Granulocyte and monocyte adsorptive apheresis (GMA) removes neutrophils and monocytes from peripheral blood, preventing their incorporation into the inflamed tissue also influencing cytokine balance. Published therapeutic efficacy in ulcerative colitis (UC) is more consistent than in Crohn's disease (CD). We assessed clinical efficacy of GMA in UC and CD 4 weeks after last induction session, at 3 and 12 months, sustained remission and corticosteroid-free remission.
Patients and methodRetrospective observational study of UC and CD patients treated with GMA. Partial Disease Activity Index-DAIp in UC and Harvey-Bradshaw Index-HBI in CD assessed efficacy of Adacolumn® with induction and optional maintenance sessions.
ResultsWe treated 87 patients (CD-25, UC-62), 87.3% corticosteroid-dependent (CSD), 42.5% refractory/intolerant to immunomodulators. In UC, remission and response were 32.2% and 19.3% after induction, 35.5% and 6.5% at 12 weeks and 29% and 6.5% at 52 weeks. In CD, remission rates were 60%, 52% and 40% respectively. In corticosteroid-dependent and refractory or intolerant to INM patients (UC-41, CD-14), 68.3% of UC achieved remission or response after induction, 51.2% at 12 weeks and 46.3% at 52 weeks, and 62.3%, 64.3% and 42.9% in CD. Maintained remission was achieved by 66.6% in CD and 53.1% in UC. Up to 74.5% of patients required corticosteroids at some timepoint. Corticosteroid-free response/remission was 17.7% in UC and 24% in CD.
ConclusionsGMA is a good therapeutic tool for both in UC and CD patients. In corticosteroid-dependent and refractory or intolerant to INM patients it avoids biological therapy or surgery in up to 40% of them in one year.
La granulomonocitoaféresis (GMA) elimina neutrófilos y monocitos de la sangre periférica, impidiendo su incorporación al tejido inflamado y modificando el equilibrio de citoquinas. Los datos publicados de eficacia en colitis ulcerosa (CU) son más consistentes que en enfermedad de Crohn (EC). Valoramos la eficacia clínica de GMA en CU y EC a las 4 semanas tras la última sesión de inducción, a 3 y 12 meses, la remisión sostenida y remisión sin corticosteroides.
Pacientes y métodoEstudio observacional retrospectivo. Pacientes con CU y EC tratados con GMA. Para evaluar la eficacia empleamos el disease activity index partial (DAIp) en CU y el índice Harvey-Bradshaw (HBI) en EC. Empleamos columnas Adacolumn® en inducción y sesiones opcionales de mantenimiento.
ResultadosTratamos 87 pacientes (EC-25, CU-62); el 87,3% eran corticodependientes, el 42,5% refractarios/intolerantes a inmunomoduladores (INM). CU: la remisión y respuesta fueron 32,2% y 19,3% tras inducción, 35,5% y 6,5% a 12 semanas y 29% y 6,5% a 52 semanas. EC: la remisión fue 60%, 52% y 40%, respectivamente. En pacientes corticodependientes y refractarios o intolerantes a INM (CU-41, EC-14), el 68,3% de CU lograron remisión o respuesta tras inducción, 51,2% a 12 semanas y 46,3% a 52 semanas, por 62,3%, 64,3% y 42,9% en EC. La remisión mantenida fue del 66,6% en EC por 53,1% en CU. El 74,5% de los pacientes requirieron corticosteroides en algún momento. La respuesta/remisión sin corticosteroides alcanzó el 17,7% en CU y el 24% en EC.
ConclusionesLa GMA es buena herramienta terapéutica tanto en pacientes con CU como con EC. En pacientes corticodependientes y refractarios o intolerantes a INM, evita la terapia biológica o la cirugía hasta en un 40% de ellos en un año.
Inflammatory bowel disease (IBD) comprises ulcerative colitis (UC), Crohn's disease (CD) and undifferentiated colitis (UDC), diseases of unknown etiology that share predisposing genetic factors and unclear environmental triggers that lead to a loss of immunological tolerance against gut luminal antigens. The result is a sustained inflammation in the intestinal wall responsible for tissue damage and increased mucosal permeability to antigens that exacerbate and maintain the immune response. Multiple cell lines of the innate and adaptive immune system are involved as well as cytokines and chemokines implicated in recruitment, activation, regulation and blocking of the immune response.
Medication used to control IBD inflammation aim to modulate or inhibit immune cell activity or block specific cytokines. Granulocyte and monocyte adsorptive apheresis (GMA) is a non-pharmacological therapy that filters the patient's peripheral blood through a cellulose acetate filter (Adacolumn, JIMRO, Takasaki, Japan) that traps neutrophils and monocytes (and to a lesser extent lymphocytes), removing them from the vascular stream, preventing their incorporation into the inflamed tissue, influencing the cytokine profile, limiting transendothelial migration of white blood cells to the inflamed tissue and increasing leukocyte apoptosis.1–5
Since first used in Japan about 20 years ago to treat IBD patients, its clinical efficacy and safety has been suggested by many clinical trials conducted both in Japan and in Europe. In spite of the American Society for Apheresis (ASFA) defined GMA as a second-line treatment (1B/2B recommendation level in UC and 1B in CD)6 and was also supported by consensus documents endorsed by the Spanish Working Group for Ulcerative Colitis and Crohn's Disease (GETECCU),7,8 latest IBD medical guidelines (ECCO-European Crohn's and Colitis Organization-2022, AGA-American Gastroenterological Association-2020, GETECCU-2020)9–13 do not comment on this treatment or define it as an alternative therapy without solid proven effectiveness. This has possibly conditioned the use of GMA in IBD, limiting it to patients in whom other treatment options have failed or are not applicable. This article provides our experience in treating patients with UC and CD using GMA.
AimOur primary objective was to assess the observed response and/or remission rates in UC and CD 4 weeks after the end of the induction treatment, and thereafter at 3 and 12 months. Secondary objectives included: response and remission in corticosteroid-dependent IBD patients refractory or intolerant to immunomodulators, sustained response and remission rates, corticosteroid-free remission rates at 12 months, comparative clinical efficacy in patients younger or older than 60 years, and the need for rescue therapy with biologics or surgery.
Patients and methodPatientsRetrospective observational case series of IBD patients treated with GMA in the Costa del Sol Universitary Hospital (Marbella, Spain) from 2004 to 2022. All patients had active disease at the beginning of treatment. Extent and severity of the disease were described according to the definitions by the World Gastroenterology Organization (OMGE) in the Montreal classification.14 Clinical indices of inflammatory activity in UC (Partial Disease Activity Index-DAIp) and CD (Harvey-Bradshaw Index – HBI), as well as serum C-reactive protein (CRP) and fecal calprotectin (FC) were recorded (when available) before GMA, 4 weeks after the last induction session (end of induction), and at 12 and 52 weeks.
Clinical efficacyIn UC patients, clinical remission was defined by a DAIp ≤1 without any subscore >1, and response when there was a drop in the DAIp ≥2 or a 30% decrease from the baseline value. In CD patients, clinical remission was defined by a HBI ≤5 and response when there was a drop ≥3 with respect to the baseline value. Patients who did not show improvement with GMA or required rescue therapy with biological drugs or surgery at some point after the completion of the induction treatment were considered non-responders.
Apheresis procedureGMA was carried out at the Day Hospital Unit. We used cellulose diacetate filters (Adacolumn®). Apheresis sessions consisted of a filtrate volume of between 1800ml and 2700ml of blood at a pumping speed of 30ml/min for 60 or 90min, depending on the technical and individual needs of each patient, performed through two antecubital vein lines with 18-gauge intravascular catheters (in cases of inadequate peripheral vein lines, a double-lumen Hickman-type central catheter was used). All patients underwent an induction protocol consisting of one weekly session for 5 weeks in UC, two weekly sessions for the first two weeks and one weekly session for three additional weeks in CD. It was left to the discretion of the treating physician to apply additional monthly maintenance sessions in case of an incomplete response after induction.
Ethical aspectsGMA treatment was conducted in accordance with the standard clinical protocols of our institution and consensus guidelines. The study was approved by the Ethical Coordinating Committee of the Andalusian Biomedical Research Institute in January 2021.
Statistical analysisDescriptive cohort analysis uses measures of central tendency, dispersion and position for quantitative variables and frequency distribution for qualitative ones. To assess differences between quantitative measurements at 4 study moments (baseline, post-induction, 12 and 52 weeks), the generalized linear model for repeated measurements with and without segmentation by need for rescue was used. To evaluate differences between dichotomized response and need for rescue we used the Chi-square test, and Fisher's exact test was used when expected frequencies were less than 5. In the different analyses, a level of statistical significance was established at p<0.05.
ResultsFrom September 2004 to April 2022, 87 IBD patients were treated with GMA in our institution, 25 patients with CD (28.7%), and 62 patients with UC (71.3%). The male/female ratio was 1:1 (44 males and 43 females). The mean age at inclusion was 41.8 years (range 14–77).
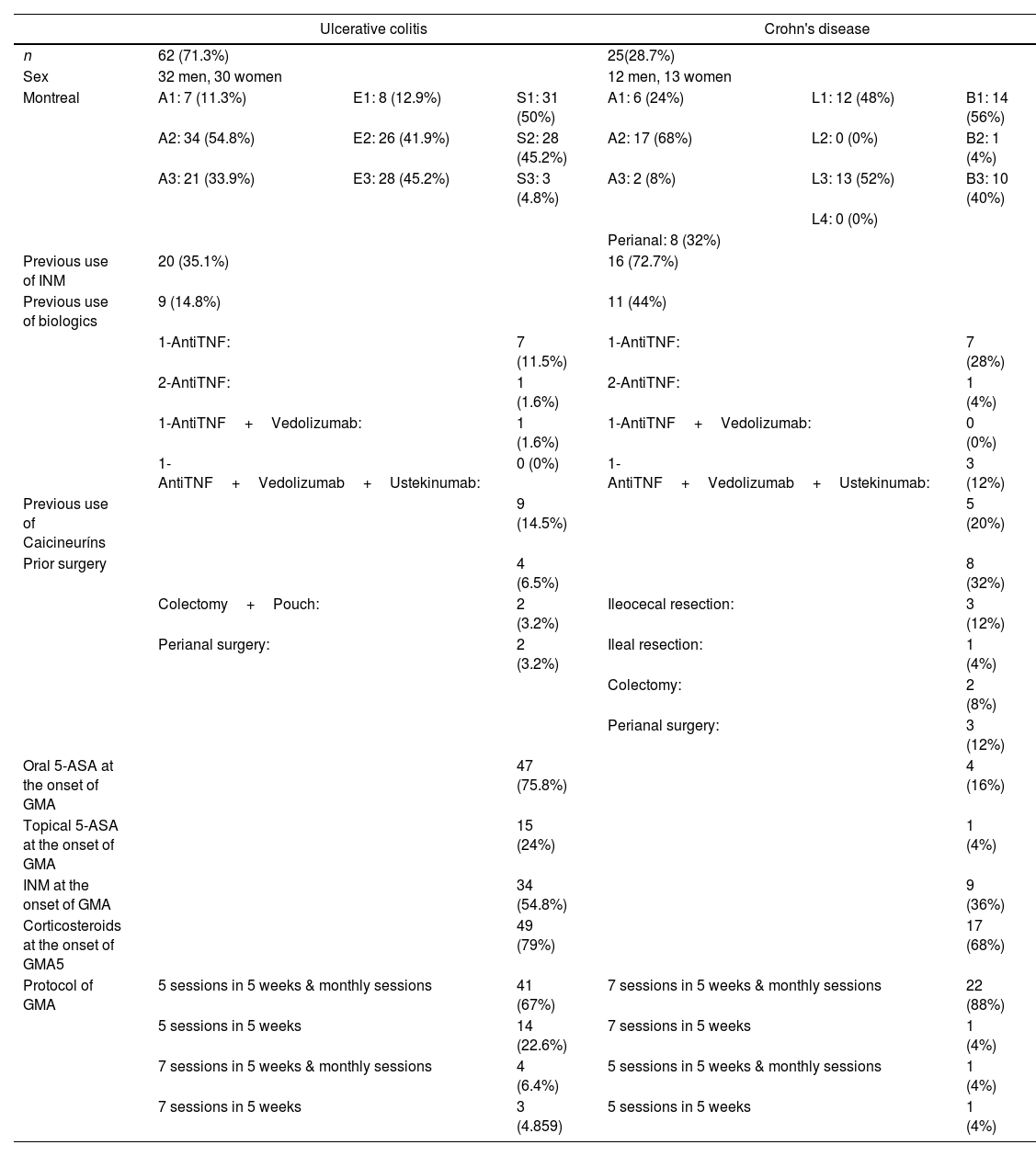
In UC, 45.2% of patients had extensive disease (E3) and 41.9% had left-sided colitis (E2), 95.2% had mild-to-moderate disease (S1-50%, S2-45.2%), 35.1% were refractory or intolerant to INM, 14.8% had lost response to biological drugs, 14.5% were also refractory to calcineurins, and 6.5% had a previous colonic surgery. In CD, ileocolonic disease was predominant (52%), with 56% of patients carrying an inflammatory pattern, penetrating in 40%, and with perianal fistula records in 32% of cases. In this group of CD patients, 72.7% were refractory or intolerant to INM, 44% experienced loss of response to biologics, and 20% refractory to calcineurins, with slightly more than a third of them having undergone previous IBD surgery (36%).
Medications used by patients at the start of GMA included corticosteroids (UC-79%, CD-68%), oral 5-ASA (UC-75.8%, CD-16%), and INM (UC-54.8%, CD-36%). All INM used at baseline were thiopurines except for one patient taking methotrexate.
GMA treatment schemes varied according to the type of IBD individual patient's circumstances. In 67% of UC patients, induction consisted of 5 sessions in 5 weeks followed by additional monthly maintenance sessions until clinical remission and normalization of biomarkers were achieved or rescue therapy was required. In 88% of CD patients induction consisted in 7 sessions in 5 weeks and were followed by monthly maintenance sessions. Most of GMA procedures (90.8%) were performed through peripheral venous access (90.3%-UC and 88%-CE), with only 8 patients (9.2%) requiring central vein lines (Table 1).
Cohort description at the start of GMA therapy.
| Ulcerative colitis | Crohn's disease | |||||
|---|---|---|---|---|---|---|
| n | 62 (71.3%) | 25(28.7%) | ||||
| Sex | 32 men, 30 women | 12 men, 13 women | ||||
| Montreal | A1: 7 (11.3%) | E1: 8 (12.9%) | S1: 31 (50%) | A1: 6 (24%) | L1: 12 (48%) | B1: 14 (56%) |
| A2: 34 (54.8%) | E2: 26 (41.9%) | S2: 28 (45.2%) | A2: 17 (68%) | L2: 0 (0%) | B2: 1 (4%) | |
| A3: 21 (33.9%) | E3: 28 (45.2%) | S3: 3 (4.8%) | A3: 2 (8%) | L3: 13 (52%) | B3: 10 (40%) | |
| L4: 0 (0%) | ||||||
| Perianal: 8 (32%) | ||||||
| Previous use of INM | 20 (35.1%) | 16 (72.7%) | ||||
| Previous use of biologics | 9 (14.8%) | 11 (44%) | ||||
| 1-AntiTNF: | 7 (11.5%) | 1-AntiTNF: | 7 (28%) | |||
| 2-AntiTNF: | 1 (1.6%) | 2-AntiTNF: | 1 (4%) | |||
| 1-AntiTNF+Vedolizumab: | 1 (1.6%) | 1-AntiTNF+Vedolizumab: | 0 (0%) | |||
| 1-AntiTNF+Vedolizumab+Ustekinumab: | 0 (0%) | 1-AntiTNF+Vedolizumab+Ustekinumab: | 3 (12%) | |||
| Previous use of Caicineuríns | 9 (14.5%) | 5 (20%) | ||||
| Prior surgery | 4 (6.5%) | 8 (32%) | ||||
| Colectomy+Pouch: | 2 (3.2%) | Ileocecal resection: | 3 (12%) | |||
| Perianal surgery: | 2 (3.2%) | Ileal resection: | 1 (4%) | |||
| Colectomy: | 2 (8%) | |||||
| Perianal surgery: | 3 (12%) | |||||
| Oral 5-ASA at the onset of GMA | 47 (75.8%) | 4 (16%) | ||||
| Topical 5-ASA at the onset of GMA | 15 (24%) | 1 (4%) | ||||
| INM at the onset of GMA | 34 (54.8%) | 9 (36%) | ||||
| Corticosteroids at the onset of GMA5 | 49 (79%) | 17 (68%) | ||||
| Protocol of GMA | 5 sessions in 5 weeks & monthly sessions | 41 (67%) | 7 sessions in 5 weeks & monthly sessions | 22 (88%) | ||
| 5 sessions in 5 weeks | 14 (22.6%) | 7 sessions in 5 weeks | 1 (4%) | |||
| 7 sessions in 5 weeks & monthly sessions | 4 (6.4%) | 5 sessions in 5 weeks & monthly sessions | 1 (4%) | |||
| 7 sessions in 5 weeks | 3 (4.859) | 5 sessions in 5 weeks | 1 (4%) | |||
Indications for GMA included exclusive corticosteroid dependence in 44.8% of patients (40.3% of UC and 56% of CD), corticosteroid dependence with refractoriness or intolerance to immunosuppressants in 42.5% (UC-48.4%, CD-28%), corticosteroid-refractoriness (UC-4.8%, CD-16%), superimposed cytomegalovirus infection in UC flare-up (3.6%), and severe UC flare-up in 1.1%.
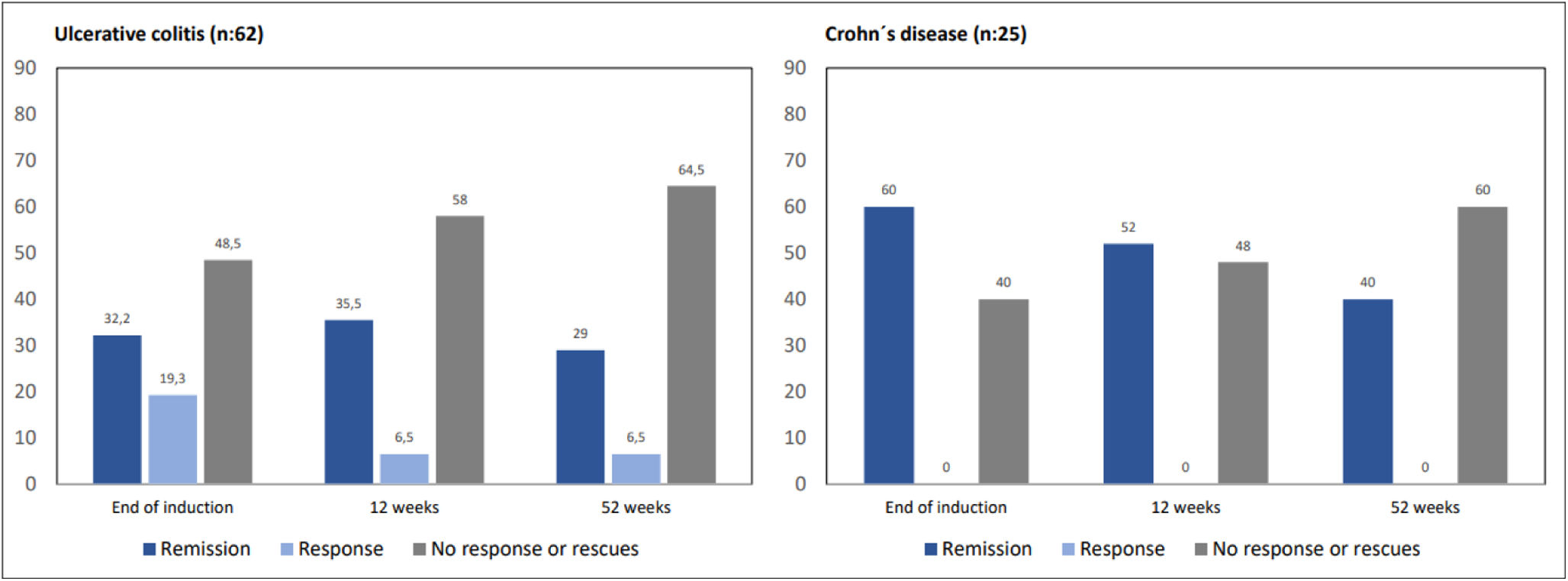
Clinical efficacyAmong UC patients, 32.2% of them achieved clinical remission and 19.3% clinical response 4 weeks after the end of induction. At 12 weeks, 35.5% of patients were in remission and 6.5% in clinical response, with 11 patients having required biologicals or surgery (17.7%). At 52 weeks, 29% of the patients were in clinical remission and 6.5% were in response, increasing to 22 patients those who had required rescue therapy with biologics or surgery (37%).
In CD patients, 60% of them achieved clinical remission at the end of the induction, while 40% had no significant clinical improvement. At 12 weeks, 52% of the patients were in remission (n=14) due to the effect of GMA, and 16% (n=4) had required biologics or surgery. At 52 weeks, 40% of CD patients were in remission (n=11), rising to 11 (43%) those who required rescue therapy (Fig. 1).
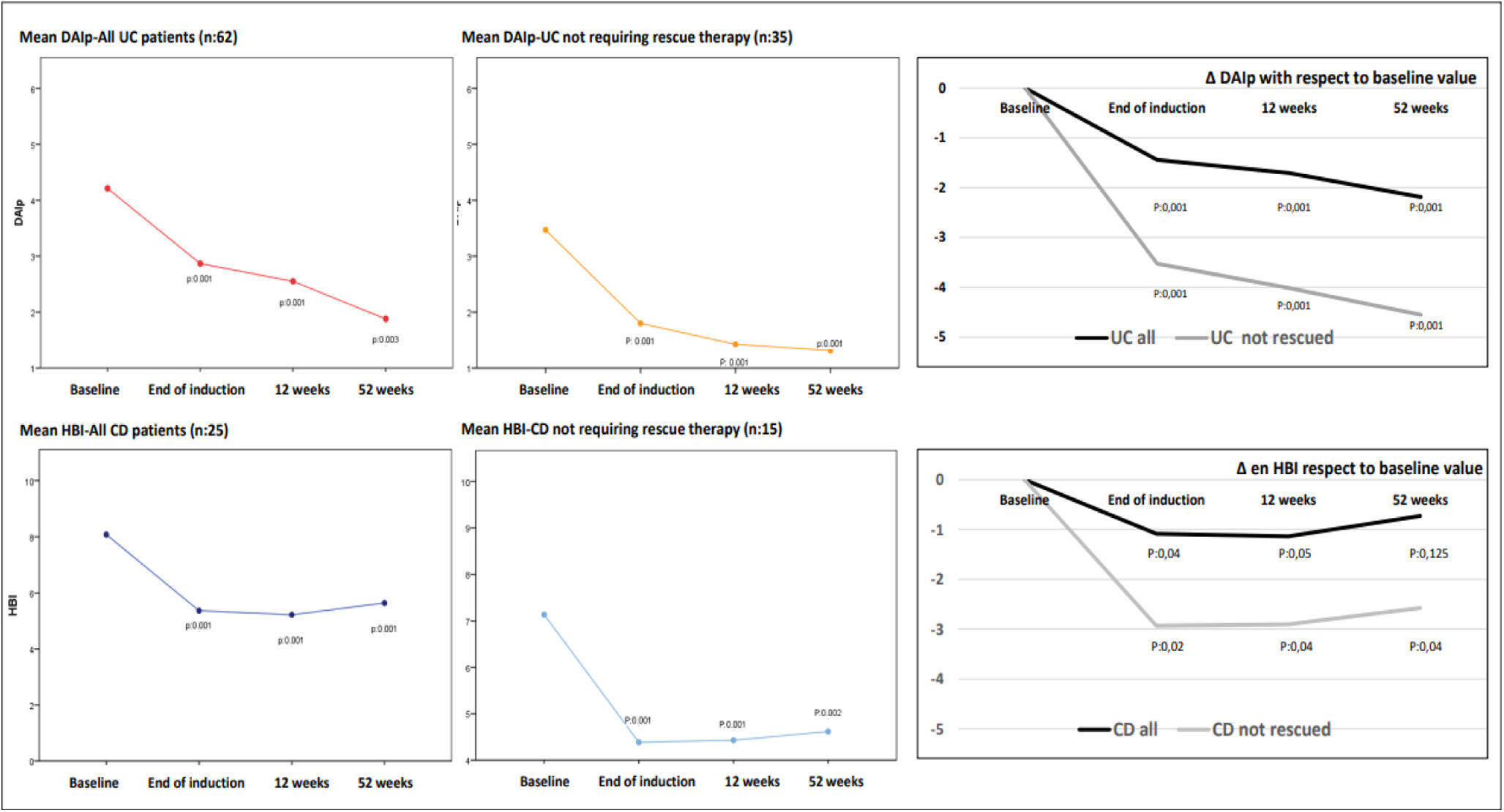
Clinical indices experienced a decrease during follow-up. Thus, mean baseline DAIp declined four weeks after induction (4.3±2.5 vs 2.8±2.7, p=0.001) as mean HBI also did (6.2±2.2 vs 5.4±2.3, p=0.001). Thereafter, this reduction was more evident in patients treated just with GMA with respect to those that required rescue therapies with biologics or surgery (at 12 weeks: DAIp 1.9±1.6 vs 5.1±3.2 and HBI 5.1±2.1 vs 6.5±2.1 in just GMA-treated patients with respect to patients needing rescue therapy, respectively. At 52 weeks: DAIp 1.5±1.4 vs 1.9±1.7 and HBI 4.7±2.6 vs 6.1±2.4 in just GMA-treated patients vs patients needing rescue therapies, respectively). In patients treated only with GMA the significative reduction in the mean values of DAIp and HBI observed after the induction phase was maintained over time throughout the follow-up (Fig. 2).
Graphs on left-side and middle show changes in the absolute mean values of DAIp and HBI indices during follow-up, including all patients with UC and CD, and separately patients who did not require rescue with biologics or surgery throughout follow-up (without rescues) as a consequence of GMA therapy. The graphs on the right side of the figure show the deltas (Δ) or relative changes of DAIp and HBI indices with respect to mean baseline values. DAIp: Disease Activity Index-partial; HBI: Harvey-Bradshaw Index.
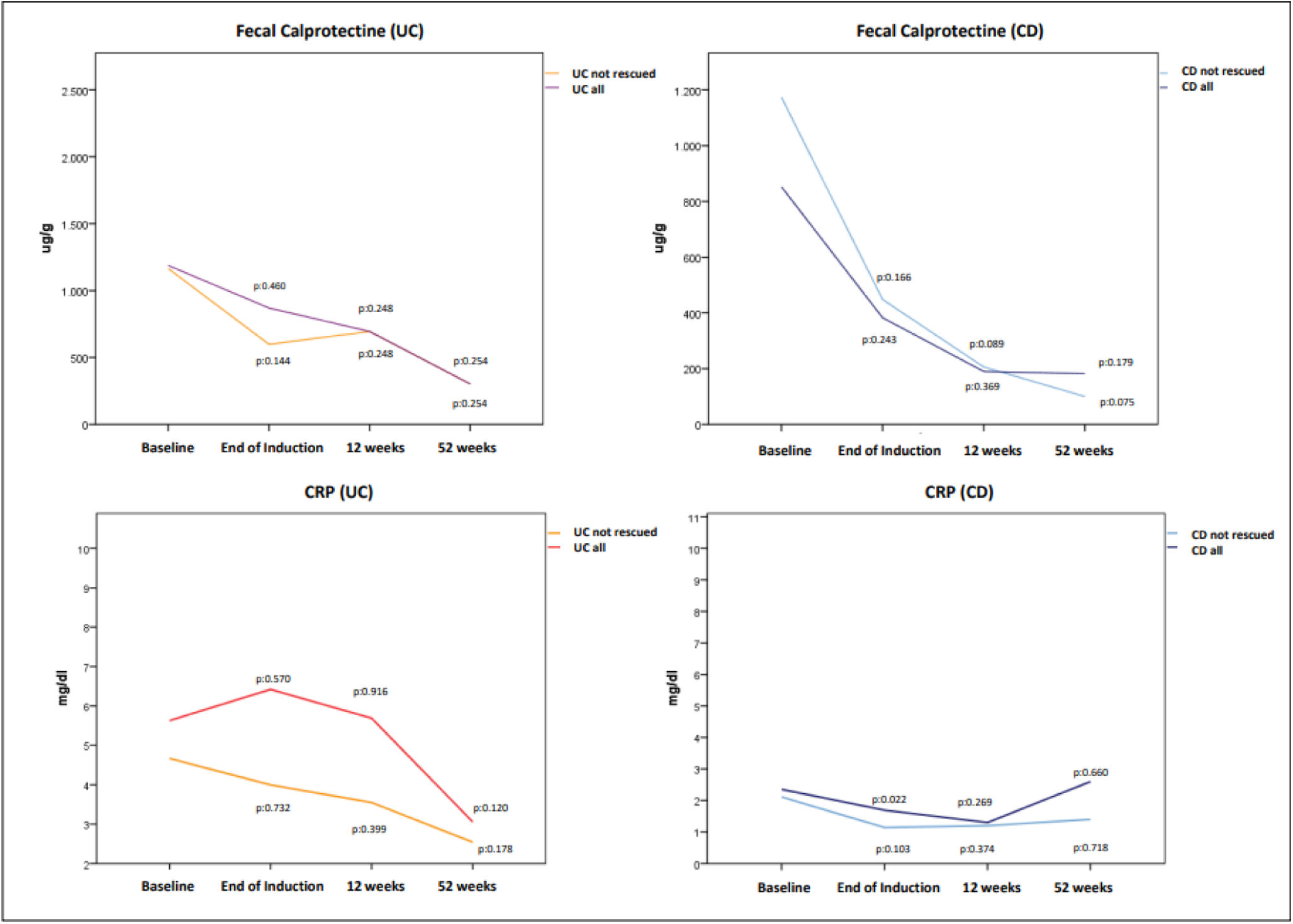
Laboratory biomarkers (CRP and FC) also decreased over time but not reaching statistical significant differences compared to baseline value, as shown in Fig. 3. The mean baseline CRP and FC in UC were 5.6±4.1mg/dl and 1187.9±1108.7μg/g being 2.3±1.8mg/dl and 802.7±772.7μg/g in CD. In patients with UC treated with GMA alone, a FC ≤250μg/g was achieved in 33.3%, 33.3% and 54.5% after induction, at 12 and 52 weeks respectively. These percentages were 16.6%, 33.3% and 50% in patients with CD.
Changes in mean fecal calprotectin (top) and C-reactive protein-CRP (bottom) values in patients with UC and CD. Only in 12 UC patients and in 7 CD patients we had calprotectin levels recorded at the different moments of assessment. As a consequence it was not possible to assess statistical significant differences. Although in the CRP analysis the number of patients with available data was higher, changes experienced in CRP both in UC and CD did not reach statistically significant difference.
Two-thirds of UC patients (66.7%) and one-third of CD patients (36.4%) were treated with a filtrated volume of 2700ml/session, instead of 1800ml/session in the rest of patients. Volume of filtrate per GMA session did not influence significantly efficacy results in UC nor CD at any of the evaluation moments.
Analysis of the efficacy of different GMA schemes was done in UC patients as 88% of CD patients had the same scheme. Thus, four weeks after induction remission and remission-or-response rates were 26.7% and 47.4% in those UC patients treated with 5 sessions/5 weeks. In those treated with 7 sessions/5 weeks these percentages were 6.7% and 6.7% respectively. Once those patients that required rescue therapies were excluded, rates of response or remission at 12 weeks were 33.3% (4 out of 12 patients) in those treated with 5 session/5 weeks, 75% (3 out of 4 patients) with 7 session/5 weeks, 45% (18 out of 40 patients) with 5 sessions/5 weeks plus maintenances and 33.3% (1 out of 3 patients) with 7 sessions/5 weeks and maintenances. These percentages at 52 weeks were 25% (3 out of 12), 25% (1 out of 4), 44.7% (17 out of 38) and 33.3% (1 out of 3) respectively. No statistical significant differences were observed probably due to the small number of patients in some GMA schemes.
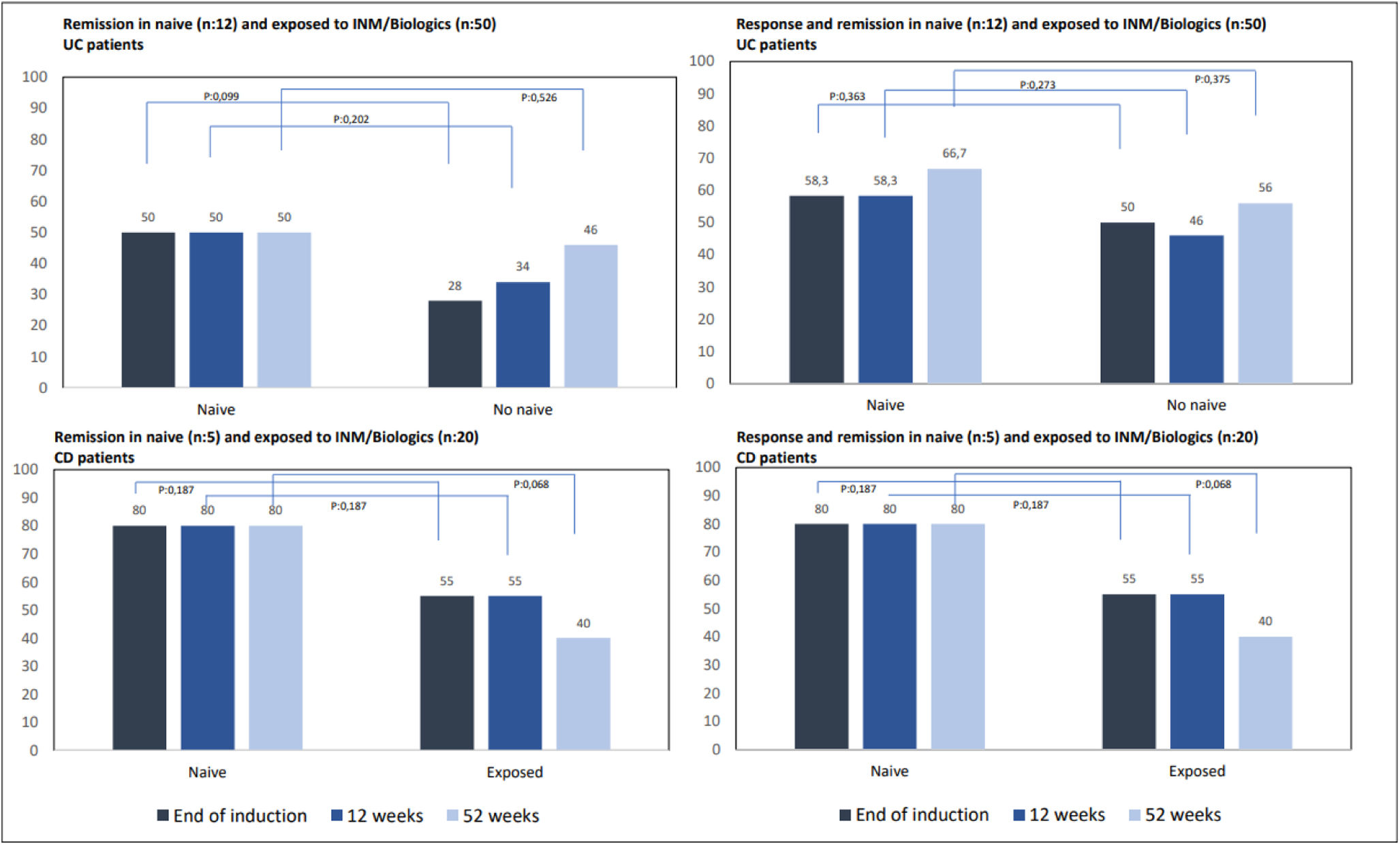
We also analyzed the response and remission rates depending on whether patients were naive to INM or biologics or had been previously exposed to any of these drugs. Even with a clear tendency for naive patients to show higher response and remission rates than those exposed to INM or biologics, no statistically significant benefit was demonstrated (Fig. 4). None of the naive patients required surgery or biological therapy, while 37 of those previously exposed (10-CD, 27-UC) did.
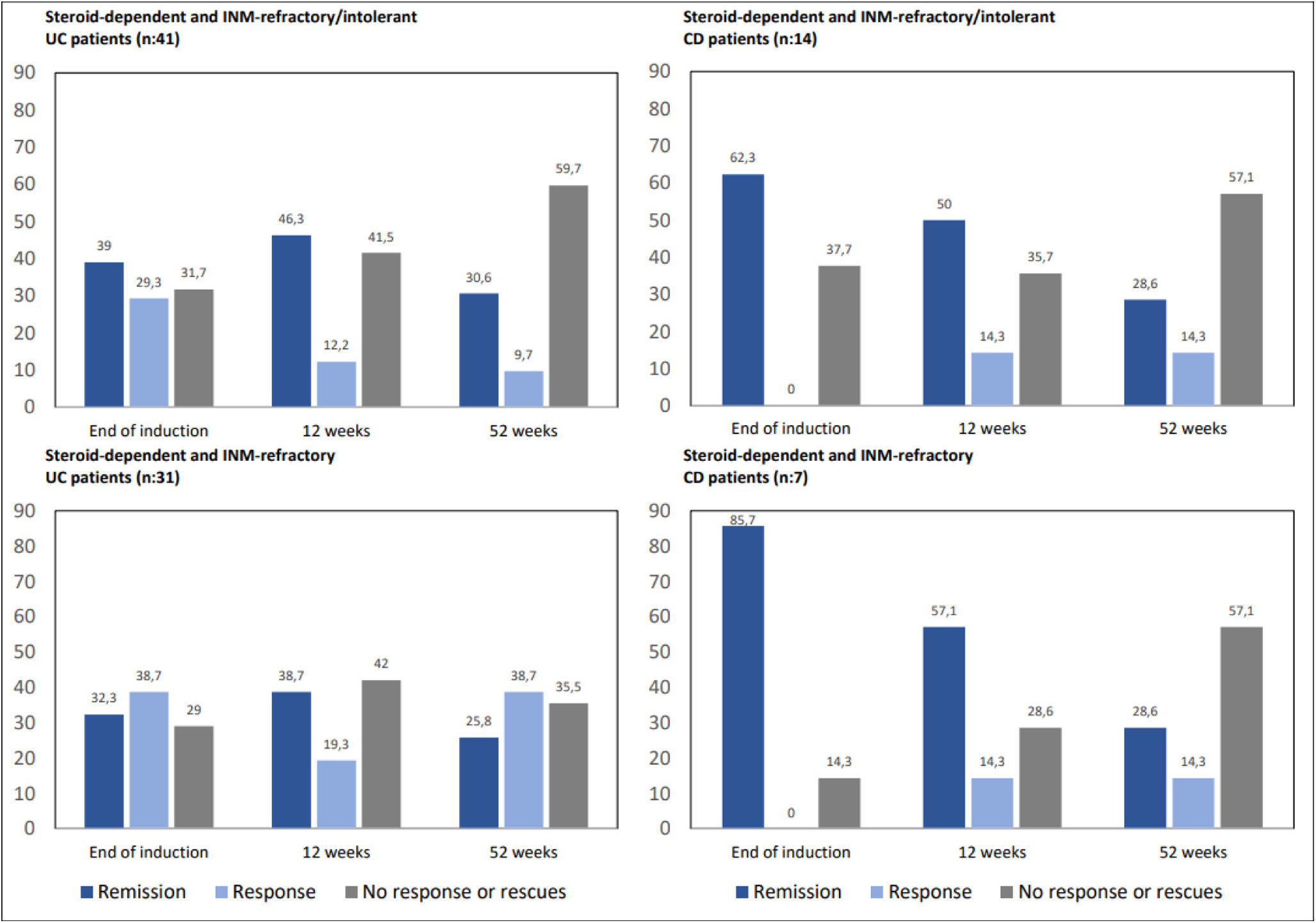
A group of special interest is corticosteroid-dependent patients who are refractory or intolerant to INM (n=55). In our series, 41 patients with UC and 14 with CD fulfilled this criteria. After induction, sixteen UC patients (39%) achieved remission and twelve clinical response (29.3%); consequently, 28 patients (68.3%) reached response or remission. At 12 weeks, the remission rate was 46.3% (n=19), with an additional 12.2% (n=5) in clinical response; in other words, 51.2% (n=24) in response or remission by GMA. At week 52, UC patients in response or remission accounted for 46.3% (n=19), 15 of whom were in remission (30.6%) and 4 in clinical response (9.7%). With regard to CD, 62.3% (n=9) achieved remission after induction. At 12 weeks, 64.3% of the patients were in clinical response or remission, 50% (n=7) in remission and 2 patients were in clinical response (14.3%). At 52 weeks, the remission rates due to GMA accounted for 28.6% of patients (n=4), 14.3% (n=2) with clinical response, and 42.9% of CD patients in response or remission. In those corticosteroid-dependent and thiopurine-refractory IBD patients (n=38; CU-31, EC-7) the addition of GMA while maintaining the INM showed even slightly better results (Fig. 5).
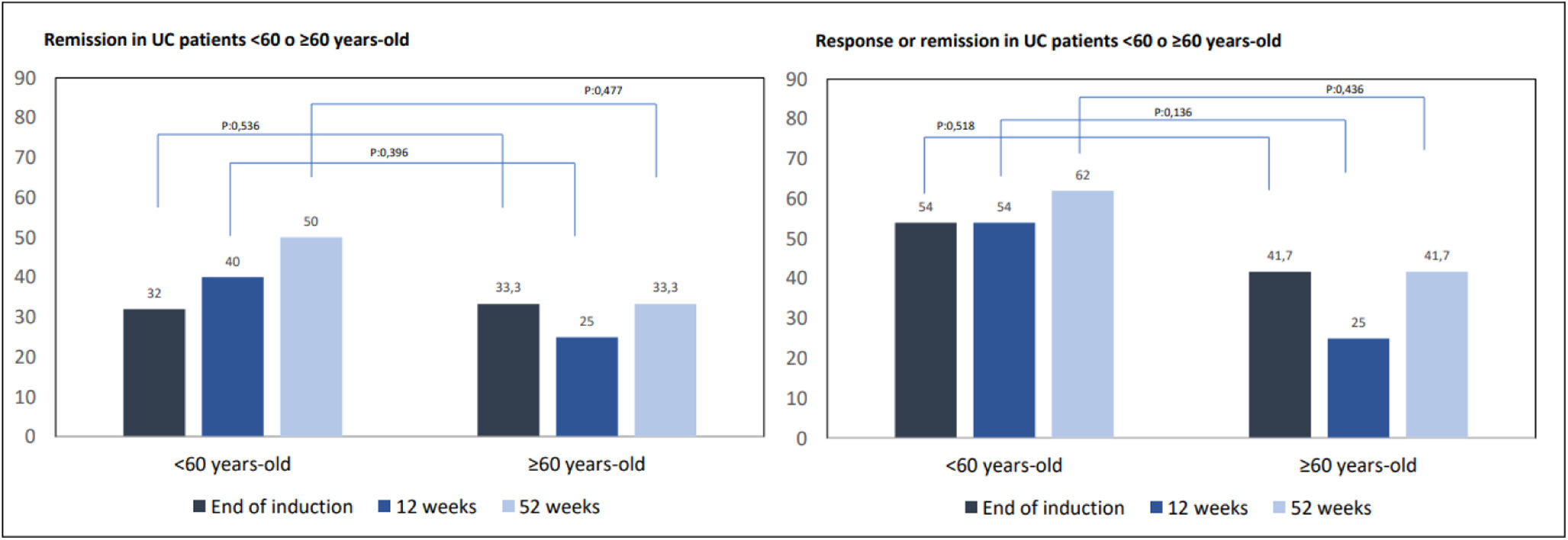
Only 14 patients in our cohort were 60 years old or older, 12 of them with UC. We assessed the efficacy of GMA in this group of >60-year-old UC patients compared to younger UC patients (n=50). After induction, 32% (n=16) of <60-year-old UC patients achieved remission, compared to 33.3% of those ≥60-year-old (n=4), p=0.536. Similar results were observed at 12 weeks (40%, n=20 vs 25%, n=3; p=0.396) and at the end of follow-up (50%, n=25 vs 33.3%, n=4; p=0.477). If response or remission is considered all together, similar results were observed. As a consequence, age did not seem to make a difference in the possibility of achieving remission (Fig. 6).
Maintenance of remissionPersistence of the therapeutic effect is a relevant aspect. In patients with clinical response or remission after induction (remission: 20 CU patients and 15 CD patients. Response: 12 UC patients and 0 CD patients. Response or remission in 47 IBD patients) we evaluated rates of maintained remission at 12 and 52 weeks without the use of rescue therapy with biologics or surgery.
Among 32 UC patients with response or remission after induction, 17 patients were in remission at 12 weeks and 52 weeks (53.1%) without any biological or surgical rescue treatment needed. In those naive to INM/Biologics (n=12), all those who achieved remission after induction maintained it throughout the follow-up.
Eleven out of 15 patients with CD in clinical response or remission after the end of induction (73.3%) were in remission at 12 weeks and 10 patients (66.7%) at 52 weeks. As in UC, those CD-naive INM/Biologics patients (n=5) who achieved remission after induction remained in remission throughout the follow-up.
Use of corticosteroids in parallel with GMAAt the start of GMA, 79% of UC and 68% of CD patients were on corticosteroids, either systemic or low-bioavailability oral corticosteroids. As GMA sessions were carried out, patients were instructed to gradually reduce the dose of corticosteroids. Overall, up to 74.5% of the patients required some dose of corticosteroids at some timepoint (we include here any type and at any dose).
More in detail, four weeks after the last induction session 75.5% of UC and 68.2% of CD patients were still using corticosteroids (p=ns). Thereafter, those patients who required rescue therapies used corticosteroids more than those treated with GMA alone. At 12 weeks, 63.6% of patients with UC who required rescues used corticosteroids compared to 35.4% of those who did not (p=0.007), while at 52 weeks these percentages were reduced to 38.5% and 28.3% respectively (p=0.23). In CD, at 12 weeks, 100% of the patients who required rescues used corticosteroids compared to 44.4% of those who did not (p=0.008), being these percentages at 52 weeks 100% and 30.8% respectively (p=0.125). At the end of the follow-up rates of corticosteroid-free remission-or-response were 17.7% in UC and 24% in CD patients.
Rescue treatmentsPatients requiring rescue treatments due to a lack of response to GMA were considered non-responders. Forty-three percent of our patients required rescue with biologics or surgery during follow-up (41.7% with CD and 43.5% with UC). Up to 36% of patients required biologics or calcineurins, including infliximab (62.1%), adalimumab (17.2%), vedolizumab (6.9%), tacrolimus (6.9%), ustekinumab (3.4%), and golimumab (3.4%). On the other hand, among the 15.3% of IBD patients requiring surgery (12.5% with CD and 15% with UC), procedures included colectomy with pouch (55.6%), colectomy without a pouch (11.1%), and small bowel resection (33.3%). Still, GMA helped up to 57% of patients avoid biologics or surgery. Even more, up to 22.1% of all patients did not require any rescue with biologics, surgery or corticosteroids after the GMA induction phase.
SafetyFive patients (5.7%) experienced adverse events (AE). In one patient with CD, the procedure had to be stopped without completing the induction due to a severe headache followed by vasovagal syncope after each of the two attempted apheresis sessions. The remaining four patients experienced headaches, abdominal pain and hypotension as AEs, all of them mild and not leading to GMA withdrawal. Safety was also similar in subjects <60 years old (8.2%) and those ≥60 years old (0%), p=0.584.
DiscussionThis paper shows our experience in real clinical practice using GMA in IBD patients throughout the last 20 years. Many of these patients had already been treated with thiopurines, biological therapies, calcineurins or surgical procedures (Table 1). Other patients did not receive INM or biologicals because they had contraindications, they refused the use of immunosuppressants, or due to patient's fragility. All of them begun GMA with an active disease and, despite the fact that many of our patients required rescue therapies during follow-up, this treatment managed to prevent 57% of patients from biological treatment or surgery, showing that in those in clinical response or remission after induction, half of UC and two-thirds of CD patients could maintain remission. These data are in line with other published series of patients with UC, highlighting, however, the good results in CD and confirming the excellent safety profile as reported in other publications.16,40
In UC, one of the first published papers from 2001 included 53 patients treated with 5 sessions in 5 weeks reporting response or remission rates of 58%, with a reduction in the need for corticosteroids.15 Along these lines, Hibi et al.,16 reported response rates of over 60% in UC patients with different degrees of severity (80.4% in mild, 65.7% in moderate, 63.2% in severe disease). Two meta-analyses by Habermalz et al.,17 and Zhu et al.,18 showed that the addition of GMA achieved better rates of response, remission, and maintenance of remission than 5-aminosalicylates alone. When compared with corticosteroids, Tominaga et al.,19 and Bresci et al.,20 described similar remission rates with GMA with fewer adverse effects, and a subsequent meta-analysis including 9 controlled trials showed higher remission rates than corticosteroids and less adverse effects.21 However, the ATICCA trial did not evidence that adding GMA to corticosteroids increased remission rates at 24 weeks, although delayed the time to clinical recurrence and lowered adverse effects due to corticosteroids.22 Further, GMA in corticosteroid-dependent and INM-refractory or intolerant UC patients avoided biologics or surgery in up to 51% of cases in the paper by Imperiali et al.,23 in one year of follow-up. Moreover, the European multicenter ART-trial showed remission rates in up to 20% of patients refractory to antiTNF at 48 weeks.24 Nonetheless, the study by Sands et al.,25 compared Adacolumn-GMA with a placebo column and described similar remission or response rates at 12 weeks, but quite interesting just 38% of the patients had erosive histological lesions at inclusion, with a post hoc analysis showing that in these patients GMA provided better outcomes compared to the placebo column (remission 23.9% vs 0%, p=0.02, and response 54.4% vs 17.7%, p=0.01).26 Our results are consistent with previously published studies with overall post-induction response or remission rates of 51.3%, with no differences between patients older or younger than 60 years of age as already demonstrated in the article by Ito et al.27
In CD, available published information is scarcer than in UC but promising.28–31 The only randomized trial to date comparing GMA to a placebo column was published in 2013 by Sands et al.32 and reported no benefit in remission or response at 12 weeks, but again surprisingly only 15% of the subjects had endoscopically proven inflammatory activity. Other published cohort or prospective studies in CD included less than 40 patients and used GMA in patients naive to INM, biologicals, and even to corticosteroids.33,34 In 2014, Fukuchi et al.35 treated 22 CD patients naive to corticosteroids, INM and biologicals (10 GMA sessions in 5 weeks) while starting azathioprine, achieving remarkable 77.2% clinical and 22.7% endoscopic remission rates at 6 weeks, with 81.8% clinical and 50% endoscopic remission rates at 52 weeks when patients were just on thiopurines. Along this line, Bresci et al.36 treated 30 patients with CD refractory to mesalazine and corticosteroids (5 sessions in 5 weeks), with remission rates of 63.3% after induction, 53.3% at 6 months, and 40% at 12 months and without the location of the disease implying differences in efficacy or risk of relapse. Interestingly Sacco et al.37 in a series of 35 patients with steroid-dependent or steroid-refractory CD naive to INM and biologicals, reported clinical remission rates of 63%, 54% and 43% at 6, 24, and 52 weeks respectively, with no significant differences due to being dependent or refractory to corticosteroids, and with rates of mucosal healing rates of 48% and 37% at 24 and 52 weeks, respectively.
Our series provide quite similar clinical outcomes to those by Bresci G. and Sacco R. with the difference that 72.7% of our patients had been previously treated with INM and up to 44% with biologics, and that received monthly maintenance sessions. In the small group of patients naïve to INM/biologics (n=5), all but one achieved remission after induction and maintained it throughout follow-up, and in those 7 patients on thiopurines when GMA was started up to 42.9% of them could avoid biologics or surgery at the end of follow-up.
We were unable to demonstrate any significant difference in efficacy between naïve patients and those previously exposed to INM or biologics, nor between different apheresis schemes. The small number of naïve patients and the fact that the majority of patients with UC and CD followed the same apheresis regimen may have influenced these results.
Most of our patients were corticosteroid-dependent, and although 74.5% required some dose of corticosteroids throughout the 52 weeks of follow-up, those refractory to GMA and with persistent or recurrent disease that required biologics or rescue surgery used them more. At the end of 52 weeks of follow-up, around one-fifth of our IBD patients treated with GMA alone were in response or remission without corticosteroids.
There are several limitations to this study. Among them, its retrospective nature, a long period of case collection and the use of maintenance sessions additional to those of induction in the majority of patients with UC and CD with the intention of consolidating the response. The number of cases included, reflects our center's commitment to this technique at a time when the star treatments have been and continue to be biological drugs. However, it is a mirror of the use we have made of this technique in real clinical practice in our center. To the best of our knowledge, apart from Spanish multicenter SIMAC registry38 including 142 UC patients from 23 hospitals, our series of patients with UC and CD treated with GMA is the longest published in our country.
Apart from the recommendation of Yamamoto et al. in patients with UC,39 we consider that GMA can also be a therapeutic option in corticosteroid-dependent patients with UC and CD (inflammatory pattern) that are refractory, intolerant or that reject the use of immunosuppressants, in fragile patients and also in those with a mild-to-moderate disease and short duration of illness.
However, a well designed placebo-controlled study with adequate patient selection is still awaited to unequivocally demonstrate the usefulness of this therapeutic procedure so that it can be available in all centers that provide specialized care to this group of patients with IBD.
FundingThis research work has not received any external funding.
Conflict of interestThe authors have no conflict of interest.