Gastrointestinal (GI) complications are common side effects related to non-steroidal anti-inflammatory drugs (NSAID) and low-dose aspirin (LDA) use. The guidelines to prevent GI complications establish that patients at high risk should receive gastroprotection. However, different reports have suggested that these strategies are not greatly executed. To determine the prevalence of use of preventive strategies to reduce GI complications in NSAID and/or LDA users in primary care in Spain, we performed an observational, cross-sectional, multicentre study in which primary care physicians from Spain participated. From January 2009 to May 2009, physicians collected demographic, clinical and treatment data from the last visit in 2008 of the first 5 consecutive patients who met the selection criteria. A multivariate logistic regression was carried out to identify independent predictors of the preventive strategies used.
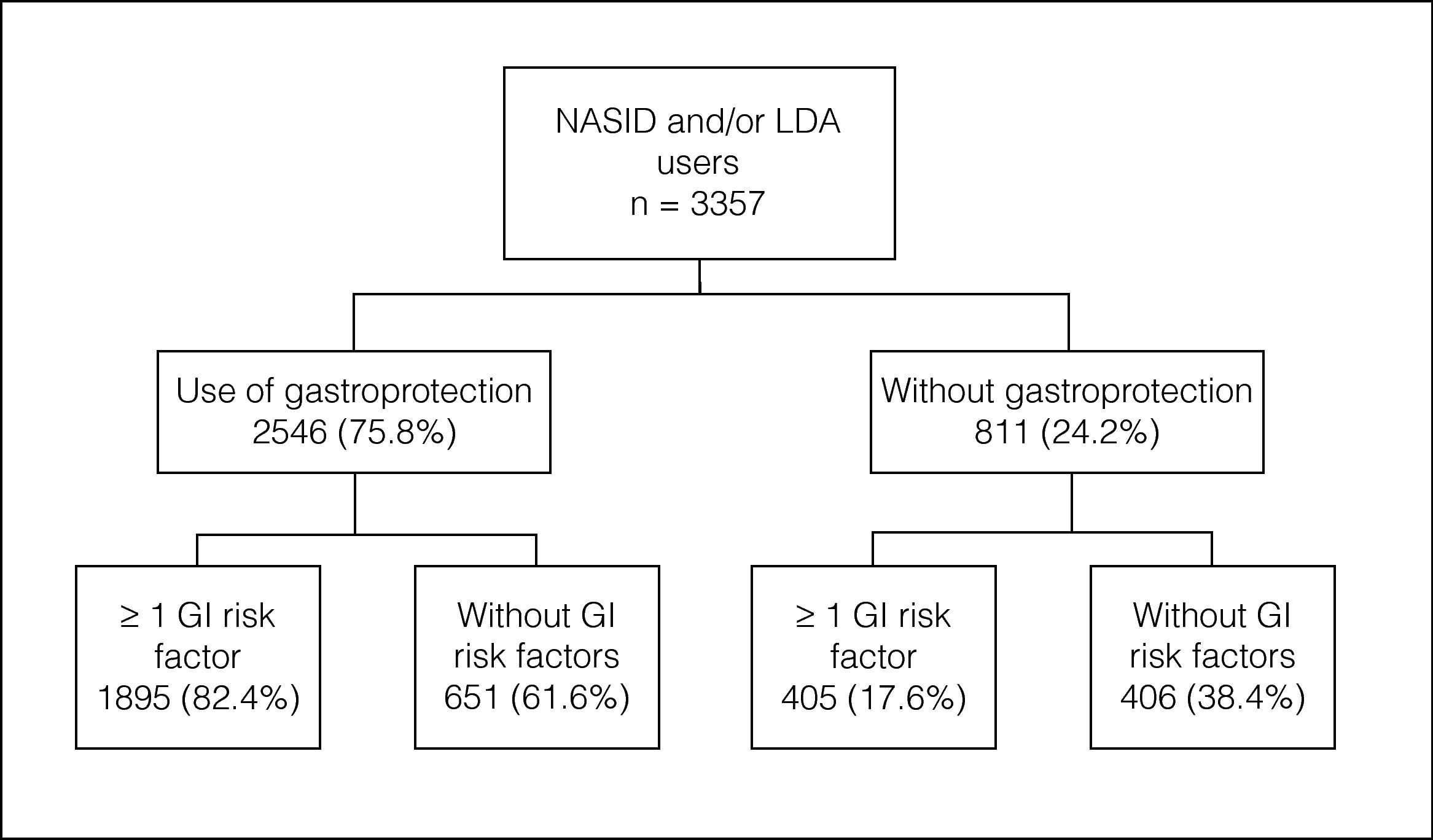
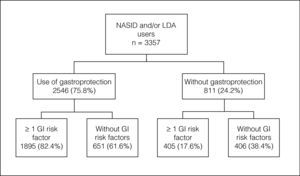
A total of 713 primary care physicians included 3357 patients: 68% NSAID users, 19.1% LDA users and 12.9% NSAID/LDA users. 31.5% of patients did not have a risk factor for GI complications, 25.6% had one risk factor and 42.9% had 2 or more risk factors. The overall prevalence of preventive strategy use was 75.8%. The prevalence of gastroprotection use increased with the number of risk factors.
The underutilization of gastroprotection in at-risk patients treated with NSAIDs is low and not as marked as those previously reported at the primary care level in other countries. We also found high rates of gastroprotection use in LDA users.
Las complicaciones gastrointestinales (GI) son un efecto secundario habitual relacionado con el uso de antiinflamatorios no esteroideos (AINE) y aspirina a dosis bajas (ADB). Las directrices para prevenir las complicaciones GI establecen que los pacientes con elevado riesgo deben recibir alguna forma de protección gástrica. Sin embargo, diversos informes sugieren que dichas estrategias no se llevan a cabo. Para determinar la prevalencia en la atención primaria española del uso de estrategias preventivas para reducir las complicaciones GI en los pacientes a los que se les ha prescrito AINE y ADB, se realizó un estudio observacional, transversal y multicéntrico en el que que participaron médicos de atención primaria. Desde enero a mayo de 2009, los médicos recogieron datos demográficos, clínicos y sobre tratamiento procedentes de la última visita en 2008 de los 5 primeros pacientes consecutivos que cumplían los criterios de selección. Se llevó a cabo una regresión logística multivariante para identificar los predictores independientes de las estrategias preventivas utilizadas.
Un total de 713 médicos de atención primaria incluyeron a 3357 pacientes: el 68% tomaba AINE, el 19,1% ADB y el 12,9% recibia AINE y ADB. El 31,5% de los pacientes no presentaba factores de riesgo de complicaciones GI, el 25,6% tenia uno y el 42,9% 2 o más factores de riesgo. La prevalencia total de uso de estrategias de prevención fue del 75,8%. La prevalencia del uso de gastroprotección incrementó con el número de factores de riesgo.
La infrautilización de protección GI en pacientes con alto riesgo tratados con AINE es baja y no es tan marcada como la que se notifica en la atención primaria de otros países. También se observó un uso elevado de gastroprotección en los pacientes que toman ADB.
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most frequently prescribed drugs worldwide due to their anti-inflammatory, analgesic and antipyretic effects. Each year, more than 25 million prescriptions for NSAIDs are dispensed in Spain.1 Low-dose aspirin (LDA) (75–325mg/day) is also highly prescribed because it reduces the risk of vascular events and death in patients with coronary and cerebrovascular disease.2
Gastrointestinal (GI) complications are the most frequent side effects associated with NSAID and LDA use, which may potentially lead to patient death.3 GI complications range from the development of dyspepsia and symptoms of drug intolerance to clinically significant gastroduodenal ulcer complications such as bleeding, obstruction and perforations. Given the long-term use of NSAIDs and LDA by millions of patients, these complications have a significant impact on the population.
Risk factors for GI complications associated with NSAIDs are well defined and include a previous GI event (especially if complicated such as bleeding, perforation or obstruction), age, concomitant use of anticoagulants, corticosteroids, other NSAIDs including LDA, high-dose NSAID therapy and chronic debilitating disorders, especially cardiovascular disease.4 Based on these risk factors, several medical societies and scientific associations have issued guidelines for the prevention of GI complications in NSAID users.4–8 According to these guidelines, patients with an increased risk of GI complications should receive gastroprotection with misoprostol, proton-pump inhibitors (PPI) or high-dose histamine-2-receptor antagonists (H2RA).
Despite these recommendations, reports have suggested that protective strategies are still greatly underprescribed in NSAID users, and that this also occurs in patients at the highest risk of NSAID-related ulcers and ulcer complications.9–11 Additionally, inappropriate use of gastroprotection also occurs in patients with no risk factors for GI complications.10,12
To date, few data are available on the actual use of protective strategies among NSAID or LDA users in daily clinical practice at primary care level in most European countries, including Spain. One nationwide study carried out in Spain indicated that specialists treating patients with rheumatic diseases were well informed of GI complications related to NSAID therapy13 and differences between indication and prescription of protective strategies among at-risk patients were minimal in Spain.13,14 However, this data cannot be extrapolated to clinical practice at the primary care level. Considering that primary care accounts for the majority of NSAID prescriptions, it is important to provide data on the use of gastroprotection at this care level.
Although LDA has fewer side effects than high-dose aspirin and other NSAIDs, the risk is also present,15,16 LDA prescription is progressively increasing as a result of increasing evidence on the benefits in both primary and secondary treatment strategies for cardiovascular disease. Many of these patients also take concomitant NSAIDs. Moreover, the risk of bleeding ulcers in patients taking NSAIDs and LDA is almost two times higher than of those taking either drug alone.16
Therefore, the purpose of this study was to determine the prevalence of use of preventive strategies to reduce GI complications in NSAID and/or LDA users in primary care in Spain. We also evaluated the relationship between risk factors for GI complications and the likelihood of receiving adequate gastroprotection.
Materials and methodsThe study was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was approved by the Regional Institutional Review Board of Aragón, Spain. We performed an observational, cross-sectional, multicentre study in which primary care physicians from all over Spain participated. Primary care physicians were randomly selected from a nationwide survey of primary care physicians. Physicians selected should include the first five consecutive patients who met the selection criteria: had completed at least one visit in 2008 in which they were taking or had been prescribed an NSAID and/or LDA. During an observational period from January 2009 to May 2009, physicians collected demographic, clinical and treatment data of the last visit in 2008 from the medical records. These data were collected using a case report form specifically designed for this study. All patients provided written informed consent to participate in the study.
For continuous variables, mean±standard deviation (SD) are presented and for categorical variables, absolute and relative frequencies were obtained. A multivariate logistic regression was carried out to identify independent predictors of the preventive strategies used. Demographic, clinical and treatment variables were included in the analysis. Risk factors of GI complications included were elderly age (≥65 years), prior history of peptic ulcer (documented by endoscopy or radiology), prior history of gastrointestinal bleeding, prescription of high-dose NSAID therapy (using at least two NSAIDs including LDA),16 concomitant use of oral corticosteroids, concomitant use of antithrombotic treatment (including other anticoagulants or antiplatelet agents) and severe concomitant disease. Information concerning Helicobacter pylori infection was collected but not considered among the risk factors of GI complications since it was thought that the number of patients who would have undergone any test to detect the infection would be very low. Sample size was estimated using data from a previous study carried out in Spain which included 200 NSAID users.17 In that study, the proportion of patients not receiving gastroprotection was 40%.
All the analyses were performed using SPSS version 16.0 or SAS version 9.1.
ResultsDemographic and clinical characteristics of the study populationA total of 713 primary care physicians included 3504 patients in the study, 3357 of whom met the inclusion criteria (had completed at least one visit in 2008 in which they were taking or had been prescribed an NSAID and/or LDA) were evaluated. Of these, 2282 (68%) were NSAID users, 641 (19.1%) LDA users and 434 (12.9%) NSAID and LDA users. Musculoskeletal and connective tissue disorders were the main reason for prescribing NSAID (n=2108, 79.1%). The second reason were injuries, poisoning and therapeutic procedures complications (n=235, 8.7%). A total of 35.9% of patients (n=1113) received long-term therapy with NSAIDs (more than 1 month) with an average duration of 23 months. LDA was mainly prescribed for the treatment of cardiac disorders (45.3%) and LDA was used as prophylaxis in 305 patients (28.4%). In this group, 92.9% of patients (n=999) received long-term therapy with LDA.
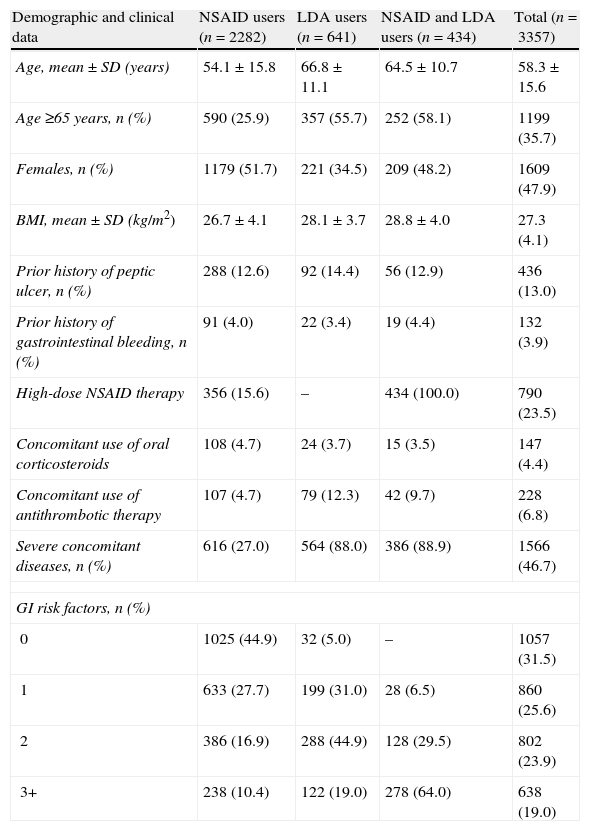
Table 1 summarises the main demographic and clinical characteristics of the study population. Mean age was 58.3±15.6 years and 47.9% of patients were women. The two most prevalent risk factors for GI complications were age (>65 years) and the presence of severe concomitant diseases. As expected, the number of patients who presented a prior history of cardiovascular disease was considerably high among LDA users: 87.5% of LDA users and 88.5% of NSAID and LDA users. The prevalence of other individual risk factors for GI complications was relatively low. In the whole population, 31.5% of patients did not have a risk factor for GI complications, 25.6% had one risk factor and 42.9% had two or more risk factors. The proportion of patients without risk factors was higher in the group of patients treated only with NSAIDs (44.9%) than in LDA users (31%).
Demographic and clinical characteristics of patients included in the study.
| Demographic and clinical data | NSAID users (n=2282) | LDA users (n=641) | NSAID and LDA users (n=434) | Total (n=3357) |
| Age, mean±SD (years) | 54.1±15.8 | 66.8±11.1 | 64.5±10.7 | 58.3±15.6 |
| Age ≥65 years, n (%) | 590 (25.9) | 357 (55.7) | 252 (58.1) | 1199 (35.7) |
| Females, n (%) | 1179 (51.7) | 221 (34.5) | 209 (48.2) | 1609 (47.9) |
| BMI, mean±SD (kg/m2) | 26.7±4.1 | 28.1±3.7 | 28.8±4.0 | 27.3 (4.1) |
| Prior history of peptic ulcer, n (%) | 288 (12.6) | 92 (14.4) | 56 (12.9) | 436 (13.0) |
| Prior history of gastrointestinal bleeding, n (%) | 91 (4.0) | 22 (3.4) | 19 (4.4) | 132 (3.9) |
| High-dose NSAID therapy | 356 (15.6) | – | 434 (100.0) | 790 (23.5) |
| Concomitant use of oral corticosteroids | 108 (4.7) | 24 (3.7) | 15 (3.5) | 147 (4.4) |
| Concomitant use of antithrombotic therapy | 107 (4.7) | 79 (12.3) | 42 (9.7) | 228 (6.8) |
| Severe concomitant diseases, n (%) | 616 (27.0) | 564 (88.0) | 386 (88.9) | 1566 (46.7) |
| GI risk factors, n (%) | ||||
| 0 | 1025 (44.9) | 32 (5.0) | – | 1057 (31.5) |
| 1 | 633 (27.7) | 199 (31.0) | 28 (6.5) | 860 (25.6) |
| 2 | 386 (16.9) | 288 (44.9) | 128 (29.5) | 802 (23.9) |
| 3+ | 238 (10.4) | 122 (19.0) | 278 (64.0) | 638 (19.0) |
Helicobacter pylori infection status was unknown in 86.9% of patients; only 7.1% of patients had a positive test result. Most patients (64.6%) were non-smokers. With regard to alcohol consumption, 35.6% of patients reported drinking 11 units a week.
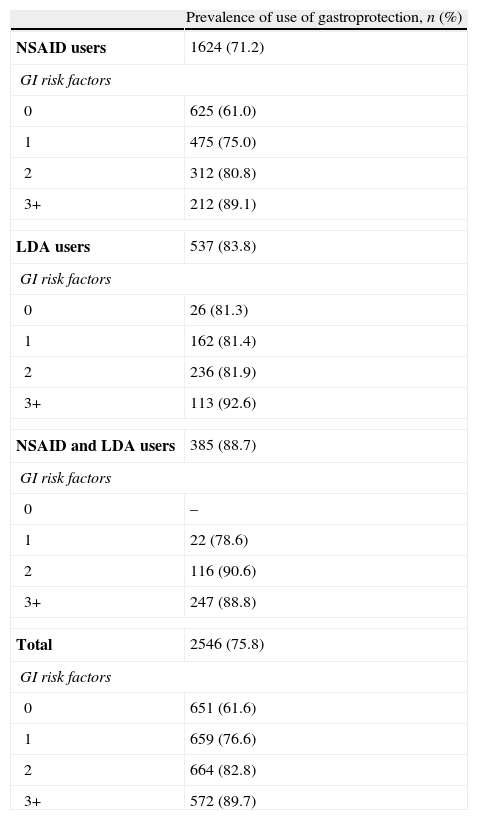
Gastroprotection use and risk factors of gastrointestinal complicationsThe overall prevalence of preventive strategy use in the study population was 75.8% (n=2546) (Fig. 1). Those patients taking concomitant NSAIDs and LDA showed the highest prevalence of gastroprotection use (88.7%), while those patients taking only NSAIDs had the lowest prevalence (71.2%). Table 2 summarises the prevalence of prophylactic therapy use according to the number of risk factors for GI complications. In the group of patients without risk factors for GI complications, the prevention rates were high (61.6% for the whole population). The prevalence of gastroprotection use also increased with the number of risk factors. Thus, in the group of patients with the highest number of risk factors (+3) practically all patients used a preventive strategy. Conversely, 17.6% (n=405) of at-risk NSAID and/or LDA users (patients with at least one risk factor for GI complications) did not receive gastroprotective agents (20.5% of NSAID users, 16.1% of LDA users and 11.3% of NSAID+LDA users). We found statistically significant differences in the prevalence of use of gastroprotection according to the number of GI risk factors between NSAID and LDA users (p<0.05). Thus, the prevalence of gastroprotection was slightly higher among LDA users.
Prevalence of preventive strategy use according to the number of risk factors for GI complications.
| Prevalence of use of gastroprotection, n (%) | |
| NSAID users | 1624 (71.2) |
| GI risk factors | |
| 0 | 625 (61.0) |
| 1 | 475 (75.0) |
| 2 | 312 (80.8) |
| 3+ | 212 (89.1) |
| LDA users | 537 (83.8) |
| GI risk factors | |
| 0 | 26 (81.3) |
| 1 | 162 (81.4) |
| 2 | 236 (81.9) |
| 3+ | 113 (92.6) |
| NSAID and LDA users | 385 (88.7) |
| GI risk factors | |
| 0 | – |
| 1 | 22 (78.6) |
| 2 | 116 (90.6) |
| 3+ | 247 (88.8) |
| Total | 2546 (75.8) |
| GI risk factors | |
| 0 | 651 (61.6) |
| 1 | 659 (76.6) |
| 2 | 664 (82.8) |
| 3+ | 572 (89.7) |
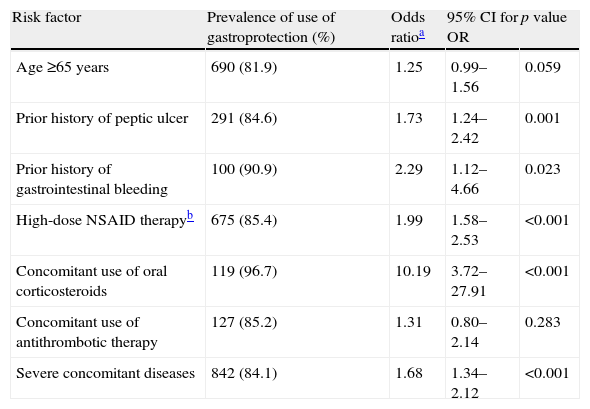
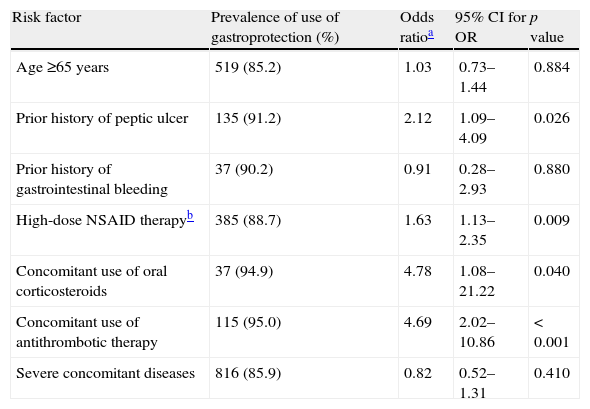
Tables 3 and 4 present the prevalence of specific risk factors with regard to the use of gastroprotection, and the association between individual risk factors and the probability of receiving this treatment in NSAID and LDA users, respectively. Among NSAID users, gastroprotection was significantly associated with prior history of peptic ulcer, prior history of gastrointestinal bleeding, high-dose NSAID therapy, concomitant use of oral corticosteroids and presence of severe concomitant diseases (Table 3). The significant variables in the logistic regression for using a preventive strategy among LDA users were prior history of peptic ulcer, high-dose NSAID therapy and concomitant use of oral corticosteroids and antithrombotics (Table 4).
Association between preventive strategy use and risk factors in NSAID users (n=2716).
| Risk factor | Prevalence of use of gastroprotection (%) | Odds ratioa | 95% CI for OR | p value |
| Age ≥65 years | 690 (81.9) | 1.25 | 0.99–1.56 | 0.059 |
| Prior history of peptic ulcer | 291 (84.6) | 1.73 | 1.24–2.42 | 0.001 |
| Prior history of gastrointestinal bleeding | 100 (90.9) | 2.29 | 1.12–4.66 | 0.023 |
| High-dose NSAID therapyb | 675 (85.4) | 1.99 | 1.58–2.53 | <0.001 |
| Concomitant use of oral corticosteroids | 119 (96.7) | 10.19 | 3.72–27.91 | <0.001 |
| Concomitant use of antithrombotic therapy | 127 (85.2) | 1.31 | 0.80–2.14 | 0.283 |
| Severe concomitant diseases | 842 (84.1) | 1.68 | 1.34–2.12 | <0.001 |
Association between preventive strategy use and risk factors in LDA users (n=1075).
| Risk factor | Prevalence of use of gastroprotection (%) | Odds ratioa | 95% CI for OR | p value |
| Age ≥65 years | 519 (85.2) | 1.03 | 0.73–1.44 | 0.884 |
| Prior history of peptic ulcer | 135 (91.2) | 2.12 | 1.09–4.09 | 0.026 |
| Prior history of gastrointestinal bleeding | 37 (90.2) | 0.91 | 0.28–2.93 | 0.880 |
| High-dose NSAID therapyb | 385 (88.7) | 1.63 | 1.13–2.35 | 0.009 |
| Concomitant use of oral corticosteroids | 37 (94.9) | 4.78 | 1.08–21.22 | 0.040 |
| Concomitant use of antithrombotic therapy | 115 (95.0) | 4.69 | 2.02–10.86 | < 0.001 |
| Severe concomitant diseases | 816 (85.9) | 0.82 | 0.52–1.31 | 0.410 |
The most commonly used strategy for prevention of GI complications was the prescription of PPI. Thus, PPIs were used by almost all patients who received gastroprotective therapy (99.6%). In contrast, H2RA only accounted for 2% of prescriptions. Approximately 50% of the gastroprotection prescriptions were for long-term therapy (>1 month).
DiscussionPreventive measures have been reported to be underused in patients treated with NSAIDs with one or more risk factors of GI complications9,11,18 However, we observed a high prevalence of preventive strategy use among NSAID and/or LDA users in primary care. The study also revealed that such prevalence was especially high in at-risk patients, with one or more risk factors for GI complications. Moreover, the prescription of gastroprotection in patients treated with NSAIDs or LDA without any other risk factor for GI complications was also high.
Medical practice differences between countries, such as access to the health system, doctor awareness of side effects, drug policies, drug price and over-the-counter NSAIDs, among other factors, should explain the results obtained in comparison with previous studies.9,11,19
Nonetheless, our results concur with other studies carried out in the same country but under a different perspective which showed high levels of gastroprotective prescription.13,19–21 One of these studies showed that specialists were aware of the risk factors and the current prevention strategies but overestimated the risk of GI complications with NSAIDs.20 In that study, PPIs were co-prescribed with NSAIDs in >80% of patients with and without risk factors. Moreover, that study suggested that a high proportion of specialists were well informed about the latest advances in the NSAID field and implemented appropriate prevention strategies in at-risk patients. Similar results were found in a study carried out among specialists in Spain.16 The GAP study indicated a high rate of gastroprotective prescription among specialists in patients with GI risk, which was especially high in patients with prior history of ulcer or gastrointestinal bleeding, use of more than one NSAID and concomitant therapy with corticosteroids.16
More than half of our population presented one or more risk factors for gastrointestinal complications and, unlike other studies,19–21 we found a positive correlation between the number of risk factors and the rate of gastroprotection use, indicating better awareness of the appropriate indication of concomitant gastroprotective therapy, according to guidelines. Similar results were found in a study carried out in the Netherlands using a primary care data base.22
Prophylactic use of gastroprotection was significantly associated with prior history of peptic ulcer, prior history of gastrointestinal bleeding, high-dose NSAID therapy, concomitant use of oral corticosteroids and the presence of severe concomitant diseases. A small but relevant proportion of at-risk NSAID users (17.6%) did not use gastroprotection, which suggests that educational programmes should continue to be offered to reduce this gap.
Very few studies have reported concomitant gastroprotective therapy in LDA users, and we are not aware of any at the primary care level. The prevalence of gastroprotection use was slightly higher in LDA users than in NSAID users. Despite the fact that LDA is associated with a lower GI risk than NSAIDs, cardiovascular co-morbidity in LDA users and the need for long-term treatment are important risk factors for GI bleeding. Thus, as shown in our study, mostly NSAID users received short-term therapy, whereas LDA users received mainly long-term therapy. In any case, high-dose NSAID therapy is significantly associated with the prophylactic use of gastroprotection in LDA or NSAID users.
A substantial number of patients with no risk factors receiving either NSAID or LDA treatment received gastroprotection, mainly PPI. Although PPI treatment is considered to be relatively safe,23 long-term treatment with these types of drugs has shown to be associated with a higher incidence of certain adverse effects, such as increased risk of GI infections (Campylobacter, Salmonella), pneumonia and even hip fracture.24–27 Since these associations have only been found in some studies, there is a need for further research to confirm these results. Therefore, efforts need to be made not only to increase the use of gastroprotection among patients with GI risk factors who are being treated with NSAIDs or LDA or both, but also to reduce prescription rates in those without risk factors.
Our study had some limitations. First, the information was not obtained from a database but from the records of participating physicians. Second, this study does not take into account the over-the-counter use of NSAIDs, which are among the most widely used drugs without a medical prescription; therefore, our conclusions are linked to clinical practice at the general practitioner level, but not to the general population. It can be assumed that over-the-counter NSAID use is not accompanied by concomitant gastroprotection in most cases.
In conclusion, our study indicates that the underutilization of gastroprotection in at-risk patients treated with NSAIDs is low and not as marked as those previously reported at the primary care level in other countries. These results suggest significant differences between countries. We also have shown high rates of gastroprotection use in patients treated with LDA, something that was rarely reported before at primary care level. Finally, we have also shown that patients with no risk factors treated with NSAIDs or LDA have a high prevalence of gastroprotection use, suggesting that continued education at primary care level is the key to implementing appropriate prevention strategies in at-risk patients and also to avoid high prescription rates in patients without risks.
FundingThis work was supported by Almirall S.A.
Conflict of interestDr. Angel Lanas has been advisor for companies as AstraZeneca, Pfizer and Bayer. Dr. Ma Josep Plazas is employee of Almirall. The other authors of this work declare no conflict of interest.
Ethical approvalThe study was approved by the Regional Institutional Review Board of Aragón, Spain.
The authors thank all the physicians and patients who participated in the study.