Cure of Helicobacter pylori infection in patients with gastric lymphoma of mucosa-associated lymphoid tissue (MALT) leads to long-term clinical remission in the initial stages. As it is a rare disease, its management in clinical practice remains largely unknown and heterogeneity of care remains a concern.
The aim was to audit the management and evolution of a large series of low-grade gastric MALT lymphomas from thirteen Spanish hospitals.
Materials and methodsMulticentre retrospective study including data on the diagnosis and follow-up of patients with gastric low-grade MALT lymphoma from January 1998 to December 2013. Clinical, biological and pathological data were analyzed and survival curves were drawn.
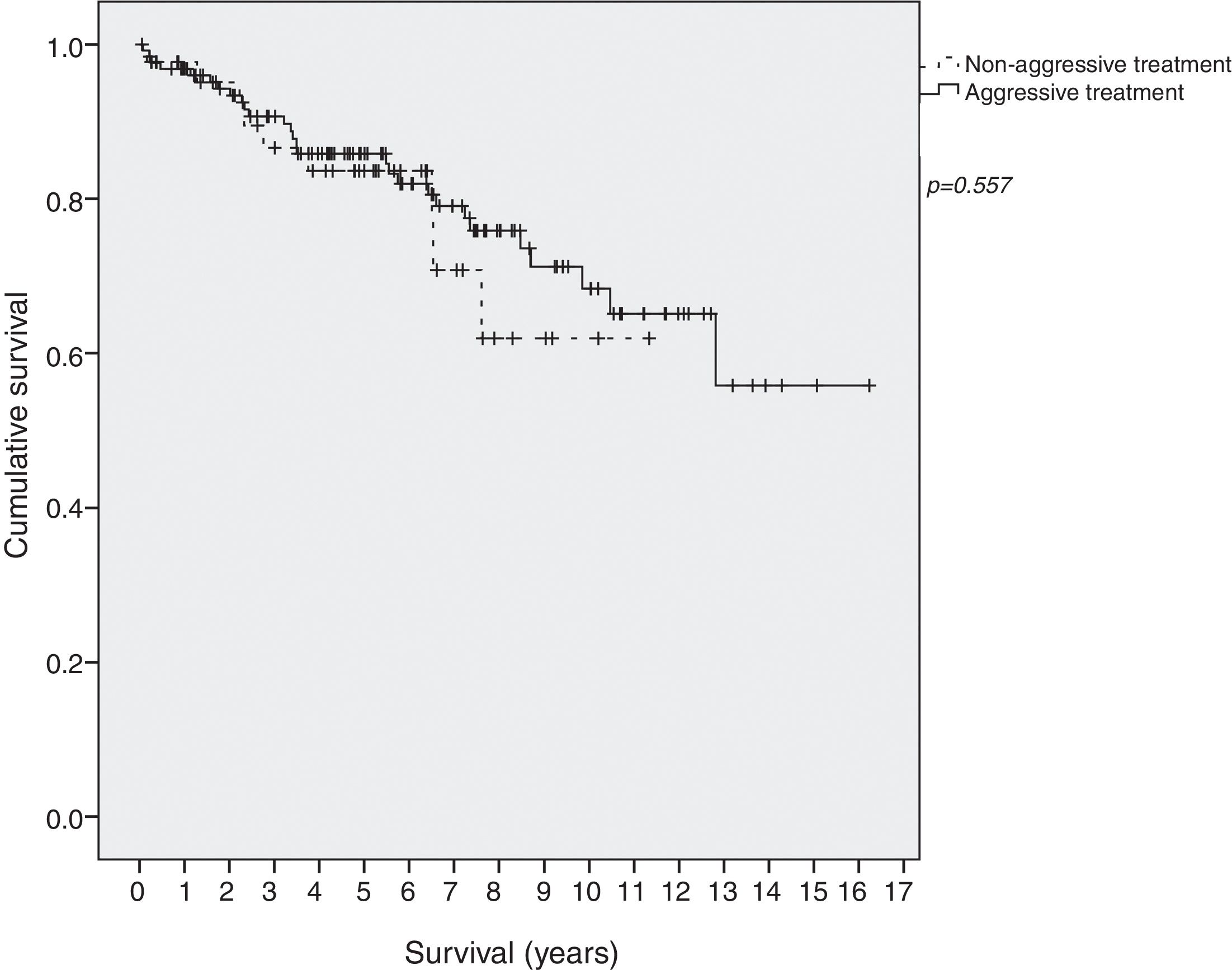
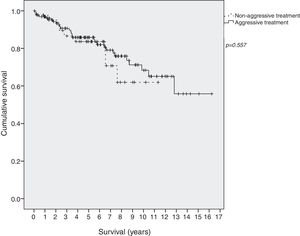
ResultsOne-hundred and ninety-eight patients were included. Helicobacter pylori was present in 132 (69%) patients and 103 (82%) in tumors confined to the stomach (stage EI) and was eradicated in 92% of patients. Chemotherapy was given in 90 (45%) patients and 43 (33%) with stage EI. Marked heterogeneity in the use of diagnostic methods and chemotherapy was observed. Five-year overall survival was 86% (89% in EI). Survival was similar in EI patients receiving aggressive treatment and in those receiving only antibiotics (p=0.577).
DiscussionGastric MALT lymphoma has an excellent prognosis. We observed, however, a marked heterogeneity in the use of diagnostic methods or chemotherapy in early-stage patients.
La cura de la infección por Helicobacter pylori (H. pylori) en pacientes con linfoma gástrico de tejido linfoide asociado mucosas (mucosa-associated lymphoid tissue [MALT]) conduce a la remisión clínica a largo plazo en los estadios iniciales. Al tratarse de una enfermedad rara, su tratamiento en la práctica clínica en muchas ocasiones se desconoce y la heterogeneidad de la atención sigue siendo motivo de preocupación.
El objetivo es auditar el tratamiento y la evolución de una gran serie de linfomas gástricos MALT de bajo grado procedentes de 13 hospitales españoles.
Materiales y métodosEstudio retrospectivo y multicéntrico que incluye datos sobre el diagnóstico y el seguimiento de pacientes con linfoma MALT gástrico de bajo grado desde enero de 1998 hasta diciembre del 2013. Se analizaron los datos clínicos, biológicos y patológicos, y se trazaron las curvas de supervivencia.
ResultadosSe incluyó a 198 pacientes. El H. pylori estaba presente en 132 (69%) de los pacientes y en 103 (82%) tumores confinados al estómago (estadio EI) y se erradicó en el 92% de los pacientes. Se administró quimioterapia a 90 (45%) de los pacientes y a 43 (33%) en estadio EI. Se observó una marcada heterogeneidad en el uso de los métodos de diagnóstico y de la quimioterapia. La supervivencia global a los 5 años fue del 86% (89% en estadio EI). La supervivencia fue similar en los pacientes en estadio EI que recibieron tratamiento agresivo y en los que recibieron solo antibióticos (p=0,577).
DiscusiónEl linfoma MALT gástrico presenta un pronóstico excelente. Sin embargo, se observó una marcada heterogeneidad en el uso de los métodos de diagnóstico o la quimioterapia en pacientes en estadio inicial.
Gastric extranodal marginal zone lymphoma of mucosal-associated lymphoid tissue (MALT) is the second most frequent gastric tumor after adenocarcinoma, and the most common form of extranodal lymphoma.1,2 One-third of MALT lymphomas present as primary gastric lymphomas associated, in at least two-thirds of cases, with chronic H. pylori infection.3 In fact, in most cases, the infection is a prerequisite for the gastric MALT lymphoma development. A striking characteristic of gastric MALT lymphoma is that eradication of H. pylori infection leads to long-term clinical remission in a large proportion of patients.2,4 Guidelines for the treatment of low-grade gastric MALT lymphoma have established that H. pylori eradication is the first-line treatment in the early stages, and radiotherapy or chemotherapy when radiotherapy is not feasible for advanced disease.5,6
A few large series of gastric MALT have been published, the two largest ones in Japan,7,8 but little is known about its management and prognosis in clinical practice, especially in western countries. In Europe, some clinical series have been published, but most of them are single-center studies including a low number of patients.9–15 The largest series published in Europe have shown a very favorable prognosis. In Portugal, Moleiro et al. included 144 patients with 105 months follow-up,12 and similar data were reported by another study in Germany with 196 patients and a shorter – follow-up of 27 months.15 However, no study has dealt with the rates of adherence to guidelines in clinical practice and/or the heterogeneity in the care for these patients.
This study aims to retrospectively audit the management and evolution of a large series of low-grade gastric MALT lymphomas from thirteen different Spanish hospitals distributed along the country.
Patients and methodsThe study was designed as a multicenter collaborative trial organized by the Asociación Española de Gastroenterología (AEG). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethical review board (Comité de Ética de la Investigación con medicamentos Parc Taulí de Sabadell, Code CEIm: 2007/508, February 7, 2007). As the study is retrospective, written informed consent could not be obtained. The study included all patients diagnosed with gastric low-grade MALT lymphoma from January 1998 to 2009. Clinical reports were revised until December 2013.
To avoid missed cases, gastric MALT lymphomas were recruited using an electronic search. As most Spanish hospitals use SNOMED to manage the pathological information, we used this database to retrieve the cases. A search was performed using the general code for lymphoma (M95903) and the topographic code for stomach (T63). A second search was also performed using the specific code for MALT (M96993) and the stomach topographic code. Retrieval of cases was as exhaustive as possible: when available, data from specific local databases were used.
Clinical data (age, sex, symptoms, physical examination), test results (blood test, H. pylori tests, endoscopy and radiology examinations), pathological data, treatments and survival were retrieved from clinical records. Data were collected anonymously in an Excel database. Data collection sheet is provided as Supplementary Document 1.
Statistical analysisCategorical variables are presented as absolute and relative frequencies. Continuous variables are summarized as mean and standard deviations (SD). Kaplan–Meyer survival curves were drawn for the whole series and combining different conditions (stage, hospital and aggressive treatment). The curves were compared using log-rank test. A p value less than 0.05 between groups was considered as significant.
ResultsBaseline characteristicsThe clinical records of 198 patients from 13 hospitals up to 2013 were retrieved for the study. The diagnosis was made between 1992 and 2009 (95% of them after 1998). Patients’ mean age was 62.8±13 years, and 110 (56%) were female.
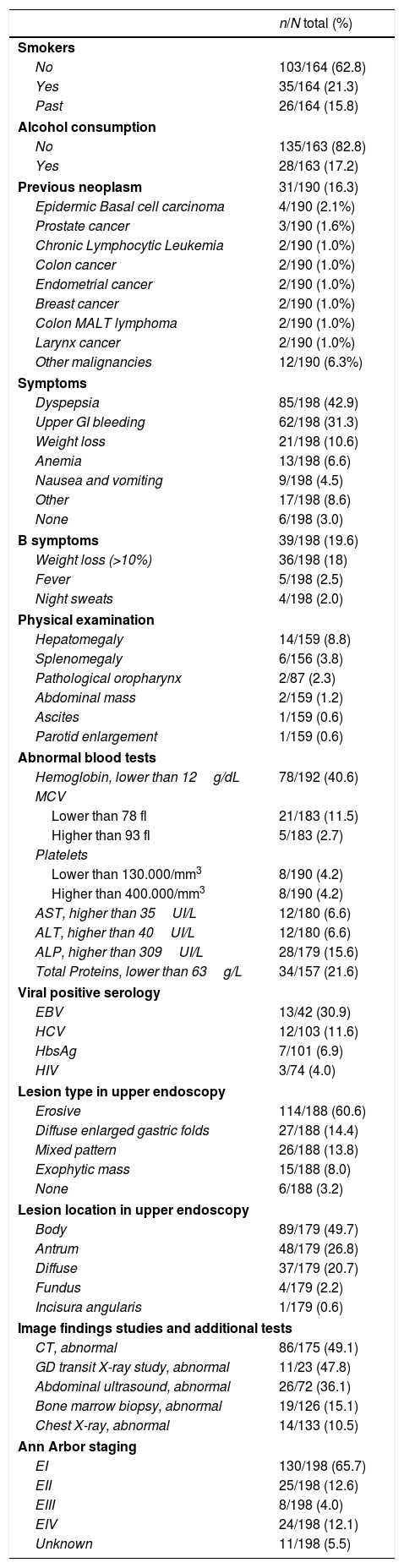
Clinical characteristics and complementary tests are shown in Table 1. Thirty-one out of 190 patients (16%) had a previous history of cancer and the types and localizations found are displayed in Table 1.
Clinical characteristics of the patients and main finding in tests.
| n/N total (%) | |
|---|---|
| Smokers | |
| No | 103/164 (62.8) |
| Yes | 35/164 (21.3) |
| Past | 26/164 (15.8) |
| Alcohol consumption | |
| No | 135/163 (82.8) |
| Yes | 28/163 (17.2) |
| Previous neoplasm | 31/190 (16.3) |
| Epidermic Basal cell carcinoma | 4/190 (2.1%) |
| Prostate cancer | 3/190 (1.6%) |
| Chronic Lymphocytic Leukemia | 2/190 (1.0%) |
| Colon cancer | 2/190 (1.0%) |
| Endometrial cancer | 2/190 (1.0%) |
| Breast cancer | 2/190 (1.0%) |
| Colon MALT lymphoma | 2/190 (1.0%) |
| Larynx cancer | 2/190 (1.0%) |
| Other malignancies | 12/190 (6.3%) |
| Symptoms | |
| Dyspepsia | 85/198 (42.9) |
| Upper GI bleeding | 62/198 (31.3) |
| Weight loss | 21/198 (10.6) |
| Anemia | 13/198 (6.6) |
| Nausea and vomiting | 9/198 (4.5) |
| Other | 17/198 (8.6) |
| None | 6/198 (3.0) |
| B symptoms | 39/198 (19.6) |
| Weight loss (>10%) | 36/198 (18) |
| Fever | 5/198 (2.5) |
| Night sweats | 4/198 (2.0) |
| Physical examination | |
| Hepatomegaly | 14/159 (8.8) |
| Splenomegaly | 6/156 (3.8) |
| Pathological oropharynx | 2/87 (2.3) |
| Abdominal mass | 2/159 (1.2) |
| Ascites | 1/159 (0.6) |
| Parotid enlargement | 1/159 (0.6) |
| Abnormal blood tests | |
| Hemoglobin, lower than 12g/dL | 78/192 (40.6) |
| MCV | |
| Lower than 78 fl | 21/183 (11.5) |
| Higher than 93 fl | 5/183 (2.7) |
| Platelets | |
| Lower than 130.000/mm3 | 8/190 (4.2) |
| Higher than 400.000/mm3 | 8/190 (4.2) |
| AST, higher than 35UI/L | 12/180 (6.6) |
| ALT, higher than 40UI/L | 12/180 (6.6) |
| ALP, higher than 309UI/L | 28/179 (15.6) |
| Total Proteins, lower than 63g/L | 34/157 (21.6) |
| Viral positive serology | |
| EBV | 13/42 (30.9) |
| HCV | 12/103 (11.6) |
| HbsAg | 7/101 (6.9) |
| HIV | 3/74 (4.0) |
| Lesion type in upper endoscopy | |
| Erosive | 114/188 (60.6) |
| Diffuse enlarged gastric folds | 27/188 (14.4) |
| Mixed pattern | 26/188 (13.8) |
| Exophytic mass | 15/188 (8.0) |
| None | 6/188 (3.2) |
| Lesion location in upper endoscopy | |
| Body | 89/179 (49.7) |
| Antrum | 48/179 (26.8) |
| Diffuse | 37/179 (20.7) |
| Fundus | 4/179 (2.2) |
| Incisura angularis | 1/179 (0.6) |
| Image findings studies and additional tests | |
| CT, abnormal | 86/175 (49.1) |
| GD transit X-ray study, abnormal | 11/23 (47.8) |
| Abdominal ultrasound, abnormal | 26/72 (36.1) |
| Bone marrow biopsy, abnormal | 19/126 (15.1) |
| Chest X-ray, abnormal | 14/133 (10.5) |
| Ann Arbor staging | |
| EI | 130/198 (65.7) |
| EII | 25/198 (12.6) |
| EIII | 8/198 (4.0) |
| EIV | 24/198 (12.1) |
| Unknown | 11/198 (5.5) |
MCV: mean corpuscular volumen of red cells; AST: aspartate transferase; ALT: alanine tranfserase; ALP: alkaline phosphatase; EBV: Epstein–Barr virus; HCV: hepatitis C virus; HIV: human immunodeficiency Virus; HbsAg: surface antigen of hepatitis B virus.
Dyspepsia and bleeding were the predominant clinical symptoms. The most frequent endoscopic lesion was erosive gastritis. Plain thorax radiology information was retrieved in 70% of the patients, abdominal ultrasound in 37%, abdominal CT in 89% and bone marrow biopsy in 65% of patients.
One-hundred and twenty six (66%) of low-grade gastric MALT lymphomas were confined to the stomach wall (stage EI in the Ann Arbor classification). The most frequent CT scan findings were: gastric wall enlargement in 51 patients (29%) and pathological lymph-node enlargement was found in 38 (22%). Bone marrow biopsy showed tumoral infiltration in 19 (15%) patients. The percentage of pathological findings at each exploration is shown in Table 1.
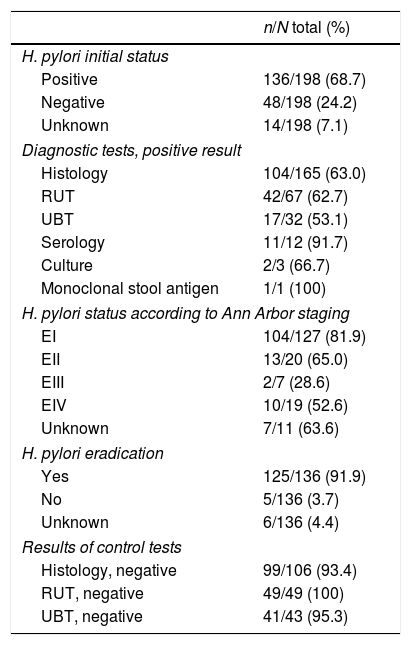
H. pylori statusH. pylori was considered to be present if at least one validated diagnostic test was positive. It was investigated in the majority of patients, but in 7% no test was performed, for a variety of reasons: surgery was the first treatment (upper GI bleeding), loss to follow-up, or advanced staging. Most patients were studied by histology and rapid urease test, and very few underwent confirmatory tests if initially negative for H. pylori. According to the Ann Arbor staging, H. pylori was present in 82% of EI, but in only 29–65% of advanced stages (Table 2). Treatment was based mainly on clarithromycin-containing triple therapy. Eradication was monitored mainly by histology and rapid urease test, and was cured in 182 (92%) patients (Table 2).
H. pylori status and tests results.
| n/N total (%) | |
|---|---|
| H. pylori initial status | |
| Positive | 136/198 (68.7) |
| Negative | 48/198 (24.2) |
| Unknown | 14/198 (7.1) |
| Diagnostic tests, positive result | |
| Histology | 104/165 (63.0) |
| RUT | 42/67 (62.7) |
| UBT | 17/32 (53.1) |
| Serology | 11/12 (91.7) |
| Culture | 2/3 (66.7) |
| Monoclonal stool antigen | 1/1 (100) |
| H. pylori status according to Ann Arbor staging | |
| EI | 104/127 (81.9) |
| EII | 13/20 (65.0) |
| EIII | 2/7 (28.6) |
| EIV | 10/19 (52.6) |
| Unknown | 7/11 (63.6) |
| H. pylori eradication | |
| Yes | 125/136 (91.9) |
| No | 5/136 (3.7) |
| Unknown | 6/136 (4.4) |
| Results of control tests | |
| Histology, negative | 99/106 (93.4) |
| RUT, negative | 49/49 (100) |
| UBT, negative | 41/43 (95.3) |
RUT: rapid urease test; UBT: urea breath test.
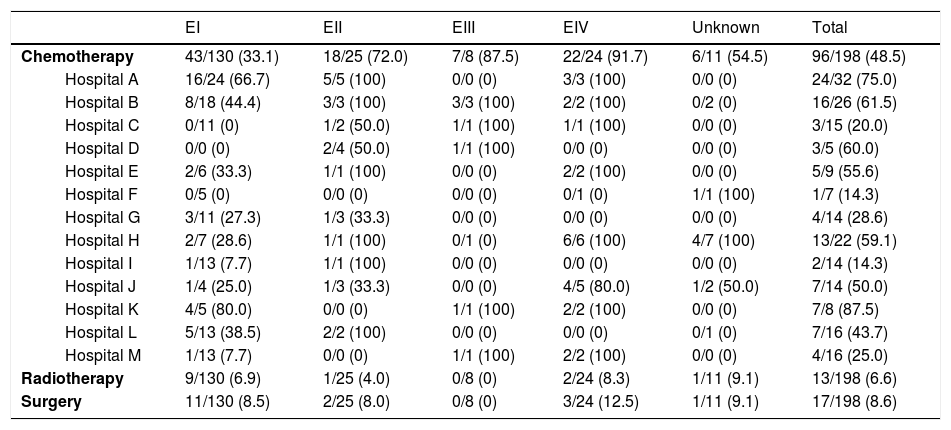
Chemotherapy was administered in 90 (45%) of patients: 43 (33%) in EI, 18 (72%) in EII, 7 (87%) in EIII and 22 (92%) in EIV. The most commonly used chemotherapy schedule was CHOP. Considerable heterogeneity was observed between the different hospitals in the administration of chemotherapy in EI patients, ranging from 0 to 80% (Table 3). In addition, in patients in an early stage (EI), chemotherapy was administered promptly during the first 180 days in 12 (34%) and during the first year in 17 (74%) of the 23 patients for whom data were available. The main reasons for early chemotherapy administration were the absence of H. pylori infection and the persistence of residual lymphoma after H. pylori eradication, although the reason was often unclear.
Additional treatments used according to the Ann Arbor staging in the different 13 hospitals participating in the study.
| EI | EII | EIII | EIV | Unknown | Total | ||
|---|---|---|---|---|---|---|---|
| Chemotherapy | 43/130 (33.1) | 18/25 (72.0) | 7/8 (87.5) | 22/24 (91.7) | 6/11 (54.5) | 96/198 (48.5) | |
| Hospital A | 16/24 (66.7) | 5/5 (100) | 0/0 (0) | 3/3 (100) | 0/0 (0) | 24/32 (75.0) | |
| Hospital B | 8/18 (44.4) | 3/3 (100) | 3/3 (100) | 2/2 (100) | 0/2 (0) | 16/26 (61.5) | |
| Hospital C | 0/11 (0) | 1/2 (50.0) | 1/1 (100) | 1/1 (100) | 0/0 (0) | 3/15 (20.0) | |
| Hospital D | 0/0 (0) | 2/4 (50.0) | 1/1 (100) | 0/0 (0) | 0/0 (0) | 3/5 (60.0) | |
| Hospital E | 2/6 (33.3) | 1/1 (100) | 0/0 (0) | 2/2 (100) | 0/0 (0) | 5/9 (55.6) | |
| Hospital F | 0/5 (0) | 0/0 (0) | 0/0 (0) | 0/1 (0) | 1/1 (100) | 1/7 (14.3) | |
| Hospital G | 3/11 (27.3) | 1/3 (33.3) | 0/0 (0) | 0/0 (0) | 0/0 (0) | 4/14 (28.6) | |
| Hospital H | 2/7 (28.6) | 1/1 (100) | 0/1 (0) | 6/6 (100) | 4/7 (100) | 13/22 (59.1) | |
| Hospital I | 1/13 (7.7) | 1/1 (100) | 0/0 (0) | 0/0 (0) | 0/0 (0) | 2/14 (14.3) | |
| Hospital J | 1/4 (25.0) | 1/3 (33.3) | 0/0 (0) | 4/5 (80.0) | 1/2 (50.0) | 7/14 (50.0) | |
| Hospital K | 4/5 (80.0) | 0/0 (0) | 1/1 (100) | 2/2 (100) | 0/0 (0) | 7/8 (87.5) | |
| Hospital L | 5/13 (38.5) | 2/2 (100) | 0/0 (0) | 0/0 (0) | 0/1 (0) | 7/16 (43.7) | |
| Hospital M | 1/13 (7.7) | 0/0 (0) | 1/1 (100) | 2/2 (100) | 0/0 (0) | 4/16 (25.0) | |
| Radiotherapy | 9/130 (6.9) | 1/25 (4.0) | 0/8 (0) | 2/24 (8.3) | 1/11 (9.1) | 13/198 (6.6) | |
| Surgery | 11/130 (8.5) | 2/25 (8.0) | 0/8 (0) | 3/24 (12.5) | 1/11 (9.1) | 17/198 (8.6) | |
n/N total (%)
In addition to eradication, 17 (9%) patients underwent surgery and 13 (7%) received radiotherapy; eleven patients (5%) received both surgery and chemotherapy.
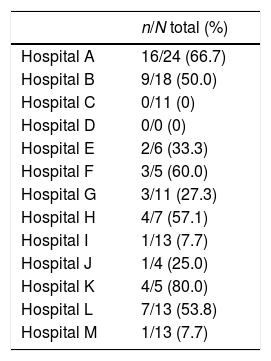
Overall, 51 out of 130 (39%) EI patients received at least one type of aggressive treatment (chemotherapy, surgery or radiotherapy). Considerable heterogeneity, ranging from 0 to 80%, was also observed between the centers (Table 4).
Different frequencies of the use of aggressive treatment chemotherapy, radiotherapy or surgery) in E1 Ann Arbor lesions according to each center.
| n/N total (%) | |
|---|---|
| Hospital A | 16/24 (66.7) |
| Hospital B | 9/18 (50.0) |
| Hospital C | 0/11 (0) |
| Hospital D | 0/0 (0) |
| Hospital E | 2/6 (33.3) |
| Hospital F | 3/5 (60.0) |
| Hospital G | 3/11 (27.3) |
| Hospital H | 4/7 (57.1) |
| Hospital I | 1/13 (7.7) |
| Hospital J | 1/4 (25.0) |
| Hospital K | 4/5 (80.0) |
| Hospital L | 7/13 (53.8) |
| Hospital M | 1/13 (7.7) |
After a mean follow-up of 8.4 years, 169 out of 198 patients (85%) were free of disease. Low-grade gastric MALT lymphoma was present at the end of follow-up in eight patients (6.1%) EI, 2 (8%) EII, 6 (75%) EIII, 11 (46%) EIV and 2 (18%) patients with unknown Ann Arbor stage. Mean follow-up in these uncured patients was 6.1 years.
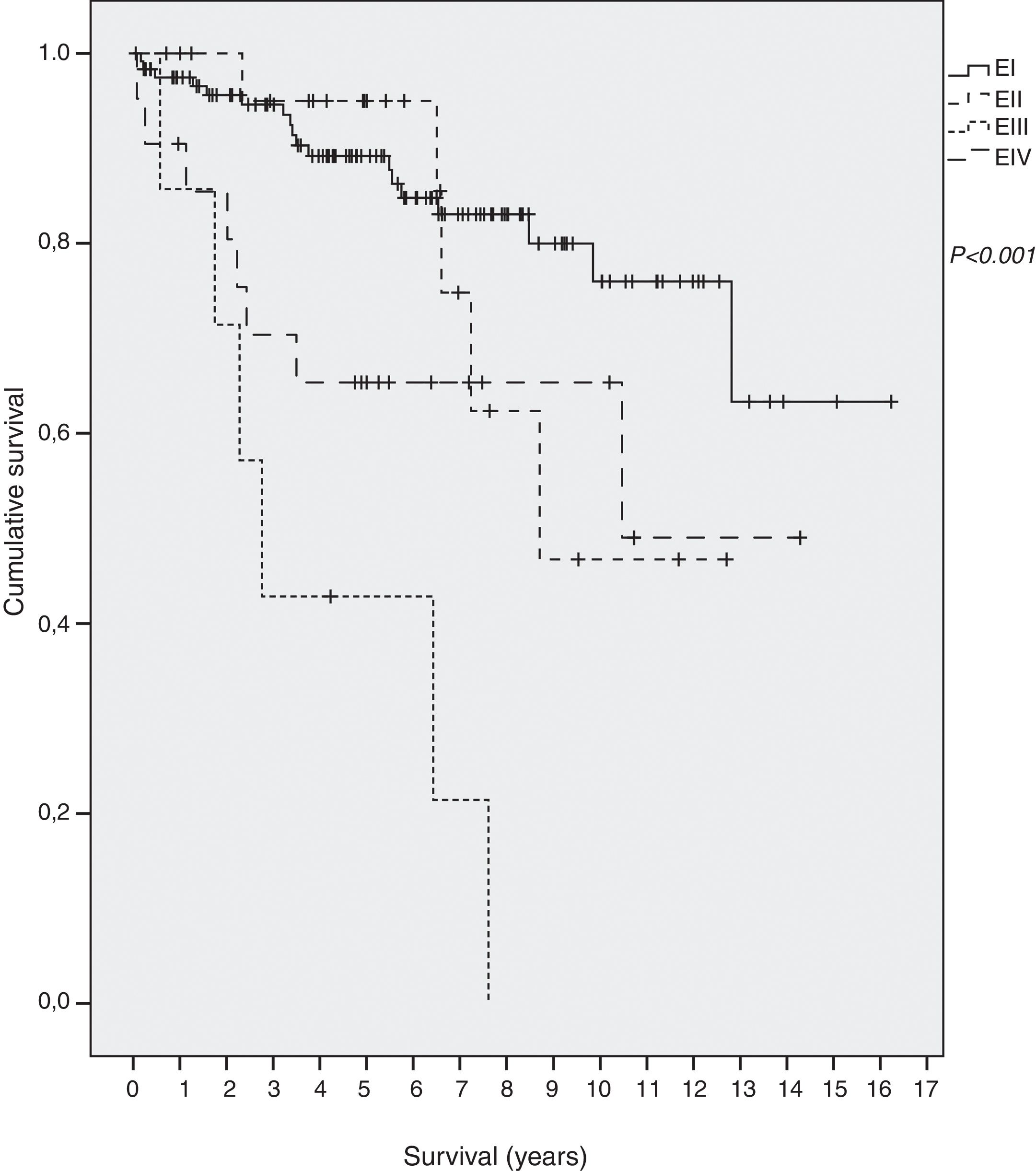
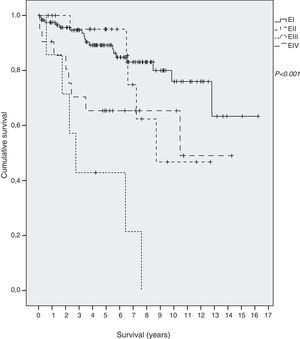
SurvivalForty-two out of 198 (21.2%) died during the follow-up period, thirteen of them (6.6%) due to the gastric MALT lymphoma. Overall five-year survival was 86%: 89% in EI, 96% in EII, 65% in EIII and 42% in EIV. Mean survival of all patients was 12.3 years (95% CI 11.0–13.5): 13.1 (95% CI 11.8–14.5) in EI, 9.6 (95% CI 7.7–11.6.) in EII, 4.1 in EIII (95% CI 1.9–6.2) and 9 in EIV (95% CI 6.6–11.9) (p<0.001) (Fig. 1).
Survival curves at each center showed considerable heterogeneity when all the patients were included, but also when only EI patients were analyzed (p=0.197). The five-year survival rate in EI patients ranged from 75% to 100%. These differences did not reach statistical significance.
Survival in EI patients treated with aggressive treatment (chemotherapy, radiotherapy or surgery) was similar to that in patients treated only with antibiotics (p=0.577) (Fig. 2).
DiscussionOur study includes one of the largest case series of gastric MALT lymphoma reported to date in Europe. There were three striking findings: first, our series confirms the good prognosis of this gastric MALT lymphoma (with a 5-year survival of 86%); second, the prevalence of H. pylori infection was 69% (82% in early stage); and third, the number of patients in whom rescue treatment was administered varied widely between hospitals.
The prevalence of H. pylori infection in this population was notably low, around 69%. Although these numbers are within the range of those published in other series and meta-analyses,16 the figure is still low especially bearing in mind that MALT gastric lymphoma is nearly always related to H. pylori infection.17 The main explanation for this finding is that extensive mucosal changes associated with the massive infiltration by neoplastic lymphocytes drastically reduce the H. pylori population, which often becomes undetectable. In this regard, the fact that only histology tests were used to investigate H. pylori infection in most patients might have contributed to the apparently low prevalence of the infection. It has been shown that the use of multiple tests markedly increases the rate of detection of H. pylori infection in these patients16,17; indeed, the Maastricht V/Florence consensus recommends performing serology and treating H. pylori in all serology-positive patients,18 and a systematic review of the published cases revealed that 17 of 110 H. pylori – negative cases (15%) achieved complete remission with antibiotic therapy alone.19
A second striking finding was the extensive use of chemotherapy, even at early stages and after successful H. pylori eradication. Furthermore, there were marked differences between centers in the proportion of patients receiving chemotherapy. Finally, there were no survival differences between patients who received chemotherapy and those who did not. Taken together, these findings suggest that aggressive treatment may have been unnecessarily administered in some patients with early-stage lesions which could have been cured with eradication treatment alone. A possible explanation is this fact that histological changes may persist for months after successful H. pylori eradication, despite the fact that the tumor may be in remission.7
Our results suggest that there is room for improvement in the treatment of low-grade gastric MALT lymphoma in Spain. Treatment schedules should be standardized, and premature chemotherapy avoided in patients with early-stage disease. Although the retrospective nature of the study did not allow us to reach definitive conclusions about the misuse of aggressive treatment, our results show a marked heterogeneity in the use of these aggressive treatments that did not seem to correlate with evident improvements in survival. Although the number of patients at each hospital is small and did not allow for comparisons, survival also shows a substantial heterogeneity between centers. Finally, the detection of H. pylori seems suboptimal. Serology was not performed in most of H. pylori-negative cases even though it is known that conventional methods do not achieve satisfactory results in the setting of extensive mucosal infiltration by the MALT lymphoma. Probably, a careful, multi-test investigation and/or the use of ultrasensitive techniques such as digital PCR20 may increase the chances of detecting the bacteria and thus of curing the disease using only a minimally invasive treatment like eradication therapy.
Our study has two main limitations: (1) Data were retrieved retrospectively and calculations were performed excluding missing data. The number of patients in whom data were available is reported in each variable; (2) Diagnosis of low-grade gastric MALT lymphoma was made based on the histology report of each hospital, and there is no information about a double check of the histology by expert pathologists and the immunohistochemistry panel used. Histology slides of low-grade EIV cases were not revised. Besides, fluorescence in situ hybridization studies for detection of t(11;18) (p21;p21) might be useful for identifying patients who are unlikely to respond to antibiotic; (3) the assessment of the gastric wall by USE was not widely used in those years and therefore, the Paris or the Lugano classification (5), that takes into account the infiltration depth and is currently recommended, is not available. Some EI patients with incomplete response to H. pylori eradication may be due to infiltration beyond the submucosa.
Results shown in this study are consistent with previous studies. A Japanese multicenter retrospective study including 420 patients with a mean follow up of 6.5 years showed that the probability of freedom from treatment failure, overall survival and event-free survival after 10 years were 90%, 95% and 86%, respectively.7 In Europe, the largest series ever published showed a complete remission rate of 85% in a retrospective analysis of a single center of 196 patients with a mean follow up of 27 months.15 However, none of these studies dealt with the heterogeneity of the treatments used in each center and the adherence to the clinical guidelines.
In conclusion, even though gastric MALT lymphoma has a good prognosis, our study suggests that there is room for improvement in its management. Ultrasound endoscopy was not widely used when the patients were diagnosed in the past decade and is currently a key point for an accurate staging. There is a need for measures directed to raise detection rates and to improve successful treatment of H. pylori infection. Furthermore, efforts to disseminate the criteria for defining remission and progression after successful H. pylori treatment are needed. This would help to avoid unnecessary aggressive treatments, especially the administration of chemotherapy in early-stage patients.
FundingThis study was supported by CIBERehd. CIBERehd is funded by the Instituto de Salud Carlos III.
Conflict of interestsThe authors declare no conflict of interest.
We thank Michael Maudsley for his help with the English