention can improve the outcomes, whereas other factors such as advance age or the presence of sterile necrosis can increase mortality, so that all these factors should be taken into account when indicating surgery.15,57 A Step up approach starting with minimally invasive surgery has also been established as the standard of care in this setting38 and Spanish studies have confirmed this approach,37 finding less mortality than classic surgery especially in patients with sterile necrosis. Probably, next steps in researching could be comparing different modalities of minimally invasive surgery inside the step-up approach given the poor results of classic surgery.
This systematic review has limitations. On the one hand, it is targeted to many different issues in AP so there is an important heterogeneity in the studies assessed and results could not be as uniform as it is desired. Many of the studies analyzed have small sample size and different inclusion criteria. Also, in terms of severity prediction different outcomes have been assessed, although those studies referring to mortality or SAP following the Revised Atlanta classification have been highlighted (Table 4).
In conclusion, our systematic review highlights Spanish research especially in terms of diagnosis of Idiopathic AP, predicting severity, management of fluids and surgical treatment, but also epidemiological analysis and some others areas related to nutrition or preventing post-ERCP AP. Many of them have been developed specifically in Spanish centers but collaboration with important international centers has also been accomplished. In the future, prospective, multidisciplinary and multicenter studies on AP management, predicting severity and cost effectiveness should be done with the cooperation of the different Spanish groups and also with other countries in order to improve outcomes for such a common, expensive, and morbid disease.
Conflict of interestThe authors declare no conflict of personal interests and no funding has been received for the present study.