The need for fluoroscopy guidance in patients undergoing endoscopic ultrasound-guided transmural drainage (EUS-TMD) of peripancreatic fluid collections (PFCs) remains unclear.
AimsThe aim of this study was to compare general outcomes of EUS-TMD of PFCs under fluoroscopy (F) vs fluoroless (FL).
MethodsThis is a comparative study with a retrospective analysis of a prospective and consecutive inclusion database at a tertiary centre, from 2009 to 2015. All patients were symptomatic pseudocyst (PSC) and walled-off pancreatic necrosis (WON). Two groups were assigned depending on availability of fluoroscopy. The groups were heterogeneous in terms of their demographic characteristics, PFCs and procedure. The main outcome measures included technical and clinical success, incidences, adverse events (AEs), and follow-up.
ResultsFifty EUS-TMD of PFCs from 86 EUS-guided drainages were included during the study period. Group F included 26 procedures, PSC 69.2%, WON 30.8%, metal stents 61.5% (46.1% lumen-apposing stent) and plastic stents 38.5%. Group FL included 24 procedures, PSC 37.5%, WON 62.5%, and metal stents 95.8% (lumen-apposing stents). Technical success was 100% in both groups, and clinical success was similar (F 88.5%, FL 87.5%). Technical incidences and intra-procedure AEs were only described in group F (7.6% and 11.5%, respectively) and none in group FL. Procedure time was less in group FL (8min, p=0.0341).
ConclusionsFluoroless in the EUS-TMD of PFCs does not involve more technical incidences or intra-procedure AEs. Technical and clinical success was similar in the two groups.
La necesidad de la ayuda de fluoroscopia en pacientes que se les realiza un drenaje transmural guiada por ecoendoscopia (USE) de colecciones pancreáticas (CP) no está claro.
ObjetivoEl objetivo de este estudio fue comparar los resultados generales del drenaje transmural de CP con ayuda de fluoroscopia (F) versus sin fluoroscopia (SF).
MétodosEstudio comparativo, análisis retrospectivo, con inclusión prospectiva y consecutiva en una base de datos específica. Estudio realizado en un centro universitario terciario, en el periodo entre 2009 y 2015. Todos los pacientes fueron seudoquistes (PSQ) o colecciones pancreáticas necróticas encapsuladas (CPN) con clínica asociada. Se asignaron 2 grupos dependiendo de la disponibilidad de la fluoroscopia. Grupos heterogéneos respecto a sus características demográficas, CP y procedimientos. El estudio analizó el éxito técnico, el éxito clínico, las incidencias, los eventos adversos y el seguimiento.
ResultadosCincuenta drenajes transmurales guiados por USE de CP, de un total de 86 drenajes por USE, fueron incluidos durante el periodo del estudio. El grupo F incluyó 26 procedimientos, PSC 69,2%, CPN 30,8%, prótesis metálicas 61,5% (46,1% prótesis de aposición luminal) y plásticas 38,5%. El grupo SF incluyó 24 procedimientos, PSQ 37,5%, CPN 62,5% y prótesis metálicas 95,8% (prótesis de aposición luminal). Éxito técnico del 100% en ambos grupos, éxito clínico clínico similar (F 88,5%, FL 87,5%). Incidencias técnicas y eventos adversos intraprocedimiento: solo descritos en grupo F (7,6% y 11.5%, respectivamente) y ninguna en el grupo SF. Tiempo del procedimiento menor en grupo SF (8min, p=0.0341).
ConclusionesEl drenaje transmural de CP sin ayuda de fluoroscopia no comportó mayor número de incidencias técnicas o eventos adversos intraprocedimiento. Los éxitos técnico y clínico fueron similares en ambos grupos.
Endoscopic ultrasound transmural drainage (EUS-TMD) has become an established treatment method for symptomatic pancreatic fluid collections (PFCs). This approach has emerged as an attractive, less invasive and cost-effective technique. A randomized trial by Varadarajulu et al. concluded that the endoscopic approach is as effective as the surgical, and was associated with shorter hospital stays, better physical and mental health of patients, and lower cost.1
This is a technically demanding procedure, which usually requires endoscopy, EUS, and fluoroscopic guidance.2–4 EUS is used routinely in the access step of the procedure, and fluoroscopy plus EUS guidance is used in the other steps of the endoscopic intervention.
Nowadays, the use of EUS guidance is practically essential because it allows identification of the most appropriate sites for drainage, avoiding major vessels, and it permits accessing non-bulging lesions, as well as control in real time of the access and changing of devices. Conversely, the use of fluoroscopy is dispensable, offering another image during the advancing of devices during the procedure, with the purpose of improving technical and clinical aspects and avoiding technical incidences and intra-procedural adverse events (AEs).4
Since the first EUS-TMD in 1992 by Grimm et al., there have been numerous technical innovations.5 Lumen-apposing metal stent (LAMS) is a good example of the appearance of new dedicated devices designed specifically for EUS-guided interventions, with the possibility of obviating the use of fluoroscopy.6–13
Until date the usual method to performing EUS-TMD of PFCs includes the systematic use of radiological control, but its use is not mandatory and fluoroless could offer other advantages. Firstly, most endoscopic rooms are not equipped with fluoroscopy. Secondly, the use of fluoroscopy carries a risk of radiation for the patient and the endoscopy staff as well.14–18 Lastly, once the endoscopy team has sufficient experience with the technique, the total duration of the procedure may be reduced, implying increased efficacy and safety.
There is a paucity of published data about EUS-TMD of PFCs without fluoroscopy, and comparative studies are lacking.19–22 The main aim of this study was to carry out a comparative analysis in terms of efficacy (clinical and technical success) and safety (intra-procedural incidences and AEs). Secondary aims were to compare stent types and the procedure durations.
Material and methodsStudy designThis study took place at Hospital Universitari de Bellvitge, a tertiary-care public institution in the Barcelona area (Catalonia, Spain), between 2009 and 2015. All patients, that underwent EUS-guided drainage, were prospectively and consecutively recruited, and analyzed retrospectively from an EUS specific database. Exclusion criterias: no peripancreatic collections; simple puncture aspiration; lack of data or follow up and patients from other centres.
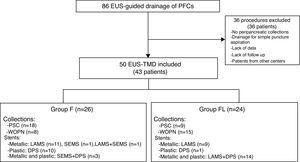
Fig. 1 is a flow diagram of the exclusion and inclusion criteria, and characteristics of the two groups compared.
Flow diagram. Number of procedures and patients, and distribution by groups. EUS, endoscopic ultrasound; PFCs, pancreatic fluid collections; EUS-TMD: EUS-guided transmural drainage; F, fluoroscopy; FL, fluoroless; PSC, pancreatic pseudocyst; WON, walled-off pancreatic necrosis; LAMS, lumen-apposing metal stent; SEMS, self-expanding metal stent; DPS, double pigtail stent.
A single interventional endoscopist (J.G.) performed all procedures. Written informed consent was obtained from all patients. Our institutional review board approved the study. The use of fluoroscopy was uncontrolled, mainly depending of the fluoroscopy arch availability. All procedures were under deep sedation or with orotracheal intubation by an anaesthesiologist. Prophylactic antibiotics were administered.
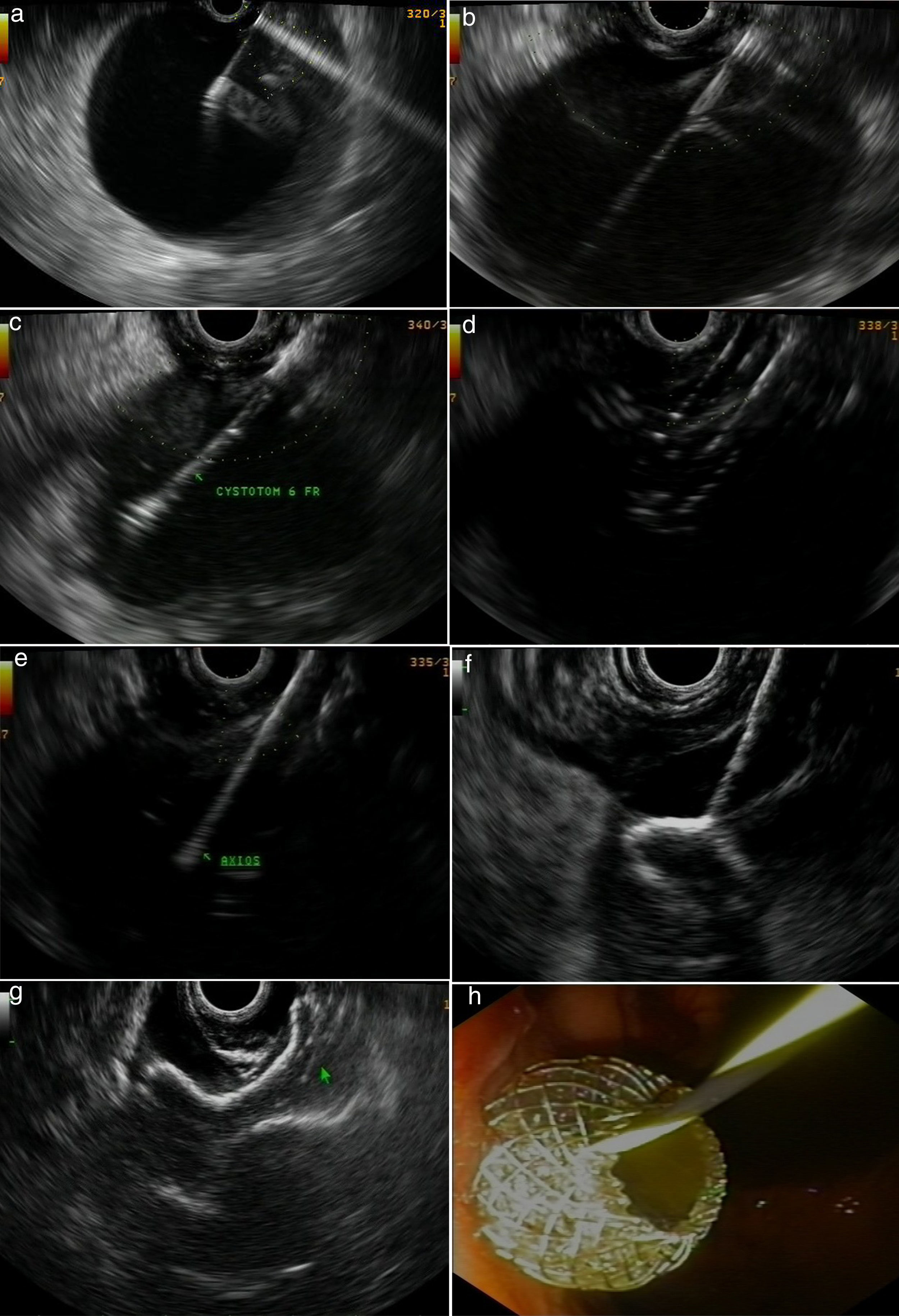
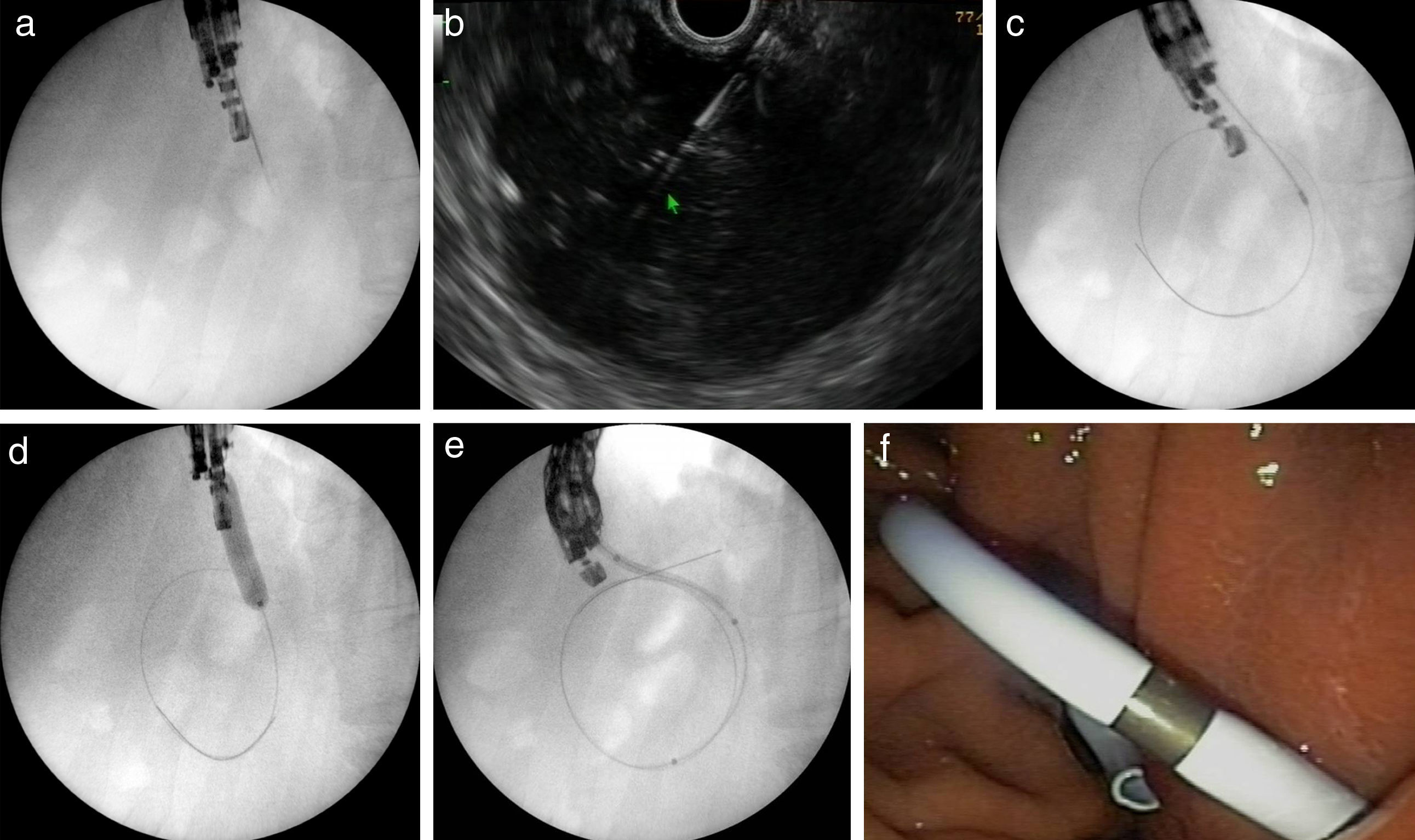
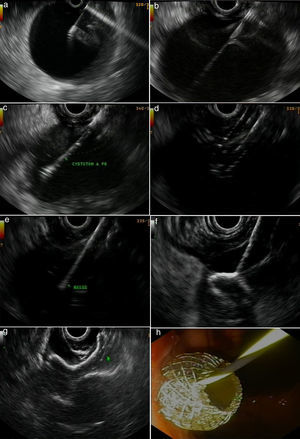
Figs. 2 and 3 represent all the steps of EUS-TMD with fluoroscopy or fluoroless guidance.
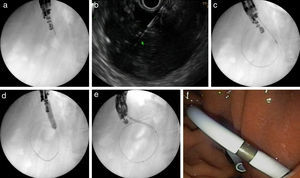
A fluoroless case, with images showing endosonography (EUS)-guided transgastric drainage of a pancreatic fluid collection (PFC) with a lumen-apposing metal stent (LAMS). (a) Puncture of a PFC using a 19-gauge fine needle (Expect Flex, Boston Sc); (b) EUS image showing the 0.035-inch guidewire advancing through the 19-gauge needle into the collection; (c) 6 Fr-cystotom inside the PFC; (d) EUS dilation of the transmural tract using a biliary balloon; (e) AXIOS catheter inserted into the PFC; (f) the distal flange of the LAMS (10×10-mm, AXIOS) inside the PFC; (g) AXIOS stent completely delivered; (h) endoscopic view of the AXIOS stent.
A fluoroscopy guidance case. Sequence of fluoroscopic and endosonography images showing transgastric drainage of a pancreatic fluid collection (PFC) with a double pigtail stent (DPS); (a) puncture of the collection using a 19G needle; (b) insertion of a guide-wire into the lesion; (c) fluoroscopy view of a 6Fr-cystotome over a guidewire; (d) radiological image of the transmural tract dilation with a biliary balloon of 8mm; (e) insertion of the DPS using fluoroscopic guidance; (f) endoscopic image of the DPS.
The key point in carrying out a fluoroless EUS-TMD is maintaining the EUS-plane image on the screen, thereby making the insertion and exchange of devices easy and safe, and reducing the risk of technical incidences. All patients were monitored and admitted to our centre for clinical observation after the procedure. PFC resolution was assessed with computed tomography (CT) scan, generally 4–6 weeks after the initial transmural drainage. When complete resolution was achieved, all stents were removed.
Outcome parameters: definitionsTechnical success was defined as achieving drainage and correct placement of the stent.
Clinical success was defined as resolution of acute symptoms, normalization of laboratory parameters, and total resolution of the collection or decrease in size by 50% at 4–6 weeks’ follow-up, as determined with imaging.
AEs were those that occurred intraprocedure and in the first 24h post-procedure since it was assumed that these could be related to the EUS-TMD procedure (when this involved a second procedure or more days of hospitalization, according to the standard lexicon for endoscopic adverse events).23
Technical incidences were failures during the interventional steps of the endoscopic drainage such as access and ostomy creation, or stent deployment-related incidences such as release failure, malposition, dislocation, and migration during the procedure. These events had no clinical consequences.
Procedure time was calculated as the time from the insertion of the first scope to the withdrawal of the last in minutes.
Fluoroscopy time was calculated as the time of radiation exposure (RE) in seconds.
Recurrence was defined as symptomatic collection diagnosed on imaging procedure following initial treatment success.
Patient follow-up was prospectively after drainage for a mean time of 39 months±26.1 based on outpatient examination findings.
Statistical analysisDiscrete variables are presented as percentage and 95% confidence interval, and continuous variables are presented as mean, standard deviation (SD) and range (or median and interquartile range for non-normally distributed data). To compare the technical and clinical success, and EAs (or incidences) of EUS-TMD with and without use of fluoroscopy, Chi-square test and Fisher exact test were used. The non-parametric Wilcoxon–Mann–Whitney test was used to compare the time, in minutes, of the entire procedure and the RE. A p<0.05 was considered a statistically significant difference. R version 3.2.5 for Windows statistical software was used for data analysis.
ResultsPatient and study characteristicsDuring this study period, 86 EUS-guided drainage of any kind of collections were performed, and a total of 36 procedures were excluded. Finally, 50 procedures (43 patients) of EUS-TMD of PFCs with stent placement and clinical follow-up at our centre were included. These collections were pseudocysts (PSC) or walled-off pancreatic necrosis (WON) defined by the revised Atlanta Criteria,2 with drainage indication according to the Working Group of IAP/APA.3 A comparative analysis of the use of fluoroscopy during the EUS-TMD was made: Group F, fluoroscopy (26 procedures) vs group FL, fluoroless (24 procedures).
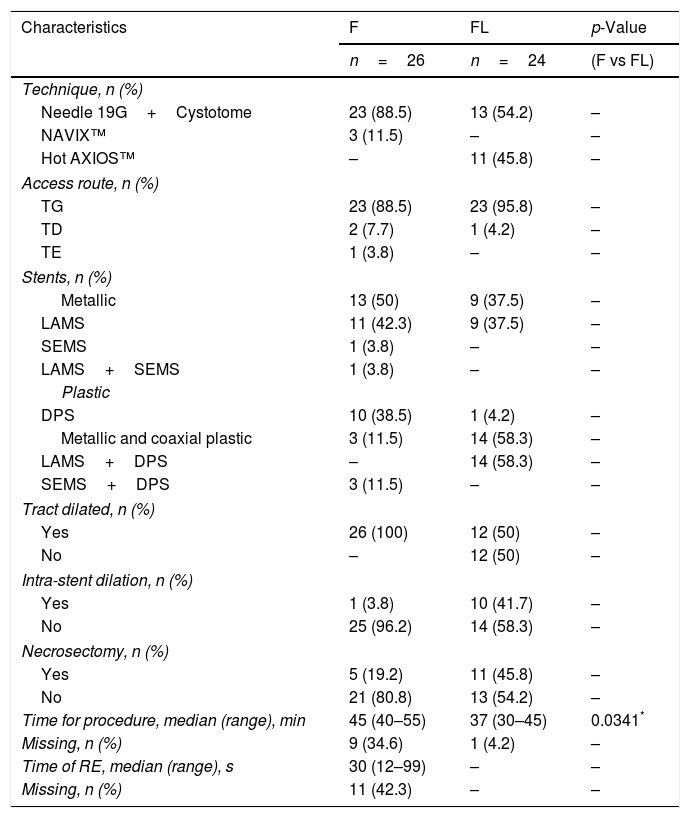
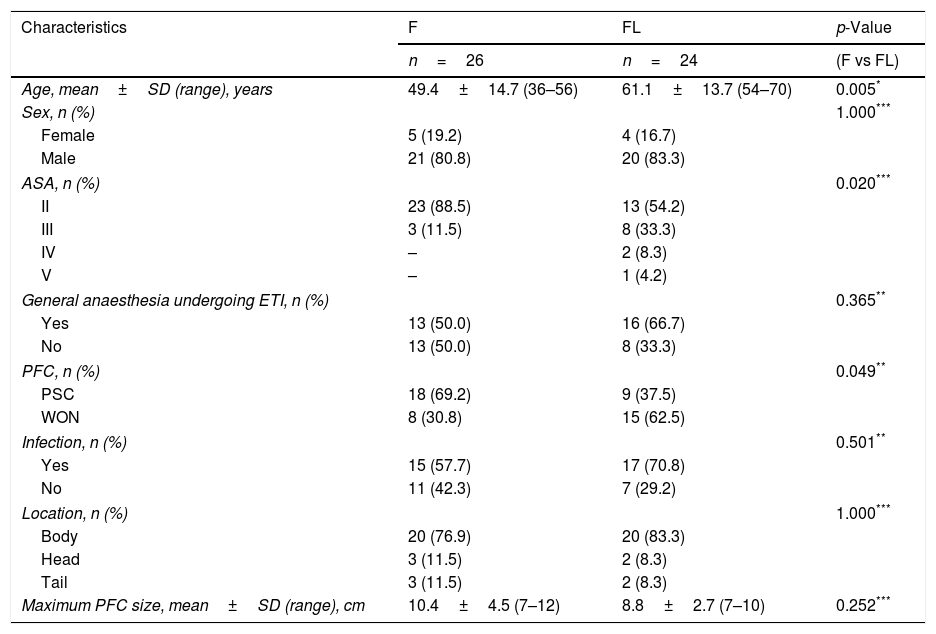
Table 1 summarizes the technical details (access route, type of device, stents and procedure duration time). Both groups were heterogeneous, and demographic data (age, sex, type of anaesthesia), characteristics, characteristics of the PFCs (type, infection, location, size) are detailed in Table 2.
Characteristics of the procedure.
| Characteristics | F | FL | p-Value |
|---|---|---|---|
| n=26 | n=24 | (F vs FL) | |
| Technique, n (%) | |||
| Needle 19G+Cystotome | 23 (88.5) | 13 (54.2) | – |
| NAVIX™ | 3 (11.5) | – | – |
| Hot AXIOS™ | – | 11 (45.8) | – |
| Access route, n (%) | |||
| TG | 23 (88.5) | 23 (95.8) | – |
| TD | 2 (7.7) | 1 (4.2) | – |
| TE | 1 (3.8) | – | – |
| Stents, n (%) | |||
| Metallic | 13 (50) | 9 (37.5) | – |
| LAMS | 11 (42.3) | 9 (37.5) | – |
| SEMS | 1 (3.8) | – | – |
| LAMS+SEMS | 1 (3.8) | – | – |
| Plastic | |||
| DPS | 10 (38.5) | 1 (4.2) | – |
| Metallic and coaxial plastic | 3 (11.5) | 14 (58.3) | – |
| LAMS+DPS | – | 14 (58.3) | – |
| SEMS+DPS | 3 (11.5) | – | – |
| Tract dilated, n (%) | |||
| Yes | 26 (100) | 12 (50) | – |
| No | – | 12 (50) | – |
| Intra-stent dilation, n (%) | |||
| Yes | 1 (3.8) | 10 (41.7) | – |
| No | 25 (96.2) | 14 (58.3) | – |
| Necrosectomy, n (%) | |||
| Yes | 5 (19.2) | 11 (45.8) | – |
| No | 21 (80.8) | 13 (54.2) | – |
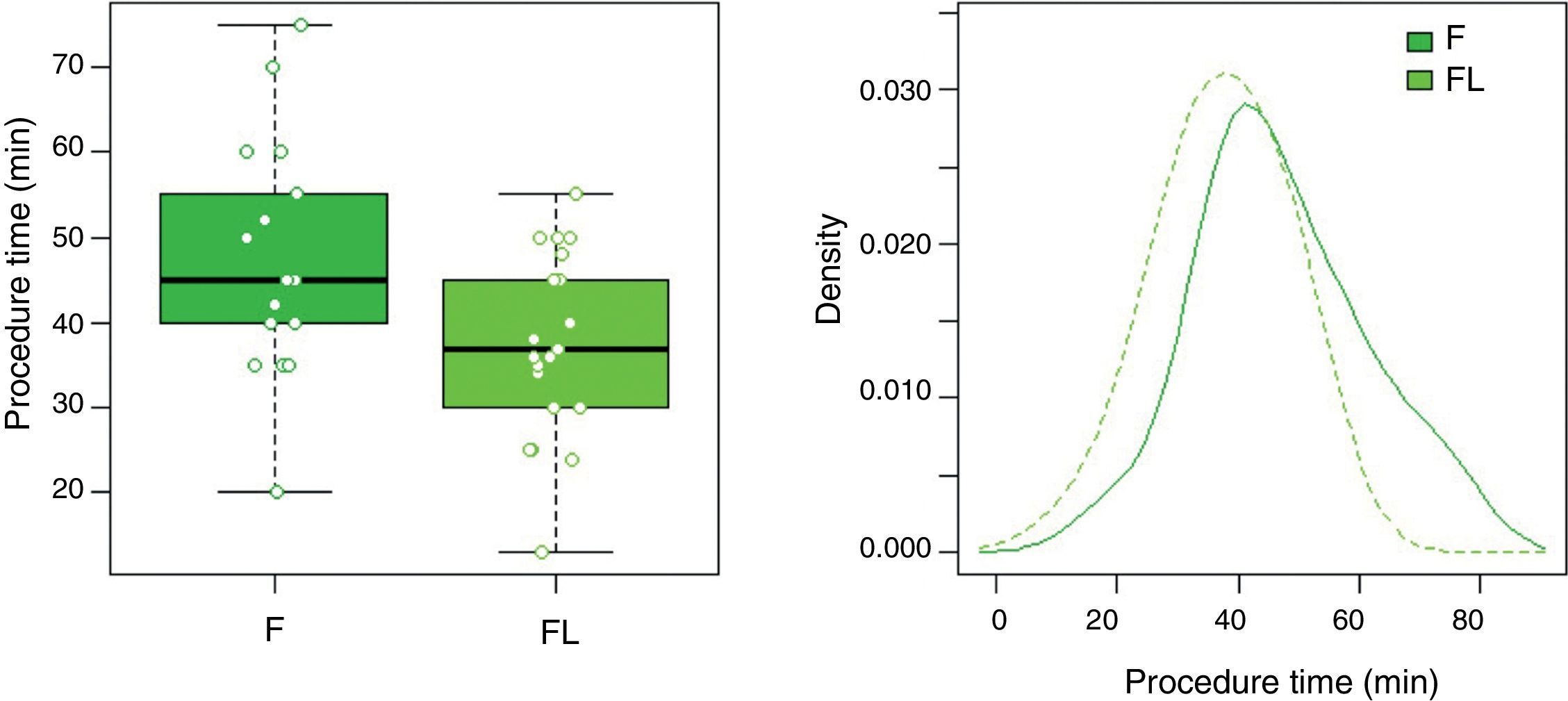
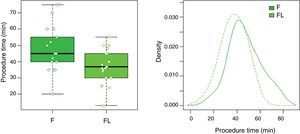
| Time for procedure, median (range), min | 45 (40–55) | 37 (30–45) | 0.0341* |
| Missing, n (%) | 9 (34.6) | 1 (4.2) | – |
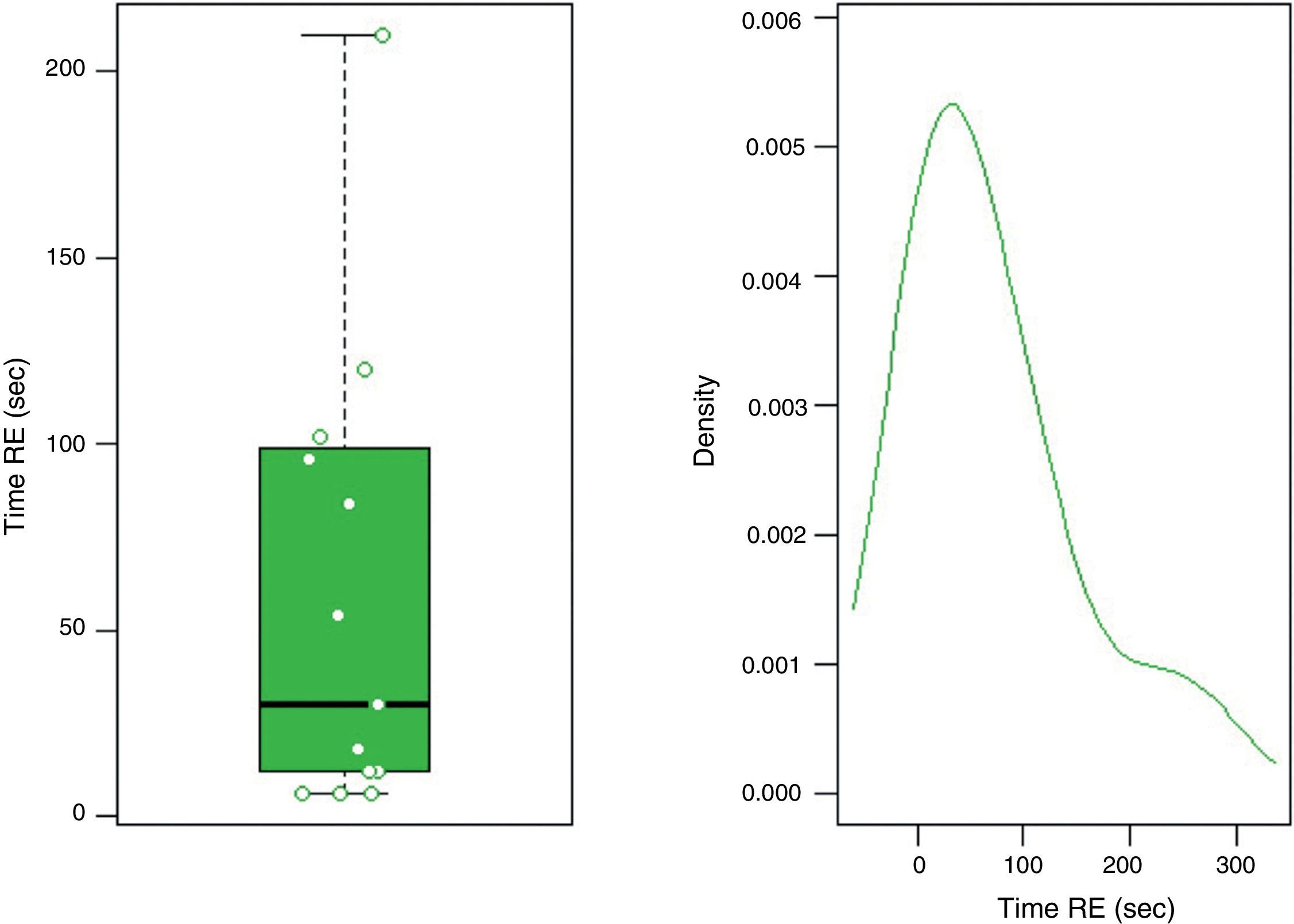
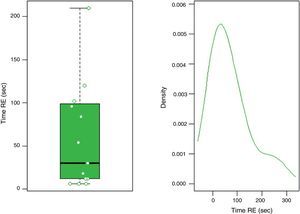
| Time of RE, median (range), s | 30 (12–99) | – | – |
| Missing, n (%) | 11 (42.3) | – | – |
F, fluoroscopy; FL, fluoroless; TG, transgastric; TD, transduodenal; TE, transesophageal; DPS, double pigtail stent; LAMS, lumen-apposing metal stent; SEMS, self-expanding metal stent; RE, radiation exposure.
Characteristics of the study participants and the PFCs.
| Characteristics | F | FL | p-Value |
|---|---|---|---|
| n=26 | n=24 | (F vs FL) | |
| Age, mean±SD (range), years | 49.4±14.7 (36–56) | 61.1±13.7 (54–70) | 0.005* |
| Sex, n (%) | 1.000*** | ||
| Female | 5 (19.2) | 4 (16.7) | |
| Male | 21 (80.8) | 20 (83.3) | |
| ASA, n (%) | 0.020*** | ||
| II | 23 (88.5) | 13 (54.2) | |
| III | 3 (11.5) | 8 (33.3) | |
| IV | – | 2 (8.3) | |
| V | – | 1 (4.2) | |
| General anaesthesia undergoing ETI, n (%) | 0.365** | ||
| Yes | 13 (50.0) | 16 (66.7) | |
| No | 13 (50.0) | 8 (33.3) | |
| PFC, n (%) | 0.049** | ||
| PSC | 18 (69.2) | 9 (37.5) | |
| WON | 8 (30.8) | 15 (62.5) | |
| Infection, n (%) | 0.501** | ||
| Yes | 15 (57.7) | 17 (70.8) | |
| No | 11 (42.3) | 7 (29.2) | |
| Location, n (%) | 1.000*** | ||
| Body | 20 (76.9) | 20 (83.3) | |
| Head | 3 (11.5) | 2 (8.3) | |
| Tail | 3 (11.5) | 2 (8.3) | |
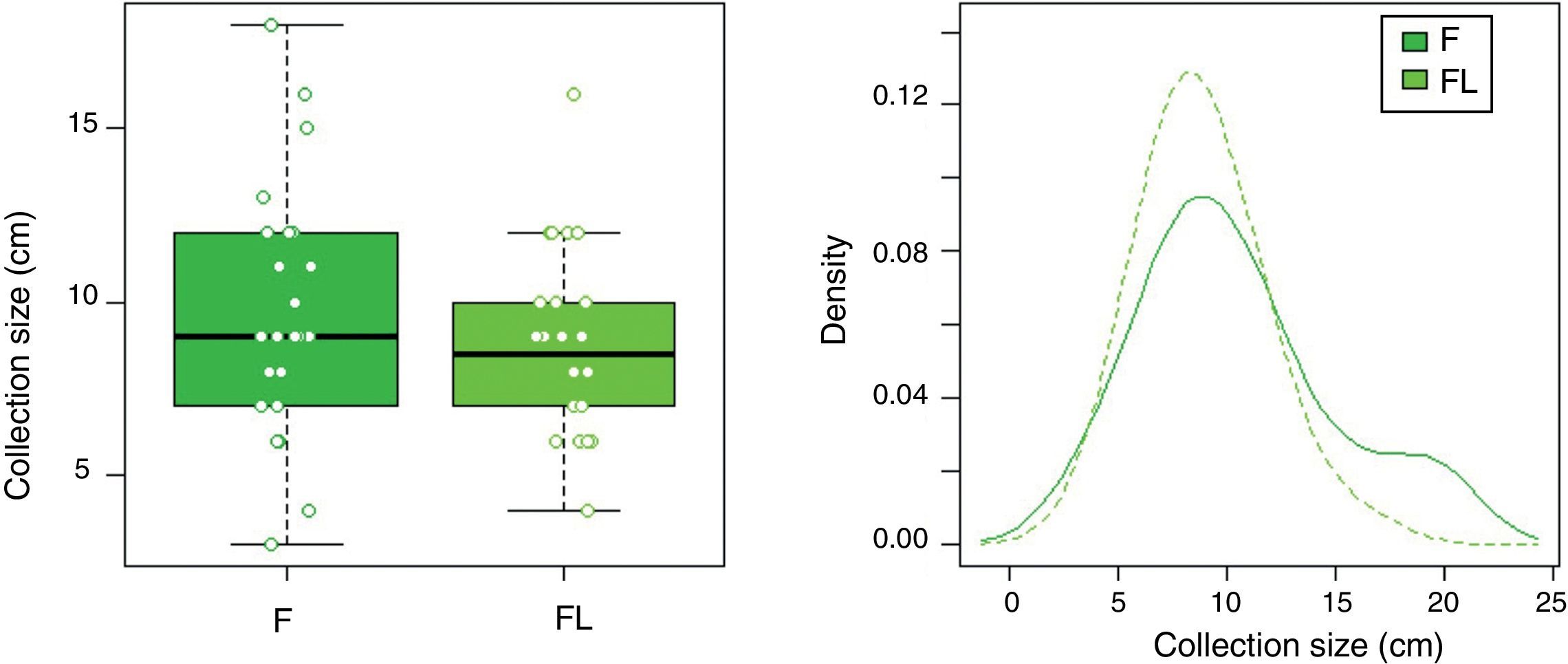
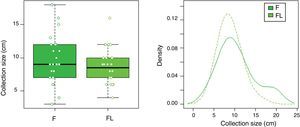
| Maximum PFC size, mean±SD (range), cm | 10.4±4.5 (7–12) | 8.8±2.7 (7–10) | 0.252*** |
PFCs, pancreatic fluid collections; F, fluoroscopy; FL, fluoroless; SD, standard deviation; ASA, American Society of Anaesthesiologists classification of physical health; ETI, endotracheal intubation; PSC, pancreatic pseudocyst; WON, walled-off pancreatic necrosis.
In group F there were larger collections that required extra time for fluid aspiration (Fig. 4). All the ostomies (tract dilation) were dilated in group F, and only half in group FL. Median procedure time of the first procedure (EUS-TMD with stent placement) was 45min (range 40–55) and 37min (range 30–45) in groups F and FL, respectively (Fig. 5), and this 8min margin in favour of the group FL was statistically significant (p=0.0341). The median time of RE was 30s (range 12–99) in group F (Fig. 6). Endoscopic necrosectomy was performed in 19.2% and 45.8% of group F and FL, respectively (Table 1). All stents were easily removed after the resolution of the lesions.
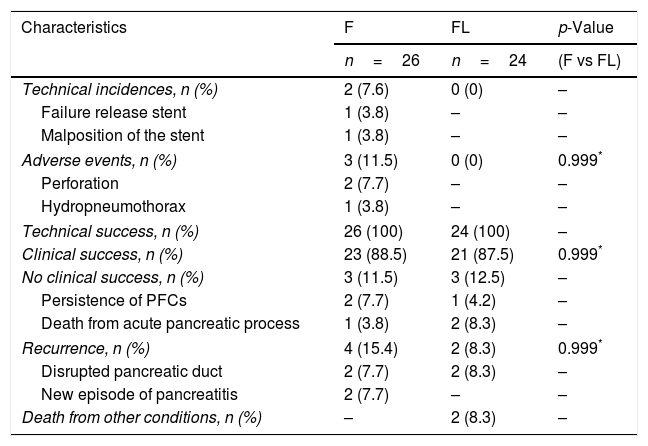
Incidences and adverse eventsTechnical incidences were 7.6% (n=2) in group F and none in group FL. One LAMS deployment failure, and one partial malposition of the inner LAMS flange in a non-adherent pancreatic collection at the gastric wall were detected.
Intra-procedure AEs were 11.5% (n=3) in group F and none in group FL. There were two cases of perforation with pneumoperitoneum, both with conservative management. Lastly, a case of hydropneumothorax that required pleural drainage occurred. All incidences showed satisfactory clinical evolution and none required surgery. A logistic model of AEs adjusted by ASA and ETI was made and no significant differences were found. Also, in the sub-analysis of AEs in terms of different types of stents and Hot AXIOS system, no significant differences were found. There were other incidences and AEs occurring up to 14 days post-procedure, but none of them was related to the initial procedure.
Outcomes and follow-upAll stents were successfully positioned in all cases, which means a technical success rate of 100% was achieved in both groups, with similar clinical success in both groups: F 88.5% (n=23) and FL 87.5% (n=21). In the group F there were two patients with persistent PFCs requiring a second drainage. One of them suffered a fatal evolution of a severe pancreatitis. In the group FL there was one case of collection infection due to external migration of the stent, requiring a second drainage, and there were two deaths from complications related to a severe pancreatic process. There was no procedure-related mortality in either group.
Six patients presented recurrence: in group F (n=4) two with suspected disrupted pancreatic duct, and two secondary to a new episode of acute pancreatitis. In group FL, (n=2) all involved suspected disrupted pancreatic duct. Incidences, AEs, outcomes, and follow-up are presented in Table 3.
Incidences, adverse events, outcomes, and follow-up.
| Characteristics | F | FL | p-Value |
|---|---|---|---|
| n=26 | n=24 | (F vs FL) | |
| Technical incidences, n (%) | 2 (7.6) | 0 (0) | – |
| Failure release stent | 1 (3.8) | – | – |
| Malposition of the stent | 1 (3.8) | – | – |
| Adverse events, n (%) | 3 (11.5) | 0 (0) | 0.999* |
| Perforation | 2 (7.7) | – | – |
| Hydropneumothorax | 1 (3.8) | – | – |
| Technical success, n (%) | 26 (100) | 24 (100) | – |
| Clinical success, n (%) | 23 (88.5) | 21 (87.5) | 0.999* |
| No clinical success, n (%) | 3 (11.5) | 3 (12.5) | – |
| Persistence of PFCs | 2 (7.7) | 1 (4.2) | – |
| Death from acute pancreatic process | 1 (3.8) | 2 (8.3) | – |
| Recurrence, n (%) | 4 (15.4) | 2 (8.3) | 0.999* |
| Disrupted pancreatic duct | 2 (7.7) | 2 (8.3) | – |
| New episode of pancreatitis | 2 (7.7) | – | – |
| Death from other conditions, n (%) | – | 2 (8.3) | – |
F, fluoroscopy; FL, fluoroless; PFCs, pancreatic fluid collections.
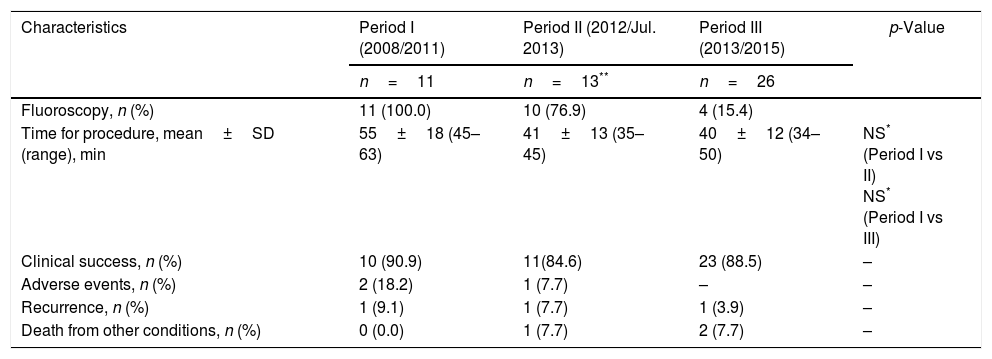
We analyzed comparative procedure time periods since the introduction of the Hot AXIOS system in our centre. No significant differences were found, although a trend towards shorter RE exposure, procedure time and AEs was observed. This is summarized in Table 4.
Comparative analysis of the use of fluoroscopy, procedure time, evolution, and adverse events by periods of study.
| Characteristics | Period I (2008/2011) | Period II (2012/Jul. 2013) | Period III (2013/2015) | p-Value |
|---|---|---|---|---|
| n=11 | n=13** | n=26 | ||
| Fluoroscopy, n (%) | 11 (100.0) | 10 (76.9) | 4 (15.4) | |
| Time for procedure, mean±SD (range), min | 55±18 (45–63) | 41±13 (35–45) | 40±12 (34–50) | NS* (Period I vs II) NS* (Period I vs III) |
| Clinical success, n (%) | 10 (90.9) | 11(84.6) | 23 (88.5) | – |
| Adverse events, n (%) | 2 (18.2) | 1 (7.7) | – | – |
| Recurrence, n (%) | 1 (9.1) | 1 (7.7) | 1 (3.9) | – |
| Death from other conditions, n (%) | 0 (0.0) | 1 (7.7) | 2 (7.7) | – |
SD, standard deviation; NS, not significant.
This is a pioneering study to specifically evaluate a comparison between the uses or not to fluoroscopy guidance in patients undergoing EUS-TMD of PFCs. This study analyzed general outcomes of this type of drainage in terms of efficacy and safety and others important aspect related to procedure, founded that Fluoroless not involve more technical incidences or intra-procedure AEs, and technical and clinical success was similar in the two groups.
Until now, only small studies describing EUS-TMD in patients with PFCs without fluoroscopy guidance have been published. One of these, by Rana et al., described 20 cases of EUS-TMD of non-bulging WON, and another by Seicean et al. reported 24 cases.19,20 Both studies described high technical and clinical success with a low rate of AEs related to the procedure.
The present study is the first to offer a comparison of fluoroscopy-assisted vs fluoroless EUS-TMD of PFCs, in one centre. The two groups had the same technical success and similar clinical success. In addition, technical incidences, AEs intra-procedure, and the procedure time were lower in the group FL. It is important to note that the groups were heterogeneous, especially concerning the WON. It has been suggested that the use of metal stents in this setting may result in better outcomes and lower cost.4–10 In this sense, the group FL had more WON cases and more infected PFCs cases, but no significant differences were found. Regarding the type of stents, in the group FL more metal stents were used, as LAMS simplify and reduce the number of steps, consequently improving technical aspects of the intervention.
Therefore, we did a comparative analysis from the introduction of the Hot AXIOS system in our centre, and we found a trend towards shorter procedure time, fluoroscopy use (RE) and AEs. For this reason, as in the previously mentioned reports, the general outcomes were satisfactory, and in our opinion after an initial learning-curve to insure sufficient experience, the direct use of the Hot AXIOS system can reduce the procedure time and the risk of procedure-related incidences. As well, fluoroscopy guidance can be avoided.
Regarding the AEs, we took in to consideration only the intra-procedure and immediately post-procedure times (<24h) that may occur in the technical process where fluoroscopy is involved and which can influence its development. Most post-procedure AEs in both groups were similar to other studies, such as infection and bleeding.6–11 As reported in a previous report by our group in Gornals et al., placing a coaxial plastic stent into the LAMS can help to reduce the risk of AEs such as external migration and occlusion of the stent, as well as the bleeding caused by the trauma of the distal flange.13 Therefore, in our study it is important to note that this technical strategy (LAMS plus coaxial plastic stent) was applied more in the group FL, yielding fewer AEs without lengthening the procedure time.
This current study has several strengths and limitations. Firstly, it is a prospective inclusion with a retrospective analysis, and the use of fluoroscopy guidance was uncontrolled, basically depending on the fluoroscopy room availability. Secondly, this study reports the endoscopic experience of a single centre with one single operator; it may be difficult to generalize these results to other centres. However, the fact that all procedures were carried out by the same interventional endoscopist means that the procedural technique and surveillance were more standardized. Thirdly, initially, during the process of learning curve, the traditional technique with fluoroscopy was predominantly used. Later, with more experience in EUS-TMD, and with the introduction of new dedicated devices, the FL technique was introduced. Finally, we are conscious that groups were heterogeneous, in this sense Group FL included cases of EUS-TMD using the Hot AXIOS system, and in group F none cases were included. This fact can carry out a technical bias and is the major weakness of this comparative study since does not allow to conclude that the FL is comparable to the usual technique F when another type of stents are used. In addition, the significant reduction in procedure time of the FL group could be related to the device Hot AXIOS used rather than to the use of fluoroscopy. For these reasons, more data are required to validate this preliminary finding.
In conclusion, the use of fluoroscopy guidance is an important tool in the EUS-TD of PFCs because it affords us an additional image during the procedure. Whether or not we use it does not seem to affect the technical and clinical success. Fluoroless guidance does not appear to involve more technical incidences or intra-procedure AEs. As well, there is no need for a fluoroscopy room, allowing the possibility of performing the procedure bedside, which is of particular utility for critically ill patients.
Conflict of interestCF Consiglieri was supported by a donation from Boston Scientific to our investigational Unit (Bellvitge Biomedical Research Institute). This funding source had no role in the design, practice, or analysis of this study. The rest of the authors declare no conflict of interest for this article.
The authors are grateful to Encarna Garcia-Recio and Lia-Tamar Sánchez-Salido (endoscopy registered nurses) for their assistance.