Hepatorenal syndrome (HRS) is the deadliest complication of cirrhosis. The purpose of this study is to analyze if the use of a protocol for HRS is associated with higher survival in these patients.
MethodsAn evidence-based protocol for the diagnosis and treatment of HRS was instituted in 2013. Data from medical records from 2010 to 2016 were obtained by searching the hospital database for patients who received terlipressin, in the three years before and after the institution of the protocol. Data were reviewed to confirm the diagnosis of HRS and multiple variables were collected. Liver-specific scores were calculated and a stepwise Cox regression approach was used for univariate and multivariate analysis.
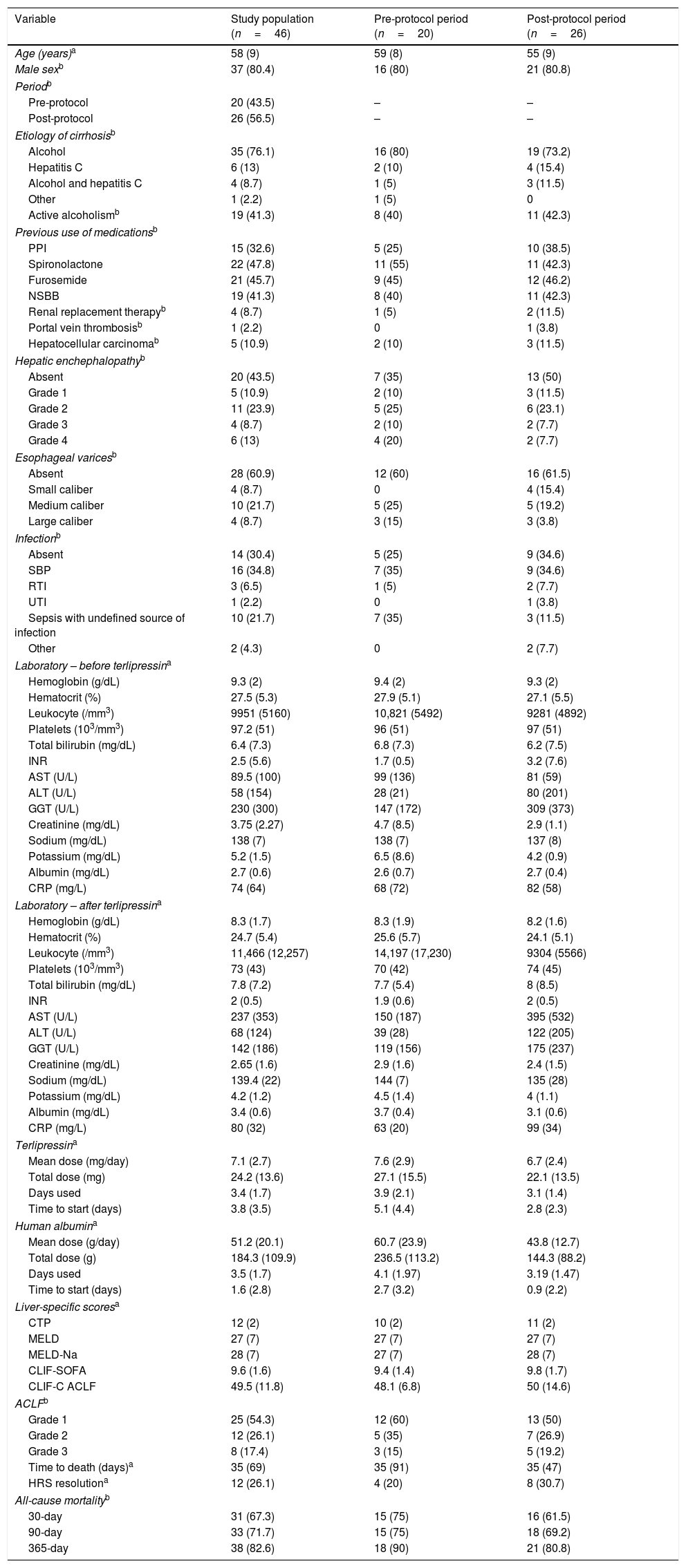
ResultsThe study included 46 patients, 20 from the pre-protocol period and 26 from the post-protocol period. Respectively, mortality at 30 days, 90 days and 365 days was 75%, 75% and 90% for the pre-protocol period, and 61%, 69% and 80% for the post-protocol period. In the multivariate analysis, an aspartate aminotransferase (AST) of <40U/L, the pre-protocol period and higher Child-Turcotte-Pugh scores were associated with higher 30-day and 90-day mortality. The total mean dose of terlipressin and human albumin used per patient was reduced from 27mg to 22mg and from 236g to 144g, respectively, after the institution of the protocol. This was not associated with higher mortality.
ConclusionThe use of an evidence-based protocol for the treatment of HRS translated into a higher survival. The authors suggest that the use of evidence-based protocols for the diagnosis and treatment of HRS could reduce cost and mortality in tertiary hospitals.
El síndrome hepatorrenal (SHR) es la complicación más mortal de la cirrosis. El objetivo de este estudio es analizar si el uso de un protocolo para el SHR se asocia a una mayor supervivencia en estos pacientes.
MétodosEn 2013 se instituyó un protocolo basado en la evidencia para el diagnóstico y tratamiento del SHR. Los datos de los registros médicos del 2010 al 2016 se obtuvieron mediante la búsqueda en la base de datos del hospital de pacientes que recibieron terlipresina, 3 años antes y después de la institución del protocolo. Se revisaron los datos para confirmar el diagnóstico de SHR y se recopilaron múltiples variables. Se calcularon las puntuaciones específicas del hígado y se utilizó un enfoque gradual de la regresión de Cox para el análisis univariado y multivariado.
ResultadosSe incluyó a 46 pacientes, 20 del período preprotocolo y 26 posprotocolo. Respectivamente, la mortalidad a los 30, 90 y 365 días fue del 75, el 75 y el 90%, respectivamente, para el período previo al protocolo y del61, el 69 y el 80%, respectivamente, para el posterior al protocolo. En el análisis multivariado, aspartato aminotransferasa (AST) <40 U/l, el período preprotocolo y las puntuaciones más altas de Child-Turcotte-Pugh se asociaron con una mayor mortalidad a los 30 y 90 días. Las dosis media total de terlipresina y albúmina humana utilizada por paciente se redujo de 27 a 22mg de terlipresina y de 236 a 144g de albúmina humana después de la institución del protocolo. Esto no se asoció con una mayor mortalidad.
ConclusiónEl uso de un protocolo basado en la evidencia para el tratamiento del SHR se tradujo en una mayor supervivencia. Los autores sugieren que el uso de protocolos basados en la evidencia para el diagnóstico y tratamiento del SHR podría reducir el costo y la mortalidad en los hospitales de tercer nivel.
Hepatorenal syndrome (HRS) is a severe complication of end-stage liver disease (ESLD), which occurs in cirrhotic patients with ascites.1 These patients generally have a marked circulatory dysfunction: the activation of cytokines and vasoactive hormones and the alteration in circulatory function in advanced cirrhosis and ascites without overt sepsis are similar to that seen in sepsis and septic shock without cirrhosis, which might cause renal hypoperfusion.2 Such circulatory dysfunction can also occur in patients with acute and acute-on-chronic liver failure (ACLF).3,4 This does not explain alone the multiorgan failure that is associated with ESLD: severe decompensation results from systemic spread of bacterial products, which cause activation of host innate immune triggers and leads to arterial vasodilatation, causing organ dysfunction through a storm of pro-inflammatory cytokines and reactive oxygen and nitrogen species – ESLD might be result of an inflammatory syndrome and not solely an hemodynamic process.5
Even though HRS is a functional syndrome, it carries a poor prognosis6 and liver transplantation (LT) is the only available definitive treatment in the long term.7 HRS is divided in two types: HRS type 1, rapidly progressive; and HRS type 2, slowly progressive; the first carrying a worse prognosis.1,8
Nevertheless, some studies have shown efficacy over placebo of diverse treatment strategies for HRS – generally the association of human albumin with a vasopressor drug,8 such as norepinephrine9,10 and terlipressin.11–14 Even though both have not been compared in a definitive head-to-head randomized clinical trial, terlipressin has been shown to reduce cost in a systematic review and meta-analysis.15 Terlipressin is, nevertheless, an expensive drug and norepinephrine, although inexpensive, requires monitoring in an intensive care unit, making both treatments costly. Also, the pharmacological treatment of HRS does not seem to increase survival in the long run except in a few patients – LT is still the choice of treatment for this severe complication of ESLD.16 Terlipressin has been shown to be superior to an association commonly used in the United States: midodrine and octreotide plus human albumin.17
The diagnosis of HRS is one of exclusion, using criteria established by the International Ascites Club. The first set of criteria were published in 1994 18 and later reviewed in 2007.19 These criteria defined in 2007 have been updated to include the concept of Acute Kidney Injury (AKI) in 2015.20 This modification, nevertheless, has not been shown to be superior in predicting adverse events when compared to the cutoff previously used of a serum creatinine above 1.5mg/dL.21
The concept of ACLF has been used as a step between decompensated cirrhosis (DC) and death, defined by the failure of multiple organic systems.22 Such concept lacked a definition when Intensive Care and Hepatology began to discuss it,23–29 but the multi-centric prospective study CANONIC published in 2013 has developed criteria based in the CLIF-SOFA score, which were shown to predict mortality.22 Hence HRS defines kidney failure, its presence already defines a patient as having ACLF.
The purpose of this study is to analyze if the use of an evidence-based protocol for diagnosis and treatment of HRS is associated with higher survival in these patients.
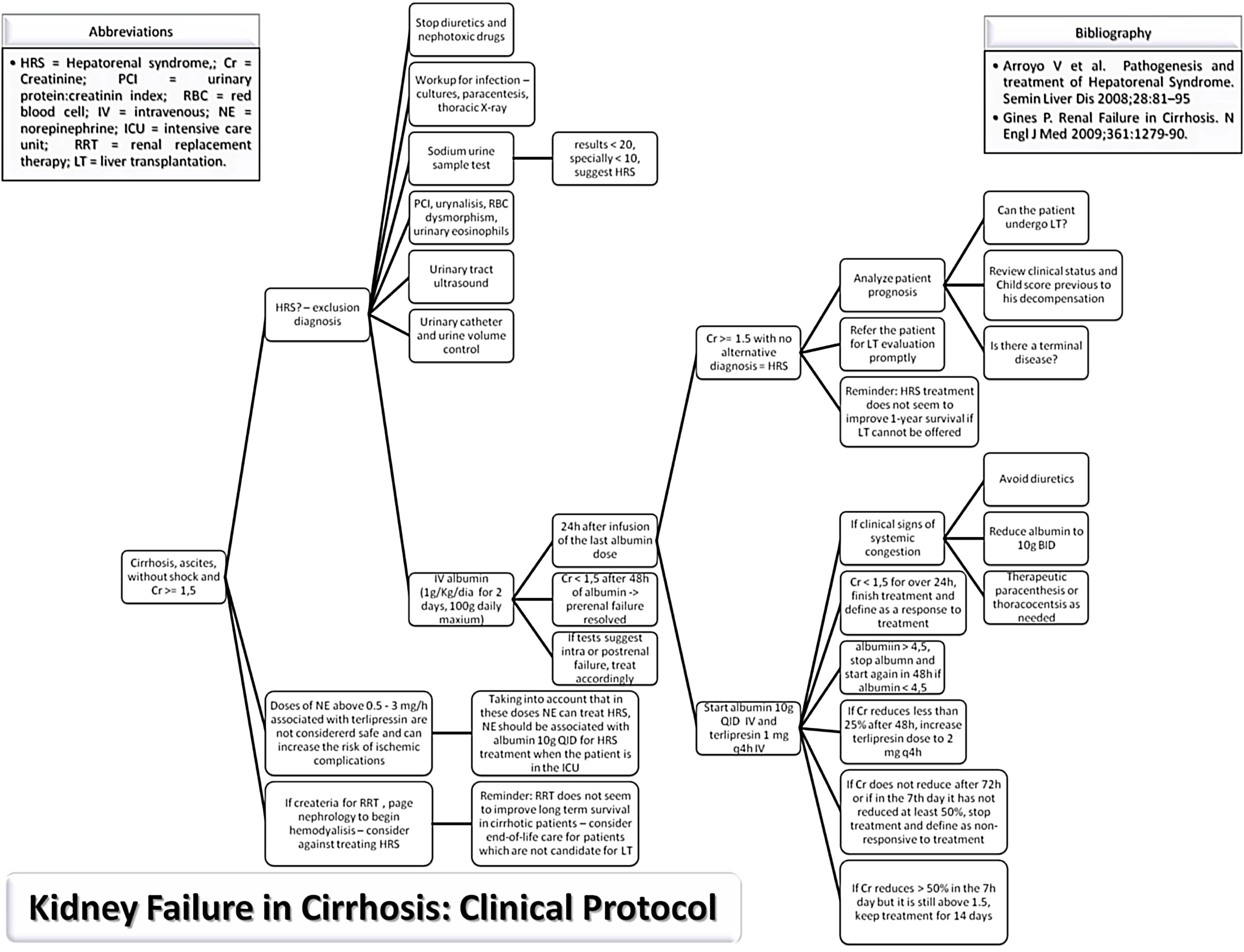
MethodsStudy populationThe study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval on June 2017 by the human research committee of the University, under protocol no. 66646617.3.0000.5341. Informed consent was waived by the human research ethics committee; since this study analyzed only medical records. An evidence-based protocol for diagnosis and treatment of HRS was developed by the Clinical Hepatology team of the University, based in the diagnostic criteria published in 2007,19,30 and instituted as standard-of-care in 12/23/2013 in the Hospital (Fig. 1). Afterwards, this protocol was used as guidance for diagnosis and treatment of HRS type 1 by every attending physician and medical resident of the team.
Data from medical records from 2010 to 2016 was obtained by searching the hospital electronic database for every patient who received terlipressin, ranging from three years prior to three years after the institution of the protocol. Electronic and physical medical records were analyzed and a data collection form was filled for each patient. Patients over 18 years old with the diagnosis of cirrhosis, ascites and AKI supported by laboratory and imaging data were included. The diagnosis of HRS type 1 was defined using the criteria published in 2007:19
- •
Cirrhosis with ascites.
- •
Serum creatinine >1.5mg/dL.
- •
No improvement of serum creatinine (decrease to a level of 1.5mg/dL or lower) after at least 2 days with diuretic withdrawal and volume expansion with human albumin. The recommended dose of human albumin is 1g/kg of body weight per day up to a maximum of 100g/day.
- •
Absence of shock.
- •
No current or recent treatment with nephrotoxic drugs.
- •
Absence of parenchymal kidney disease as indicated by proteinuria >500mg/day, microhaematuria (>50 red blood cells per high power field) and/or abnormal renal ultrasonography.
Patients were excluded if they did not have a diagnosis of cirrhosis, had incomplete medical records or the absence of ascites and kidney failure, or if they used terlipressin because of acute variceal bleeding. Data regarding clinical and laboratory variables were gathered in order to calculate liver-specific scores. The patients were stratified as pre-protocol and post-protocol, according to the date of the diagnosis of HRS and the adherence of the attending physician to the protocol, which was 96% in the post-protocol period – only one patient admitted to the Nephrology ward before the Clinical Gastroenterology Team was called had a substantial delay to start the protocol. HRS resolution was defined as a discharge creatinine of less than 1.5mg/dL, for patients who survived the hospital stay.
VariablesData was gathered through the analysis of electronic and physical medical records. Clinical data was obtained and each case was individually assessed. Standardized imaging criteria were used for the diagnosis of hepatocellular carcinoma.31,32 Hepatic encephalopathy was defined and stratified according to West-Haven criteria.33 Laboratory data is expressed in units commonly used in the hospital. Days to be started on human albumin or terlipressin was defined as the days it took after the result of the first creatinine equal or higher than 1.5mg/dL during that hospital stay for the patient to receive the first dose of human albumin and terlipressin.
Liver-specific scoresCommonly used liver-specific scores were calculated to analyze their accuracy into predicting mortality. Child-Turcotte-Pugh (CTP) is a score used in the clinical care for cirrhotic patients that aims to predict 1-year mortality for compensated and decompensated cirrhosis.34,35 CTP was calculated through an online calculator.
Model for End-Stage Liver Disease (MELD)36 and Model for End-Stage Liver Disease – Sodium (MELD-Na)37 are scores currently used for organ allocation in liver transplantation, developed to predict 90-day mortality for cirrhotic patients. Both were calculated using online calculators.
Chronic Liver-Failure – Sequential Organ Failure Assessment (CLIF-SOFA) is a score developed by the European Society for the Study of the Liver – Chronic Liver Failure (EASL-CLIF) group, adapted from the Sequential Organ Failure Assessment (SOFA) score used in intensive care (Supplementary Table 1). It aims to define ACLF and divides it in three grades.22 Both CLIF-SOFA and ACLF grade were calculated using an online calculator developed by the CLIF Research Group (https://www.clifresearch.com/ToolsCalculators.aspx). These criteria already define HRS as ACLF. Therefore, ACLF was stratified in grade 1, 2 and 3 for this study:
- -
ACLF grade 1: isolated kidney failure.
- -
ACLF grade 2: two organ failures.
- -
ACLF grade 3: three organ failures.
Another scores developed by the EASL-CLIF group, CLIF Consortium Acute Decompensation (CLIF-C AD) score and CLIF-C ACLF, were developed with the purpose of predicting expected mortality for 30-day, 90-day, 180-day and 365-day for DC and ACLF patients.38 CLIF-C ACLF was calculated using the online calculator developed by the EASL-CLIF Group.
OutcomeDeath from all causes was used as main outcome. Data was gathered using medical records and searching through national death databases (https://www.falecidosnobrasil.org.br/). If the patient was admitted to the hospital more than once for HRS, data regarding only the first admission was collected.
Statistical analysisStatistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 15.0. Categorical variables are described using frequency and corresponding percentage and continuous variables by mean and standard deviation. Cox regression was used for univarate analysis and a multivariate analysis was performed using a stepwise progression to the Cox regression. All statistical tests performed for the analysis of variables excluded missing data. Kaplan–Meier curves were used for the graphical description of survival.
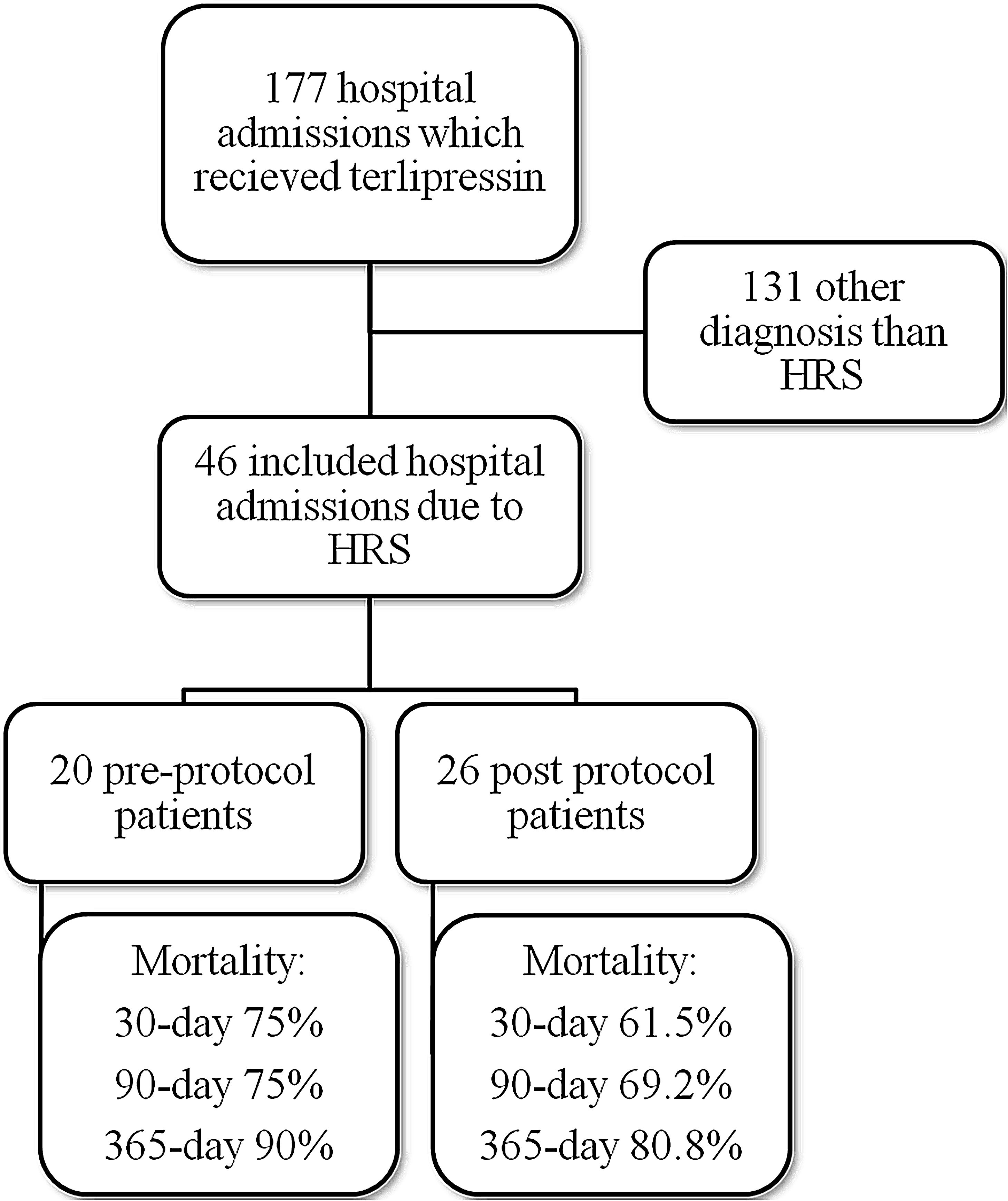
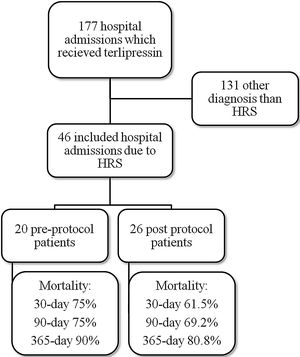
ResultsMedical record analysis retrieved 177 hospital admissions of patients who received terlipressin. Of these, 46 admissions were diagnosed as HRS type 1 and included in the study – 20 patients from the pre-protocol and 26 from the post-protocol period (Fig. 2). The remaining 131 patients that received terlipressin was because of suspected or confirmed acute esophageal variceal hemorrhage. Demographic, clinical and laboratorial data are described in Table 1 for the study population. Mean age was 58 years-old and 80% were male. The most common cause of cirrhosis was alcohol abuse (76%).
Demographic, clinical and laboratory findings of the study population and for each protocol period.
| Variable | Study population (n=46) | Pre-protocol period (n=20) | Post-protocol period (n=26) |
|---|---|---|---|
| Age (years)a | 58 (9) | 59 (8) | 55 (9) |
| Male sexb | 37 (80.4) | 16 (80) | 21 (80.8) |
| Periodb | |||
| Pre-protocol | 20 (43.5) | – | – |
| Post-protocol | 26 (56.5) | – | – |
| Etiology of cirrhosisb | |||
| Alcohol | 35 (76.1) | 16 (80) | 19 (73.2) |
| Hepatitis C | 6 (13) | 2 (10) | 4 (15.4) |
| Alcohol and hepatitis C | 4 (8.7) | 1 (5) | 3 (11.5) |
| Other | 1 (2.2) | 1 (5) | 0 |
| Active alcoholismb | 19 (41.3) | 8 (40) | 11 (42.3) |
| Previous use of medicationsb | |||
| PPI | 15 (32.6) | 5 (25) | 10 (38.5) |
| Spironolactone | 22 (47.8) | 11 (55) | 11 (42.3) |
| Furosemide | 21 (45.7) | 9 (45) | 12 (46.2) |
| NSBB | 19 (41.3) | 8 (40) | 11 (42.3) |
| Renal replacement therapyb | 4 (8.7) | 1 (5) | 2 (11.5) |
| Portal vein thrombosisb | 1 (2.2) | 0 | 1 (3.8) |
| Hepatocellular carcinomab | 5 (10.9) | 2 (10) | 3 (11.5) |
| Hepatic enchephalopathyb | |||
| Absent | 20 (43.5) | 7 (35) | 13 (50) |
| Grade 1 | 5 (10.9) | 2 (10) | 3 (11.5) |
| Grade 2 | 11 (23.9) | 5 (25) | 6 (23.1) |
| Grade 3 | 4 (8.7) | 2 (10) | 2 (7.7) |
| Grade 4 | 6 (13) | 4 (20) | 2 (7.7) |
| Esophageal varicesb | |||
| Absent | 28 (60.9) | 12 (60) | 16 (61.5) |
| Small caliber | 4 (8.7) | 0 | 4 (15.4) |
| Medium caliber | 10 (21.7) | 5 (25) | 5 (19.2) |
| Large caliber | 4 (8.7) | 3 (15) | 3 (3.8) |
| Infectionb | |||
| Absent | 14 (30.4) | 5 (25) | 9 (34.6) |
| SBP | 16 (34.8) | 7 (35) | 9 (34.6) |
| RTI | 3 (6.5) | 1 (5) | 2 (7.7) |
| UTI | 1 (2.2) | 0 | 1 (3.8) |
| Sepsis with undefined source of infection | 10 (21.7) | 7 (35) | 3 (11.5) |
| Other | 2 (4.3) | 0 | 2 (7.7) |
| Laboratory – before terlipressina | |||
| Hemoglobin (g/dL) | 9.3 (2) | 9.4 (2) | 9.3 (2) |
| Hematocrit (%) | 27.5 (5.3) | 27.9 (5.1) | 27.1 (5.5) |
| Leukocyte (/mm3) | 9951 (5160) | 10,821 (5492) | 9281 (4892) |
| Platelets (103/mm3) | 97.2 (51) | 96 (51) | 97 (51) |
| Total bilirubin (mg/dL) | 6.4 (7.3) | 6.8 (7.3) | 6.2 (7.5) |
| INR | 2.5 (5.6) | 1.7 (0.5) | 3.2 (7.6) |
| AST (U/L) | 89.5 (100) | 99 (136) | 81 (59) |
| ALT (U/L) | 58 (154) | 28 (21) | 80 (201) |
| GGT (U/L) | 230 (300) | 147 (172) | 309 (373) |
| Creatinine (mg/dL) | 3.75 (2.27) | 4.7 (8.5) | 2.9 (1.1) |
| Sodium (mg/dL) | 138 (7) | 138 (7) | 137 (8) |
| Potassium (mg/dL) | 5.2 (1.5) | 6.5 (8.6) | 4.2 (0.9) |
| Albumin (mg/dL) | 2.7 (0.6) | 2.6 (0.7) | 2.7 (0.4) |
| CRP (mg/L) | 74 (64) | 68 (72) | 82 (58) |
| Laboratory – after terlipressina | |||
| Hemoglobin (g/dL) | 8.3 (1.7) | 8.3 (1.9) | 8.2 (1.6) |
| Hematocrit (%) | 24.7 (5.4) | 25.6 (5.7) | 24.1 (5.1) |
| Leukocyte (/mm3) | 11,466 (12,257) | 14,197 (17,230) | 9304 (5566) |
| Platelets (103/mm3) | 73 (43) | 70 (42) | 74 (45) |
| Total bilirubin (mg/dL) | 7.8 (7.2) | 7.7 (5.4) | 8 (8.5) |
| INR | 2 (0.5) | 1.9 (0.6) | 2 (0.5) |
| AST (U/L) | 237 (353) | 150 (187) | 395 (532) |
| ALT (U/L) | 68 (124) | 39 (28) | 122 (205) |
| GGT (U/L) | 142 (186) | 119 (156) | 175 (237) |
| Creatinine (mg/dL) | 2.65 (1.6) | 2.9 (1.6) | 2.4 (1.5) |
| Sodium (mg/dL) | 139.4 (22) | 144 (7) | 135 (28) |
| Potassium (mg/dL) | 4.2 (1.2) | 4.5 (1.4) | 4 (1.1) |
| Albumin (mg/dL) | 3.4 (0.6) | 3.7 (0.4) | 3.1 (0.6) |
| CRP (mg/L) | 80 (32) | 63 (20) | 99 (34) |
| Terlipressina | |||
| Mean dose (mg/day) | 7.1 (2.7) | 7.6 (2.9) | 6.7 (2.4) |
| Total dose (mg) | 24.2 (13.6) | 27.1 (15.5) | 22.1 (13.5) |
| Days used | 3.4 (1.7) | 3.9 (2.1) | 3.1 (1.4) |
| Time to start (days) | 3.8 (3.5) | 5.1 (4.4) | 2.8 (2.3) |
| Human albumina | |||
| Mean dose (g/day) | 51.2 (20.1) | 60.7 (23.9) | 43.8 (12.7) |
| Total dose (g) | 184.3 (109.9) | 236.5 (113.2) | 144.3 (88.2) |
| Days used | 3.5 (1.7) | 4.1 (1.97) | 3.19 (1.47) |
| Time to start (days) | 1.6 (2.8) | 2.7 (3.2) | 0.9 (2.2) |
| Liver-specific scoresa | |||
| CTP | 12 (2) | 10 (2) | 11 (2) |
| MELD | 27 (7) | 27 (7) | 27 (7) |
| MELD-Na | 28 (7) | 27 (7) | 28 (7) |
| CLIF-SOFA | 9.6 (1.6) | 9.4 (1.4) | 9.8 (1.7) |
| CLIF-C ACLF | 49.5 (11.8) | 48.1 (6.8) | 50 (14.6) |
| ACLFb | |||
| Grade 1 | 25 (54.3) | 12 (60) | 13 (50) |
| Grade 2 | 12 (26.1) | 5 (35) | 7 (26.9) |
| Grade 3 | 8 (17.4) | 3 (15) | 5 (19.2) |
| Time to death (days)a | 35 (69) | 35 (91) | 35 (47) |
| HRS resolutiona | 12 (26.1) | 4 (20) | 8 (30.7) |
| All-cause mortalityb | |||
| 30-day | 31 (67.3) | 15 (75) | 16 (61.5) |
| 90-day | 33 (71.7) | 15 (75) | 18 (69.2) |
| 365-day | 38 (82.6) | 18 (90) | 21 (80.8) |
PPI=proton pump inhibitor; NSBB=non-selective beta-blockers; SBP=spontaneous bacterial peritonitis; RTI=respiratory tract infection; UTI=urinary tract infection; INR=international normalized ratio; AST=aspartate transaminase; ALT=alanine transaminase; GGT=gamma-glutamyl transferase; CRP=C-reactive protein; CTP=Child-Turcotte-Pugh score; MELD=Model for End-Stage Liver Disease; MELD-Na=Modified Model Including Sodium; CLIF-SOFA=Chronic Liver Failure Sequential Organ Failure Assessment; CLIF-C ACLF=CLIF Consortium Acute Decompensation Acute-on-chronic liver failure.
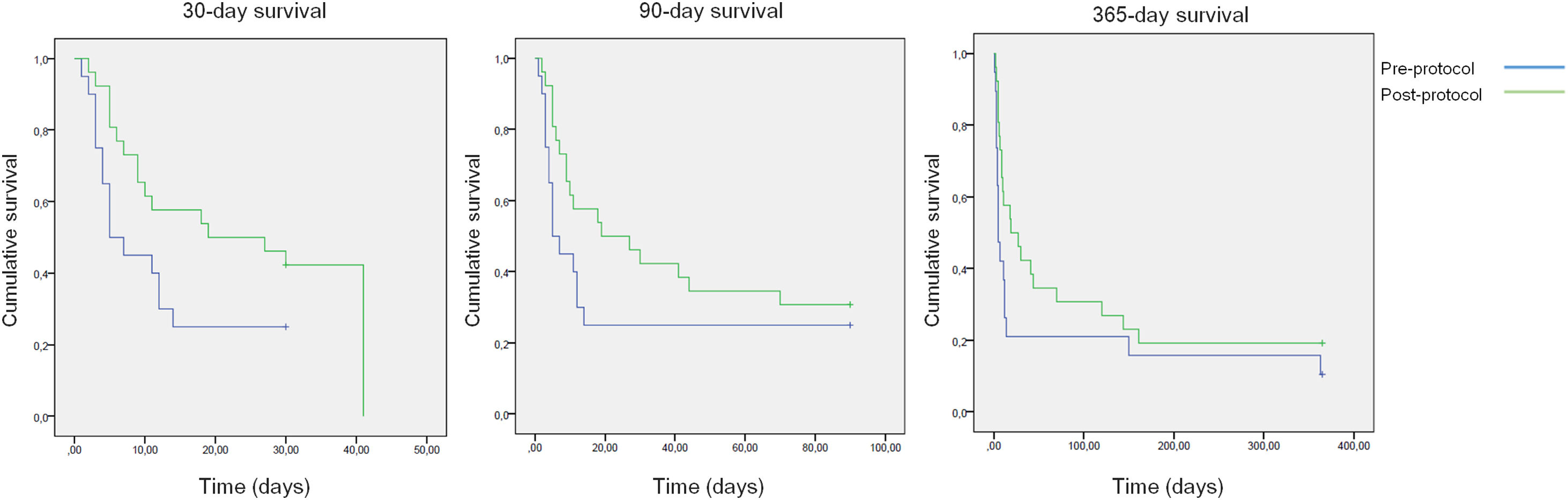
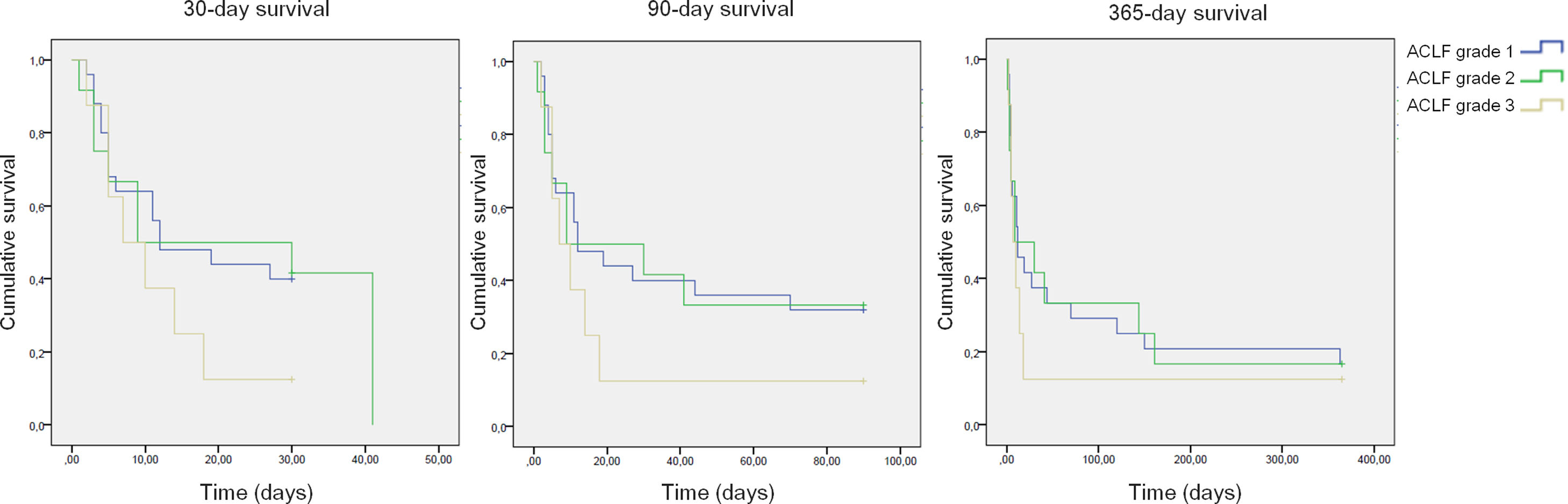
All-cause mortality for 30-day, 90-day and 365-day was 75%, 75% and 90% for the pre-protocol period and 61%, 69% and 80% for the post-protocol period, respectively (Fig. 3). ACLF grade 1 was present in 25 patients, grade 2 in 12 and grade 3 in 8. All-cause mortality for 30-day, 90-day and 365-day was 60%, 68% and 83.3% for ACLF grade 1 patients, 66.6%, 66.6% and 83.3% for ACLF grade 2 patients and 87.5%, 87.5% and 87.5% for ACLF grade 3 patients, respectively (Supplementary Figure 1).
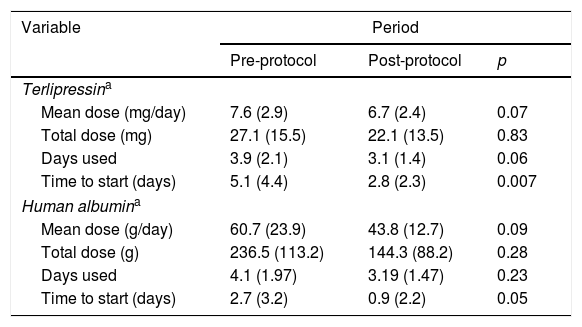
There was a trend toward the reduction of the total mean dose of terlipressin and human albumin used per patient with the institution of the protocol, reducing from 27 to 22mg of terlipressin and from 236 to 144g of human albumin per patient. This was not associated with higher mortality and was able to reduce the cost of HRS treatment (Table 2). Also, the patients in the post-protocol group were started earlier on albumin and terlipressin than the patients in the pre-protocol group – such difference was statically significant.
Human albumin and terlipressin use for HRS patients.
| Variable | Period | ||
|---|---|---|---|
| Pre-protocol | Post-protocol | p | |
| Terlipressina | |||
| Mean dose (mg/day) | 7.6 (2.9) | 6.7 (2.4) | 0.07 |
| Total dose (mg) | 27.1 (15.5) | 22.1 (13.5) | 0.83 |
| Days used | 3.9 (2.1) | 3.1 (1.4) | 0.06 |
| Time to start (days) | 5.1 (4.4) | 2.8 (2.3) | 0.007 |
| Human albumina | |||
| Mean dose (g/day) | 60.7 (23.9) | 43.8 (12.7) | 0.09 |
| Total dose (g) | 236.5 (113.2) | 144.3 (88.2) | 0.28 |
| Days used | 4.1 (1.97) | 3.19 (1.47) | 0.23 |
| Time to start (days) | 2.7 (3.2) | 0.9 (2.2) | 0.05 |
HRS=hepatorenal syndrome.
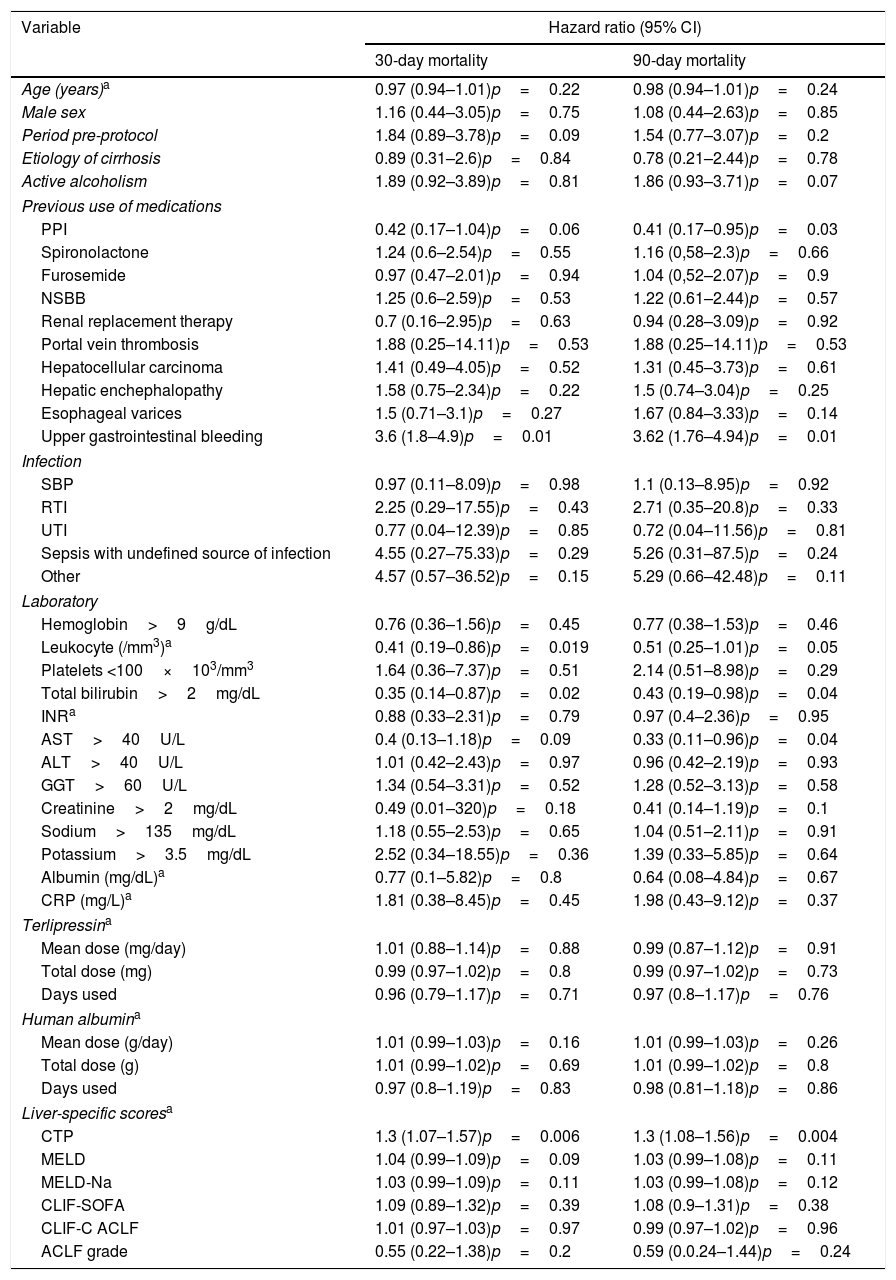
An univariate analysis was performed for 30- and 90-day mortality. Creatinine >2mg/dL, bilirubin >2mg/dL, leukocytes >10,000/mm3, platelets <100×103/mm3, AST <40U/L, pre-protocol period, absence of use of proton pump inhibitors (PPI), presence of infection, upper gastrointestinal bleeding, higher CTP, MELD and MELD-Na were associated to higher 30-day mortality. Also, creatinine >2mg/dL, bilirubin >2mg/dL, leukocytes >10,000/mm3, platelets <10×103/mm3, AST <40U/L, pre-protocol period, absence of use PPI, presence of esophageal varices, presence of infection, upper gastrointestinal bleeding, higher CTP, MELD and MELD-Na were associated to higher 90-day mortality (Table 3). Each one of these variables was used for the multivariate analysis in a stepwise progression to the Cox regression.
Univariate analysis for 30- and 90-day mortality, comparing pre and post-procotol period.
| Variable | Hazard ratio (95% CI) | |
|---|---|---|
| 30-day mortality | 90-day mortality | |
| Age (years)a | 0.97 (0.94–1.01)p=0.22 | 0.98 (0.94–1.01)p=0.24 |
| Male sex | 1.16 (0.44–3.05)p=0.75 | 1.08 (0.44–2.63)p=0.85 |
| Period pre-protocol | 1.84 (0.89–3.78)p=0.09 | 1.54 (0.77–3.07)p=0.2 |
| Etiology of cirrhosis | 0.89 (0.31–2.6)p=0.84 | 0.78 (0.21–2.44)p=0.78 |
| Active alcoholism | 1.89 (0.92–3.89)p=0.81 | 1.86 (0.93–3.71)p=0.07 |
| Previous use of medications | ||
| PPI | 0.42 (0.17–1.04)p=0.06 | 0.41 (0.17–0.95)p=0.03 |
| Spironolactone | 1.24 (0.6–2.54)p=0.55 | 1.16 (0,58–2.3)p=0.66 |
| Furosemide | 0.97 (0.47–2.01)p=0.94 | 1.04 (0,52–2.07)p=0.9 |
| NSBB | 1.25 (0.6–2.59)p=0.53 | 1.22 (0.61–2.44)p=0.57 |
| Renal replacement therapy | 0.7 (0.16–2.95)p=0.63 | 0.94 (0.28–3.09)p=0.92 |
| Portal vein thrombosis | 1.88 (0.25–14.11)p=0.53 | 1.88 (0.25–14.11)p=0.53 |
| Hepatocellular carcinoma | 1.41 (0.49–4.05)p=0.52 | 1.31 (0.45–3.73)p=0.61 |
| Hepatic enchephalopathy | 1.58 (0.75–2.34)p=0.22 | 1.5 (0.74–3.04)p=0.25 |
| Esophageal varices | 1.5 (0.71–3.1)p=0.27 | 1.67 (0.84–3.33)p=0.14 |
| Upper gastrointestinal bleeding | 3.6 (1.8–4.9)p=0.01 | 3.62 (1.76–4.94)p=0.01 |
| Infection | ||
| SBP | 0.97 (0.11–8.09)p=0.98 | 1.1 (0.13–8.95)p=0.92 |
| RTI | 2.25 (0.29–17.55)p=0.43 | 2.71 (0.35–20.8)p=0.33 |
| UTI | 0.77 (0.04–12.39)p=0.85 | 0.72 (0.04–11.56)p=0.81 |
| Sepsis with undefined source of infection | 4.55 (0.27–75.33)p=0.29 | 5.26 (0.31–87.5)p=0.24 |
| Other | 4.57 (0.57–36.52)p=0.15 | 5.29 (0.66–42.48)p=0.11 |
| Laboratory | ||
| Hemoglobin>9g/dL | 0.76 (0.36–1.56)p=0.45 | 0.77 (0.38–1.53)p=0.46 |
| Leukocyte (/mm3)a | 0.41 (0.19–0.86)p=0.019 | 0.51 (0.25–1.01)p=0.05 |
| Platelets <100×103/mm3 | 1.64 (0.36–7.37)p=0.51 | 2.14 (0.51–8.98)p=0.29 |
| Total bilirubin>2mg/dL | 0.35 (0.14–0.87)p=0.02 | 0.43 (0.19–0.98)p=0.04 |
| INRa | 0.88 (0.33–2.31)p=0.79 | 0.97 (0.4–2.36)p=0.95 |
| AST>40U/L | 0.4 (0.13–1.18)p=0.09 | 0.33 (0.11–0.96)p=0.04 |
| ALT>40U/L | 1.01 (0.42–2.43)p=0.97 | 0.96 (0.42–2.19)p=0.93 |
| GGT>60U/L | 1.34 (0.54–3.31)p=0.52 | 1.28 (0.52–3.13)p=0.58 |
| Creatinine>2mg/dL | 0.49 (0.01–320)p=0.18 | 0.41 (0.14–1.19)p=0.1 |
| Sodium>135mg/dL | 1.18 (0.55–2.53)p=0.65 | 1.04 (0.51–2.11)p=0.91 |
| Potassium>3.5mg/dL | 2.52 (0.34–18.55)p=0.36 | 1.39 (0.33–5.85)p=0.64 |
| Albumin (mg/dL)a | 0.77 (0.1–5.82)p=0.8 | 0.64 (0.08–4.84)p=0.67 |
| CRP (mg/L)a | 1.81 (0.38–8.45)p=0.45 | 1.98 (0.43–9.12)p=0.37 |
| Terlipressina | ||
| Mean dose (mg/day) | 1.01 (0.88–1.14)p=0.88 | 0.99 (0.87–1.12)p=0.91 |
| Total dose (mg) | 0.99 (0.97–1.02)p=0.8 | 0.99 (0.97–1.02)p=0.73 |
| Days used | 0.96 (0.79–1.17)p=0.71 | 0.97 (0.8–1.17)p=0.76 |
| Human albumina | ||
| Mean dose (g/day) | 1.01 (0.99–1.03)p=0.16 | 1.01 (0.99–1.03)p=0.26 |
| Total dose (g) | 1.01 (0.99–1.02)p=0.69 | 1.01 (0.99–1.02)p=0.8 |
| Days used | 0.97 (0.8–1.19)p=0.83 | 0.98 (0.81–1.18)p=0.86 |
| Liver-specific scoresa | ||
| CTP | 1.3 (1.07–1.57)p=0.006 | 1.3 (1.08–1.56)p=0.004 |
| MELD | 1.04 (0.99–1.09)p=0.09 | 1.03 (0.99–1.08)p=0.11 |
| MELD-Na | 1.03 (0.99–1.09)p=0.11 | 1.03 (0.99–1.08)p=0.12 |
| CLIF-SOFA | 1.09 (0.89–1.32)p=0.39 | 1.08 (0.9–1.31)p=0.38 |
| CLIF-C ACLF | 1.01 (0.97–1.03)p=0.97 | 0.99 (0.97–1.02)p=0.96 |
| ACLF grade | 0.55 (0.22–1.38)p=0.2 | 0.59 (0.0.24–1.44)p=0.24 |
CI=confidence interval; PPI=proton pump inhibitor; NSBB=non-selective beta-blockers; SBP=Spontaneous Bacterial Peritonitis; RTI=Respiratory Tract Infection; UTI=Urinary Tract Infection; INR=international normalized ratio; AST=aspartate transaminase; ALT=alanine transaminase; GGT=gamma-glutamyl transferase; CRP=C-reactive protein; CTP=Child-Turcotte-Pugh score; MELD=Model for End-Stage Liver Disease; MELD-Na=Modified Model Including Sodium; CLIF-SOFA=Chronic Liver Failure Sequential Organ Failure Assessment; CLIF-C ACLF=CLIF Consortium Acute Decompensation Acute-on-chronic liver failure.
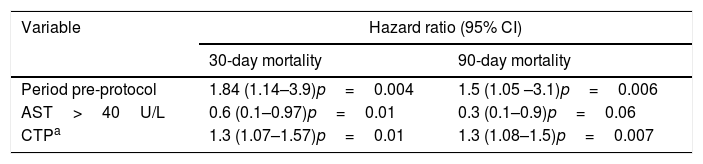
In the multivariate analysis, AST <40U/L, pre-protocol period and higher CTP scores were associated with higher 30-day and 90-day all-cause mortality (Table 4).
Multivariate analysis for 30- and 90-day mortality.
| Variable | Hazard ratio (95% CI) | |
|---|---|---|
| 30-day mortality | 90-day mortality | |
| Period pre-protocol | 1.84 (1.14–3.9)p=0.004 | 1.5 (1.05 –3.1)p=0.006 |
| AST>40U/L | 0.6 (0.1–0.97)p=0.01 | 0.3 (0.1–0.9)p=0.06 |
| CTPa | 1.3 (1.07–1.57)p=0.01 | 1.3 (1.08–1.5)p=0.007 |
CI=confidence interval; AST=aspartate transaminase; CTP=Child-Turcotte-Pugh score.
HRS type 1 is the most severe complication of ESLD, determining an expected 90-day mortality of around 90% if not treated.1,39 Although treatment has progressed in the last couple of decades, it is yet to show a major impact on survival.8 The present study has sought to show that the use of an evidence based protocol for treatment and diagnosis of HRS can reduce drug consumption and mortality.
In the present study, 30-day mortality for all ACLF patients was 67.3%. This is higher than the one described in the CANONIC study (33.9%)22 and other Brazilian studies with mortality rates of 39%.40–42 This is because the presence of AKI translates into a reduction in survival in ACLF patients – a meta-analysis has described an odds ratio of 3.98 and 4.98 for 30- and 90-day mortality, respectively.43 Nevertheless, the grade of ACLF did not impact mortality in the present study, probably due to the small number of patients.
Hospital consumption of human albumin and terlipressin for the treatment of HRS decreased with the adoption of the protocol in the present study, reducing total costs for HRS treatment, without impairing outcomes. This is a very important matter, since all treatments for HRS are costly. For example, in France, a previous study has shown a low compliance to current guidelines in human albumin prescription for cirrhotic patients.44 For HRS, the compliance to current guidelines when prescribing human albumin was higher for senior practitioners in teaching hospitals. On the other hand, an Italian study which took place in a tertiary teaching hospital has shown non-compliance to be under 10%.45 The adoption of evidence-based local protocols might improve compliance to adequate use of these drugs, mitigating the high cost of HRS treatment. In the present study, there was a high compliance to the studied protocol. This was secondary to the fact that the studied hospital has a small team of registrars and every physician from it agreed on the protocol, and the residents were very aware of its existence and use.
The problem of the cost of terlipressin and human albumin has been studied in two systematic reviews, comparing to the cost of norepinephrine plus human albumin.15,46 Since norepinephrine requires hospitalization in an intensive care unit, terlipressin was shown to be less costly, hence it can be used in the infirmary. Two studies have addressed the economic burden of HRS treatment in the United States. Both have concluded that, for the private sector and Medicare patients, the burden of the cost of HRS treatment is very high, delivering poor results. Therefore, there is an unmet need of an effective and cheaper treatment,47,48 which could improve prognosis in a large scale. In the present study, we have demonstrated a lower use of human albumin and terlipressin in patients with HRS after the institution of a protocol – this might translate into reduction of cost for these patients.
LT is the only definitive treatment for HRS, improving mortality and mitigating the risk for the need for long term renal replacement therapy (RRT).7 Although HRS has been shown to be reversed in around 83% of patients which undergo LT, it still impacts negatively post-LT survival for up to five years post-LT.49 Also, the most important risk factor for the need for long-term RRT is actually the need of RRT previously to LT.7 In the present study, the values of creatinine alone was not an isolated risk factor for mortality.
AKI is a rather common complication of cirrhosis, occurring in 20% of patients admitted to the hospital.50 The most common causes of AKI for cirrhotic patients are considered bacterial infections, followed by pre-renal kidney failure and HRS.51,52 The current criteria defined by the International Ascites Club uses the concept of AKI for the diagnosis of HRS.53 Although, it does not seem to be superior to the previous cut-off used in the criteria published in 2007.21 In the present study, the cut-off value of serum creatinine for HRS diagnosis used was 1.5mg/dL.
CTP score has been used for decades to predict mortality for cirrhotic patients. It has been shown to be useful to predict adverse events for patients admitted to the intensive care unit54 and for HRS.55,56 In the present study, it was independently associated with mortality. The grade of ACLF has been associated with higher mortality and changed response to HRS treatment in a previous study developed by the EASL-CLIF.57
The median for survival for HRS is two weeks if not treated58 and, therefore, all patients with renal and liver dysfunction should be evaluated for LT, with RRT as a bridge to it.59 AKI, and therefore HRS, can be triggered by precipitating events: the most important of these are infections (most importantly spontaneous bacterial peritonitis), gastrointestinal bleeding, use of vasodilators (such as angiotensin-converting enzyme inhibitors) and large-volume paracentesis without human albumin administration.60–65 Because of this high mortality associated with HRS, it is crucial to develop protocols to guide care and improve outcomes. In our study, the institution of the protocol resulted in a shorter time to start human albumin and terlipressin. The smaller delay to receive appropriate care probably caused the higher survival in the post-protocol period.
Since infections are a major concern, they ought to be suspected when AKI occurs, collecting blood, urine and ascites cultures66; although, empirical antibiotics should not be used in the absence of infection. Diuretics ought to be discontinued.66 Non-selective beta-blockers discontinuation is still a controversial subject – initially, it was believed that it might increase the risk for HRS, especially in patients with spontaneous bacterial peritonitis67; currently it is not believed to impact mortality.68–70 Nevertheless, it should be used with extreme caution or discontinued to avoid hypotension – it is well documented that when vasopressors cause an increase in mean arterial blood pressure, the reversal of HRS is more likely.71,72 Also, in order to avoid hypotension, if RRT is necessary, continuous hemodiafiltration seems to be superior in severe kidney failure for unstable cirrhotic patients.73 It seems reasonable; therefore, that hypotension needs to be avoided. In the present study, the use of non-selective beta-blockers did not impact mortality.
The use of PPI have been associated with higher mortality and decompensation in previous studies,74 translating into a higher risk for the development of spontaneous bacterial peritonitis and adverse events.75–77 Nevertheless, other studies have suggested that this finding only occurred because of the retrospective nature of the previous papers.78–80 MELD and MELD-Na scores are used to allocate organs for LT and are useful tools to predict 90-day mortality for ESLD, even for HRS patients.81 In the present study, the absence of use of PPI and higher MELD and MELD-Na scores were associated with higher 30- and 90-day mortality in the univariate analysis, but not in the multivariate analysis.
The largest drawback of the present study is the small sample size. This probably happened because HRS is not a very common complication of cirrhosis. Most studies in this subject are generally multi-centric, which helps to gather more data. Nevertheless, the extensive data accumulated has allowed a deep study of the population, providing an evidence-based protocol which has been shown to improve survival when compared to a historical cohort from the same hospital. Another limitation to the study is the use of the criteria published in 2007 as definition for HRS: the criteria which include AKI definitions were published in 2015, after the protocol was already in place and being used. Nevertheless, AKI criteria has not been shown to be superior to the cutoff previously used of a serum creatinine above 1.5mg/dL.21
ConclusionIn conclusion, the adoption of an evidence-based protocol for the diagnosis and treatment of HRS translated into a higher survival rate. Also, it was associated with lower total drug use. The authors suggest that, taking into account the high mortality and cost of HRS treatment, the use of evidence-based protocols for diagnosis and treatment of HRS could reduce cost and mortality in tertiary hospitals.
Key summaryHepatorenal syndrome (HRS) is one of the deadliest complications of cirrhosis and carries a high mortality. An evidence-based protocol for diagnosis and treatment of HRS was instituted as standard-of-care in 2013 in the studied hospital. Data from medical records from 2010 to 2016 were obtained by searching the hospital electronic database for every patient who received terlipressin, ranging from three years prior and after the institution of the protocol. A stepwise approach to the Cox regression was used for univariate and multivariate analysis. It was included 46 patients who were diagnosed with HRS, 20 from pre-protocol and 26 post-protocol period. Respectively, mortality for 30-day, 90-day and 365-day was 75%, 89% and 89% for the pre-protocol period and 61%, 69% and 80% for the post-protocol period. In multivariate analysis, AST <40U/L, pre-protocol period and higher Child-Pugh-Turcotte scores were associated with higher 30-day and 90-day all-cause mortality. Also, the total mean dose of terlipressin and human albumin used per patient reduced with the institution of the protocol, reducing from 27 to 22mg of terlipressin and from 236 to 144g of human albumin per patient. This was not associated with higher mortality. In conclusion, the use of an evidence-based protocol in the treatment of HRS translated in a higher survival. Also, it was associated with lower drug used for treatment of HRS.
Main points- •
Hepatorenal syndrome (HRS) is the deadliest complication of cirrhosis, generally treated with the association of terlipressin plus human albumin.
- •
An evidence-based protocol for diagnosis and treatment of HRS was instituted in 2013 in the studied Hospital and data was gathered from 3 years prior to 3 years after the institution of the protocol.
- •
In multivariate analysis, AST <40U/L, pre-protocol period and higher Child scores were associated with higher 30-day and 90-day mortality.
- •
Total mean dose of terlipressin and human albumin used per patient reduced after the institution of the protocol and such was not associated with higher mortality. Also, time to start human albumin and terlipressin reduced in the post-protocol period.
- •
The use of evidence-based protocols for diagnosis and treatment of HRS could reduce cost and mortality in tertiary hospitals.
Terres AZ – Design, data collection, writing and review.
Balbinot RS – Data collection, writing and review.
Muscope ALF – Data collection, writing and review.
Longen ML – Data collection, writing and review.
Schena B – Data collection, writing and review.
Cini BT – Data collection, writing and review.
Rost Jr. GL – Data collection, writing and review.
Balensiefer JIL – Data collection, writing and review.
Eberhardt LZ – Data collection, writing and review.
Balbinot RA – Design, writing and review.
Balbinot SS – Design, writing and review.
Soldera J – Design, statistical analysis, translation, writing and review.
None.
Conflict of interestNone.
Previous presentation: Residency conclusion thesis for Alana Zulian Terres in 2018. Partial data presented as an oral presentation in Semana Brasileira do Aparelho Digestivo, November-2018. Complete data presented as poster in UEG Week, 2019.