Post-operative morbidity of pancreatectomies occurs in up to 40–50% of patients, even in modern series. There is a need to find a simple scale in order to identify patients with increased risk of developing major post-operative complications after pancreatic resections. Many studies have been published on sarcopenia and surgical outcomes.
Aspects of sarcopenia are presented, along with a systematic review using PRISMA guidelines, in order to search for articles about sarcopenia and pancreatic surgery.
The impact of sarcopenia on morbidity and mortality in pancreatic resections is still unclear. The studies presented have been carried out over long periods of time, and many of them compare patients with different diseases. There are also different definitions of sarcopenia, and this can influence the results, as some of the reviewed articles have already shown. It is necessary to unify criteria, both in the definition and in the cut-off values. Prospective studies and consensus on sarcopenia diagnosis should be achieved.
La morbilidad postoperatoria de las pancreatectomías alcanza hasta el 40-50% de los pacientes, incluso en series modernas. Es necesaria una escala simple, capaz de identificar a los pacientes con mayor riesgo de desarrollar complicaciones postoperatorias después de las resecciones pancreáticas. Se han publicado múltiples estudios sobre sarcopenia y resultados quirúrgicos.
En este trabajo revisamos aspectos sobre la sarcopenia, realizando una revisión sistemática, de acuerdo con las guías PRISMA, buscando artículos sobre sarcopenia y cirugía pancreática.
El impacto de la sarcopenia en la morbimortalidad tras pancreatectomías aún no está claro. Los estudios presentados se han llevado a cabo en largos períodos de tiempo, muchos de ellos comparan pacientes con diferentes enfermedades. Además, la definición de sarcopenia es variada, pudiendo influir en los resultados como ya demuestran algunos de los artículos revisados. Deben realizarse estudios prospectivos, siendo necesario también unificar criterios en la definición y puntos de corte de la sarcopenia.
Main indications for pancreas resection are chronic pancreatitis (CP) and premalignant and malignant pancreatic lesions. Surgical resection is the only potentially curative treatment for pancreatic cancer.1 Almost half of patients with chronic pancreatitis (CP) require surgery during the course of the disease.2
Pancreatic surgery has historically been associated with high morbidity and mortality rates.3 As perioperative care has improved, mortality has widely decreased over the years, whereas the morbidity rate is still up to 50%.4 Pancreatoduodenectomy (PD) is one of the most complex abdominal operations, lasting on average 6–7h, with high blood loss even in experienced centers.5
Patients undergoing pancreas surgery assume a high risk of serious complications that considerably reduce survival, such as pancreatic fistula, gastrojejunostomy or biliary leakage, and surgical site infection (SSI).6 Perioperative complications affect both patient recovery and quality of life, and they also may delay oncological treatment, thus potentially affecting long-term survival.7 Outcomes after surgery are influenced by many factors including the disease process, surgeon or center volume, pancreas texture, and the patient's physical condition and comorbidities. Identification of specific risk factors that could predict complications after pancreatic surgery has been difficult and has been the focus of recent literature.3
Sarcopenia has received increasing attention as an important predictor of the postoperative outcomes following major surgery. This term includes sarcopenia, defined as the loss in muscle mass and muscle strength associating with aging, and secondary sarcopenia, defined as the loss of muscle mass and muscle strength that accompanies underlying diseases such as chronic kidney disease, chronic liver diseases, advanced malignancies and malnutrition (inadequate protein or caloric intake like patients with CP).8,9
Some investigators have proposed the use of sarcopenia as predictor of morbidity. Previous data have suggested that sarcopenia may be associated with worse outcomes among patients being treated with chemotherapy for pancreatic, breast, prostate, and renal cell cancer.1 Recently, mounting evidence associates sarcopenia with worsened prognosis after multiple abdominal operations, including gastric, liver, and emergency surgery.6 It also has recently been described as independent predictors of clinically relevant pancreatic fistula in patients undergoing pancreatoduodenectomy, but there is a lack of evidence of their effect on postoperative mortality.10
Studying muscle mass in patients undergoing pancreatectomy could be an effective way to stratify risk, as this patient group is at high risk for sarcopenia due to their underlying disease (cancer or chronic pancreatitis).3 Understanding such changes might allow better patient selection for surgery, help determine response to neoadjuvant therapies, or inform the design of novel preoperative adjuvant “prehabilitation” strategies employing exercise, dietary modifications, or drugs.11
Only a few studies have examined the association between sarcopenia and outcomes following pancreatic surgery. Our aim in this paper is to review sarcopenia and the effect of itself on morbidity and mortality of pancreas surgery.
We reviewed aspects about sarcopenia and performed a systematic review of the literature about sarcopenia in pancreatic surgery (effects on surgical outcomes).
Concept of sarcopeniaIn 1989, Rosenberg proposed the term ‘sarcopenia’ (Greek ‘sarx’ or flesh+‘penia’ or loss) to describe this age-related decrease of muscle mass.12,13 Before that, in 1931, Critchley shown the loss of muscle mass and function in the extremities due to aging using indirect methods in his study.14
Since then, there are multiple definitions of sarcopenia. Sarcopenia was defined by a consensus report published by the European Working Group on Sarcopenia in Older People (EWSGOP) at 2010. According to EWSGOP; evaluation of muscle mass, muscle strength and physical performance is necessary to diagnose sarcopenia. EWSGOP define sarcopenia as the presence of low skeletal muscle mass and either low muscle strength or low muscle performance; when all three conditions are present, severe sarcopenia may be diagnosed.15 In the same year, the European Society for Clinical Nutrition and Metabolism Special Interest Groups (ESPEN-SIG) define sarcopenia as the presence of low skeletal muscle mass and low muscle strength, assessed by walking speed.16 One year later the International Working Group on Sarcopenia (IWGS) describes sarcopenia as the presence of low skeletal muscle mass and low muscle function, assessed by walking speed, and that sarcopenia is associated with muscle mass loss alone or in conjunction with increased fat mass.17
Finally, in September 2016, sarcopenia has become a disease entity with the awarding of an ICD-10-CM (M62.84). This goal can lead to an accelerated interest of physicians diagnosing sarcopenia and pharmaceutical companies accelerating interest in developing drugs to treat sarcopenia.14
This year, the group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) have published a new revised European consensus on definition and diagnosis of sarcopenia, this updated recommendations aim to increase awareness of sarcopenia and its risk. With these new recommendations, EWGSOP2 calls for healthcare professionals who treat patients at risk for sarcopenia to take actions that will promote early detection and treatment.18
Based on the available literature, it would appear that sarcopenia is present in 5 to 10% of persons 65 years of age or older.14
Although sarcopenia is primarily a disease of the elderly, its development may be associated with conditions that are not exclusively seen in older persons, like disuse, malnutrition and cachexia. Like osteopenia, it can also be seen in younger patients such as those with inflammatory or chronic diseases. Muscle accounts for 60% of the body's protein stores. Muscle mass decrease is directly responsible for functional impairment with loss of strength, increased likelihood of falls, and loss of autonomy.13
The emphasis on a definition is because a disease definition is a prerequisite and a necessary keystone for any drug development program aiming to bring a new drug to the market. Federal regulations require that indications for therapeutic pharmaceuticals closely reflect recognized medical conditions. Specifically, Section 201.57 of the Code of Federal Regulations mandates that the Full prescribing information section of the physician label “must state that the drug is indicated for the treatment, prevention, mitigation, cure, or diagnosis of a recognized disease or condition, or of a manifestation of a recognized disease or condition, or for the relief of symptoms associated with a recognized disease or condition”.19
Sarcopenia quantificationThe parameters of sarcopenia are the amount of muscle and its function. The measurable variables are mass, strength and physical performance. The challenge is to determine how best to measure them accurately.15
As there has been a lot of controversy about sarcopenia definition, it is not easy to get a universal way to measure muscle mass. Methods for assessing sarcopenia are either complex, time consuming, or poorly validated.20
Most of articles published about surgical outcomes in patients with sarcopenia are retrospective articles, so they define sarcopenia with imagine techniques.
There are multiples radiological measures that have been approved to define sarcopenia.15 Computed tomography (CT), magnetic resonance imaging (MRI) and dual energy X-ray absorptiometry (DXA) can be used to estimate muscle mass15; the first two, CT and MRI, are the current gold standard in body composition evaluation.21 Multiples radiological measures have been approved to define sarcopenia.15 They usually use a single CT slice at the third lumbar vertebra (L3) due that it is strongly correlated with total body skeletal muscle (TSM) area.22
There are two ways used to define muscle mass with one CT slice:
- -
The psoas muscle-only approach. Psoas muscle at L3 psoas muscle is believe to be correlated with the total body skeletal area.1 That is the reason why some authors use total psoas index/total psoas area (TPI/TPA).
- -
The total abdominal muscle area/skeletal muscle index (TAMA/SMI), which includes the psoas, paraspinal, and abdominal wall muscles and excludes intra-abdominal visceral muscles.8
Some studies defend that only one CT slice may not be enough so they plead to measure the entire volume of psoas muscle, total psoas volume (TPV). TPV is calculated with total psoas length and not just one CT slice.23
However, measuring only the area of skeletal muscle using CT imaging may not be sufficient to evaluate muscle quality because we could not distinguish muscle from adipose tissue with that method, which might mislead us to evaluate low lean muscle mass with much adipose tissue as normal skeletal muscle mass.24 Intramuscular adipose tissue content (IMAC), the accumulation of fat in the skeletal muscle define a deterioration in the skeletal muscle quality and an essential component of sarcopenia.9 A few studies defined sarcopenia based on both psoas muscle area and psoas muscle density, expressed in Hounsfield Units. Psoas muscle density or total psoas density is a proxy for muscle quality as it accounts for fatty infiltration of muscle tissue. This is also known as the Hounsfield Unit Average Calculation, or HUAC.25
To date, no consensus exists on the optimal cut points to define secondary sarcopenia. Therefore, careful consideration should be given to the choice of cut point to define sarcopenia. Several factors can influence muscularity of patients (age, obesity, ethnicity, gender, socio-economic factors, and dietary habits), on which the cut point is dependent and should be taken into account.26
The other two measures, strength and physical performance can only be measure prospectively.
There are fewer well-validated techniques to measure muscle strength. One of the options to calculate it is the handgrip strength test, which can be quantified by measuring the amount of static force that the hand can squeeze around a dynamometer (massy westropp). It can be a reliable surrogate for more complicated measures of muscle strength in the lower arms or legs. It important to know that factors unrelated to muscle, e.g. motivation or cognition, may hamper the correct assessment of muscle strength.15
Multiple test can be used to calculate physical performance, the short physical performance battery (SPPB) is one of those test, measuring balance, gait speed, lower limb strength and endurance. It is quick to perform, easily replicable, requires little additional equipment, and with basic training can be performed by most healthcare personnel. SPPB test is validated to define sarcopenia in older people but it is not that much validated in secondary sarcopenia.27
Prevalence of sarcopenia in patients with pancreatic diseasesPancreatic cancerSarcopenia prevalence in pancreatic cancer has been reported in multiple studies. Prevalence variation was wide, ranging from 24.2% in a cohort with patients with resectable pancreatic adenocarcinoma4 to 86.3% in patients with recurrent or metastatic pancreatic adenocarcinoma.28 When regarding patients undergoing curative surgery the variation is generally lower (24.2%–40.6%),1,3,4,6,8,23,25,29–33 but Pecorelli et al.10 and Ninoyima et al.33 present prevalence of 66.83% and 64.15% which is much higher than the other 11 studies.
The administration of neoadjuvant therapy before planned pancreatectomy is increasingly gaining acceptance for patients with both resectable and borderline resectable pancreatic cancer.11 To date, only Cooper et al. have published the changed in sarcopenia prevalence before and after neoadjuvant therapy. They reported 4% increase, from 55% to 59% prevalence of sarcopenia.11 Sarcopenia is also important in patients undergoing neoadjuvant treatment because the ability to improve muscle indices is associated with response to treatment, an improvement of sarcopenia was associated with a higher likelihood of achieving resection.34
Chronic pancreatitisHigh prevalence of malnutrition, around 44–65%, in patients with CP is well known. This is due to the pancreatic exocrine insufficiency, to toxic habits (alcohol is the single most common etiologic for CP) and to the social and psychological factors frequently associated with the disease.34 Even though it is known that CP patients are in high risk of presenting sarcopenia, there are few studies in this field.35 To our knowledge, before 2019, the only ones reported were 52% reported by OConnor et al.36 and 18.3% presented by Olesen et al.37 Throughout the year 2019 several studies have been published about the prevalence and risk factors for sarcopenia in patients with CP and how does it affects the quality of life and treatment outcomes of this patients. Ozola et al. present a retrospective multicenter study of 265 patients with CP were the prevalence of sarcopenia was 20.4%.38 A prospective cohort study of CP outpatients, was published by Olensen et al.,39 with sarcopenia's prevalence of 17%, during the follow up, sarcopenia was associated with a worsened clinical outcome, including increased hospitalization rates and reduced survival.
Pancreas transplantationTo our knowledge, only two studies about the clinical impact of preoperative sarcopenia or PMI on the postoperative outcomes after pancreas transplantation have been published.9,40 Fukuda et al. describe a prevalence of 26.82% for sarcopenia and there was no significant differences regarding duration of diabetes, duration of hemodialysis or biochemical analysis (HbA1c, albumin) between patients with and without sarcopenia. Noguchi et al. concludes that a low PMI was not a significant predictive factor for acute rejection, but was an independent predictive factor for graft survival after the first acute rejection.40
Is it possible to minimize the impact of sarcopenia on patients undergoing pancreatic surgery?Sarcopenia is a modifiable condition. Although the treatment strategies are similar between primary and secondary sarcopenia, the treatment of the underlying illnesses for secondary sarcopenia is of outstanding importance.41 So treatment of the pancreatic disease is essential, though sometimes difficult.
Treatment options for sarcopenia comprise physical training, modifications of nutritional intake and pharmacological treatment.41
The most effective interventions to date are physical exercise and adequate nutritional protein intake.42 Yoh et al. have started the first prospective randomized trial to examine the influence of exercise therapy on sarcopenia in patients with chronic pancreatitis.43 Despite these clinical benefits, there are limited data available regarding patients with pancreatic cancer undergoing exercise therapy.43
Pharmacological therapies for sarcopenia including inhibitors of myostatin, testosterone, selected androgen receptor modulators, ghrelin agonists, and angiotensin-converting enzyme (ACE) inhibitors have been evaluated, but preliminary trials have found that they are less effective than postulated.42
Since EPI has been demonstrated as an independent risk factor for sarcopenia,39 the benefits of pancreatic enzyme replacement therapy to correct EPI and malnutrition should also be considered as part of sarcopenia treatment, but further studies are required to determine optimal regimens, the impact of health inequalities and long-term effects on nutrition.44
Sarcopenia and pancreas resection: systematic reviewIn the last years, surgical risk stratification has gained popularity because of the growing number of patients with advanced age and multiple comorbidities undergoing major surgery.5 Assessment and stratification of the surgery-related risk may allow clinicians to manage patients through different pathways and candidate-specific cohorts to prehabilitation programs before surgery.7 Pancreatic surgery needs a simple score able to identify patients with increased risk of developing all major postoperative complications.5
Non-modifiable factors such as patients’ age, BMI higher than 40, comorbidity, ASA score, texture of the pancreatic remnant, major pancreatic duct diameter and the underlying pathology that indicate surgery may be inherently associated with risk.5,45 Since it is not possible to change that factors, in the last years to identify modifiable high risk factor has been progressively gained interest. Specifically, sarcopenia has gained significant recognition as an important prognostic factor for both complications and survival in cancer patients.46
In the last years multiple studies about sarcopenia and surgical outcomes have been published. The problem is that there is a wide variation between them, some of them include different types of cancer,46 other ones include in the same study patients with malign and benign pathologies or patients with neoadjuvancy, radiotherapy, palliative surgery, etc.
We therefore performed a systematic literature review, in accordance with the PRISMA guidelines, on patients with sarcopenia who had undergone pancreatic surgery up to November 2019.
The search items were the following MESH terms: ((Sarcopenia) AND (Pancreas) OR (Pancreatic Neoplasms) OR (Pancreas Transplantation) OR (Carcinoma, Pancreatic Ductal) OR (Pancreatitis) OR (Neoplasm, Pancreatic) OR (Pancreatic Neoplasm) OR (Pancreas Neoplasms) OR (Neoplasm, Pancreas) OR (Neoplasms, Pancreas) OR (Pancreas Neoplasm) OR (Neoplasms, Pancreatic) OR (Cancer of Pancreas) or (Pancreas Cancers) OR (Pancreas Cancer) OR (Cancer, Pancreas) OR (Cancers, Pancreas) OR (Pancreatic Cancer) OR (Cancer, Pancreatic) OR (Cancers, Pancreatic) OR (Pancreatic Cancers) OR (Cancer of the Pancreas)).
The articles were included or rejected based on the information in the title and summary, and in case of doubt, after reading the complete article.
Eligibility criteria were any type of article that included patients with sarcopenia who had undergone pancreatic surgery, excluding series where all patients were treated with neoadjuvant therapy or receiving palliative surgery. We did not include studies carried out in patients with preoperative chemotherapy or radiotherapy because we aimed to determine whether sarcopenia is an independent prognostic factor for pancreatic surgery rather than a prognostic factor associated with various treatments.
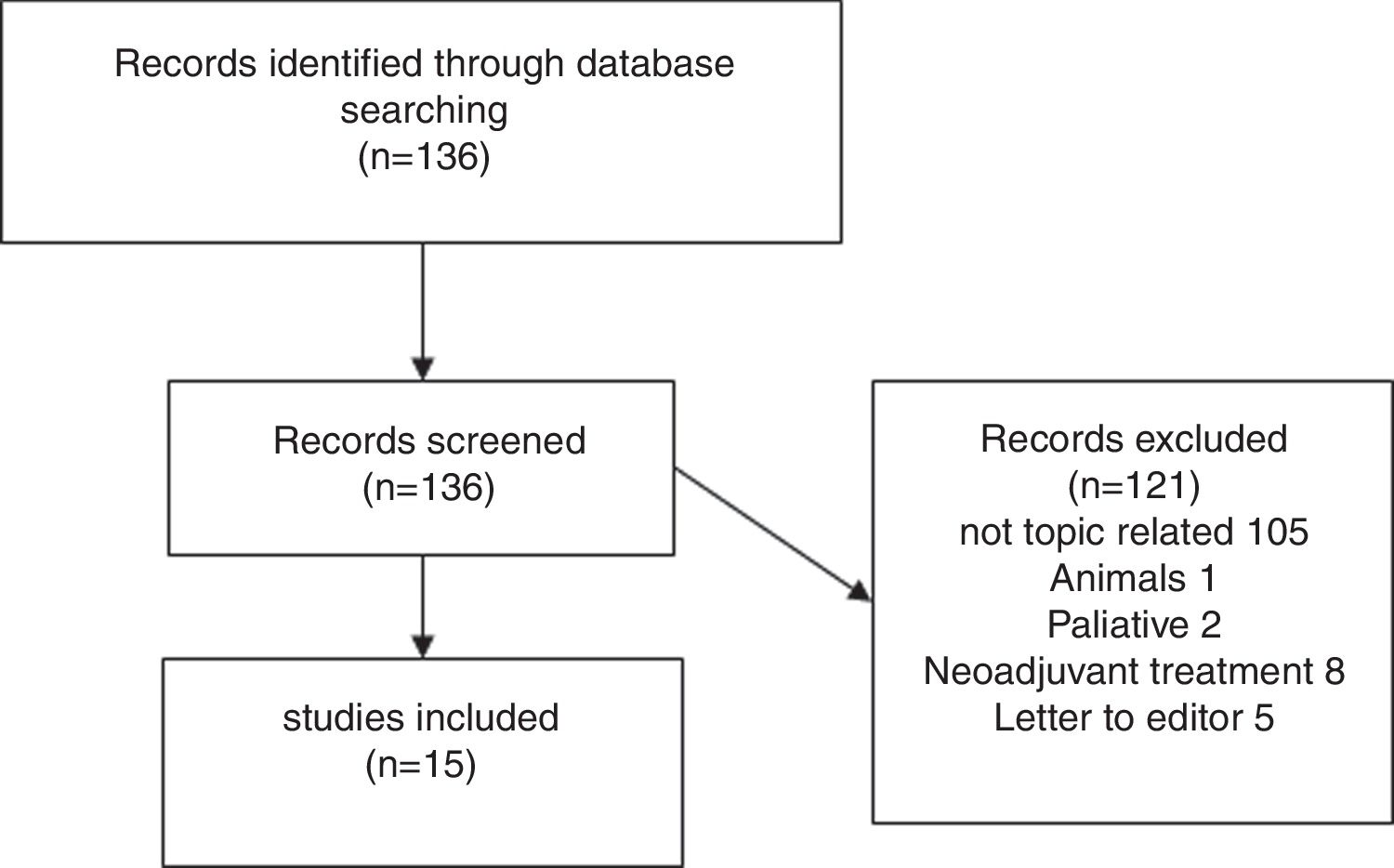
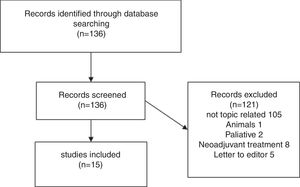
Results of systematic reviewFig. 1 presents a flowchart of systematic review of patients with sarcopenia who had undergone pancreatic surgery. The initial search yielded, 95 136 articles, but only 15 (11%) met the search criteria (Fig. 1).
Fifteen articles comparing patients undergoing pancreatic surgery with and without sarcopenia were included. All of them were case series, with a total of 3675 patients, 1202 (32.7%) were classified like sarcopenic patients.
Amini et al.23 reported 763 pancreatectomies that represent the higher number in studies that only include pancreatectomies.
Eight of the included studies were retrospective,1,3,8,9,23,25,32,49 only five of them collected the data prospectively in databases and the files were retrospectively extracted4,10,31,48,50 and in the other 2 articles it was not specify.33,47
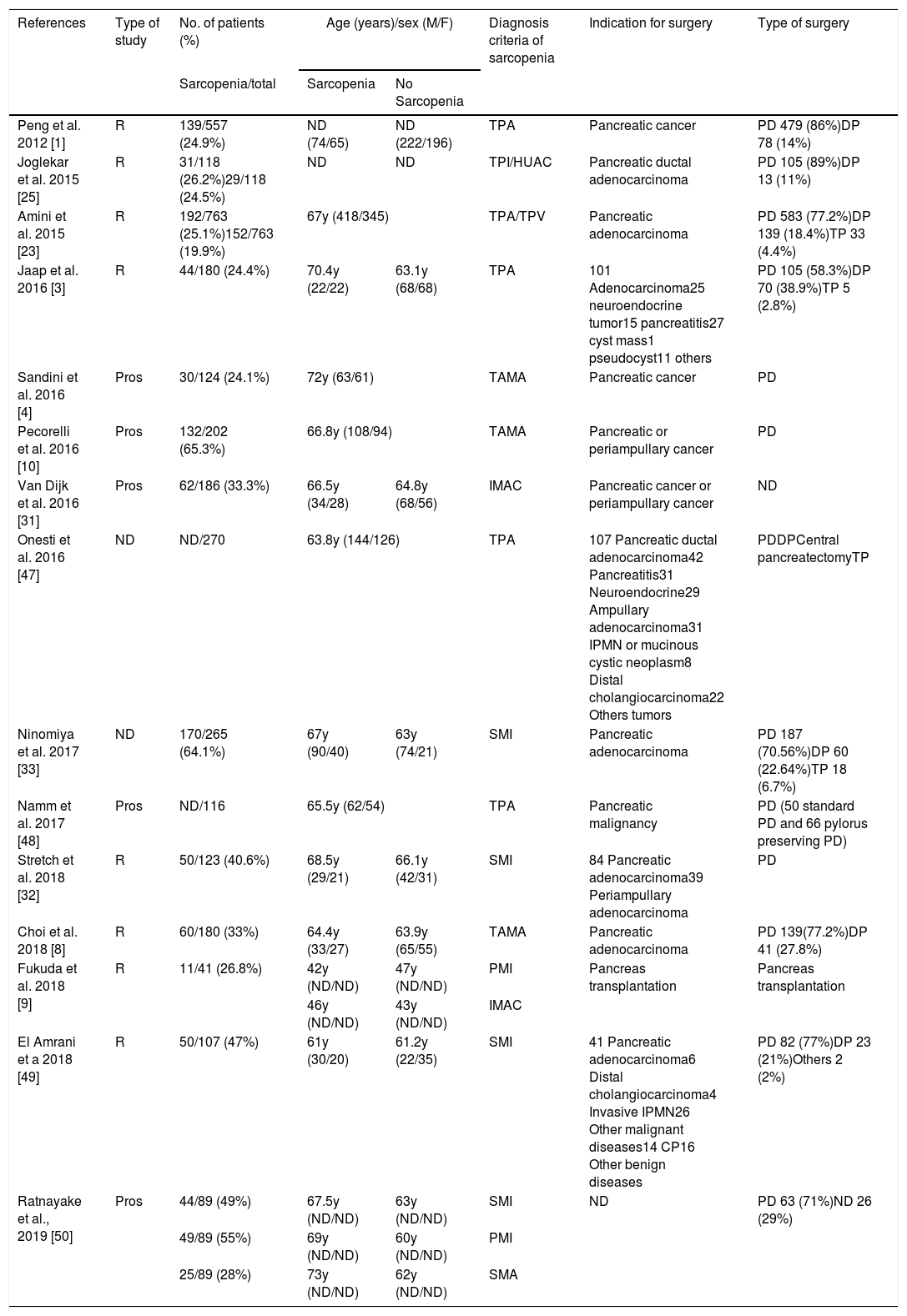
Demographic and clinical characteristics (Table 1)Most of patients were on 6th or 7th decade of life except for Fukuda et al.9 were medium age were 44 years. The pooled age trended toward a higher age in sarcopenic patients but did not reached statistical significance.
Demographic and clinical characteristics.
| References | Type of study | No. of patients (%) | Age (years)/sex (M/F) | Diagnosis criteria of sarcopenia | Indication for surgery | Type of surgery | |
|---|---|---|---|---|---|---|---|
| Sarcopenia/total | Sarcopenia | No Sarcopenia | |||||
| Peng et al. 2012 [1] | R | 139/557 (24.9%) | ND (74/65) | ND (222/196) | TPA | Pancreatic cancer | PD 479 (86%)DP 78 (14%) |
| Joglekar et al. 2015 [25] | R | 31/118 (26.2%)29/118 (24.5%) | ND | ND | TPI/HUAC | Pancreatic ductal adenocarcinoma | PD 105 (89%)DP 13 (11%) |
| Amini et al. 2015 [23] | R | 192/763 (25.1%)152/763 (19.9%) | 67y (418/345) | TPA/TPV | Pancreatic adenocarcinoma | PD 583 (77.2%)DP 139 (18.4%)TP 33 (4.4%) | |
| Jaap et al. 2016 [3] | R | 44/180 (24.4%) | 70.4y (22/22) | 63.1y (68/68) | TPA | 101 Adenocarcinoma25 neuroendocrine tumor15 pancreatitis27 cyst mass1 pseudocyst11 others | PD 105 (58.3%)DP 70 (38.9%)TP 5 (2.8%) |
| Sandini et al. 2016 [4] | Pros | 30/124 (24.1%) | 72y (63/61) | TAMA | Pancreatic cancer | PD | |
| Pecorelli et al. 2016 [10] | Pros | 132/202 (65.3%) | 66.8y (108/94) | TAMA | Pancreatic or periampullary cancer | PD | |
| Van Dijk et al. 2016 [31] | Pros | 62/186 (33.3%) | 66.5y (34/28) | 64.8y (68/56) | IMAC | Pancreatic cancer or periampullary cancer | ND |
| Onesti et al. 2016 [47] | ND | ND/270 | 63.8y (144/126) | TPA | 107 Pancreatic ductal adenocarcinoma42 Pancreatitis31 Neuroendocrine29 Ampullary adenocarcinoma31 IPMN or mucinous cystic neoplasm8 Distal cholangiocarcinoma22 Others tumors | PDDPCentral pancreatectomyTP | |
| Ninomiya et al. 2017 [33] | ND | 170/265 (64.1%) | 67y (90/40) | 63y (74/21) | SMI | Pancreatic adenocarcinoma | PD 187 (70.56%)DP 60 (22.64%)TP 18 (6.7%) |
| Namm et al. 2017 [48] | Pros | ND/116 | 65.5y (62/54) | TPA | Pancreatic malignancy | PD (50 standard PD and 66 pylorus preserving PD) | |
| Stretch et al. 2018 [32] | R | 50/123 (40.6%) | 68.5y (29/21) | 66.1y (42/31) | SMI | 84 Pancreatic adenocarcinoma39 Periampullary adenocarcinoma | PD |
| Choi et al. 2018 [8] | R | 60/180 (33%) | 64.4y (33/27) | 63.9y (65/55) | TAMA | Pancreatic adenocarcinoma | PD 139(77.2%)DP 41 (27.8%) |
| Fukuda et al. 2018 [9] | R | 11/41 (26.8%) | 42y (ND/ND) | 47y (ND/ND) | PMI | Pancreas transplantation | Pancreas transplantation |
| 46y (ND/ND) | 43y (ND/ND) | IMAC | |||||
| El Amrani et a 2018 [49] | R | 50/107 (47%) | 61y (30/20) | 61.2y (22/35) | SMI | 41 Pancreatic adenocarcinoma6 Distal cholangiocarcinoma4 Invasive IPMN26 Other malignant diseases14 CP16 Other benign diseases | PD 82 (77%)DP 23 (21%)Others 2 (2%) |
| Ratnayake et al., 2019 [50] | Pros | 44/89 (49%) | 67.5y (ND/ND) | 63y (ND/ND) | SMI | ND | PD 63 (71%)ND 26 (29%) |
| 49/89 (55%) | 69y (ND/ND) | 60y (ND/ND) | PMI | ||||
| 25/89 (28%) | 73y (ND/ND) | 62y (ND/ND) | SMA | ||||
Pros: prospective; R: retrospective; M: male; F: female; ND: not described; TPA: total psoas muscle area; TPI: total psoas index; HUAC: Hounsfield Unit Average Calculation; TPV: total psoas volume; TAMA: total abdominal muscle area; SMI: skeletal muscle index; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; TP: total pancreatectomy; PD: pancreatoduodenectomy; DP: distal pancreatectomy; CCR: colorectal; SMA: skeletal muscle attenuation.
Preoperative CT imaging was used to determine sarcopenic status. Although the terminology varied to asses sarcopenia. TPA/TPI was used in 6 studies1,3,23,47,48,50; 7 studies used TAMA/SMI4,8,10,32,49,50; HUAC, IMAC or SMA9,15,16,50 and TPV.14
Joglekar et al.,25 Amini et al.,23 Fukuda et al.,9 and Ratnayake et al.50 use more than one method to define sarcopenia and all of them realize a multiple study based on the different groups of sarcopenia, HUAC vs. TPI; TPA vs. TPI and IMAC; SMI vs. PMI vs. SMA respectively.
Cut off values were also diverse, one of the studies49 chose predefine cut off values by Prado et al.51; three of them4,9,50 used cut off values published by Martin et al.52 and the rest created their own cut off value based on quartiles or tertiles.1,3,8,10,23,25,31–33,47,49
Ten of the studies were carried out exclusively in oncological patients.1,4,8,10,23,25,31–33,48 Only 3 studies include patients with benign pathology undergoing pancreas resection in addition to oncological patients. Benign pathologies represent a 2.15% of the studied population (98/4562).3,47,49 Ratnayate et al. include patients with pancreatic adenocarcinoma and others patients undergoing surgery but do not reflect the cause of the surgery.50 Finally Fukuda et al. report the only study about sarcopenia influence in pancreatic transplantation.9
In total 3229 pancreatectomies were reported, PD was the most commonly performed pancreatic resection (82.4%).
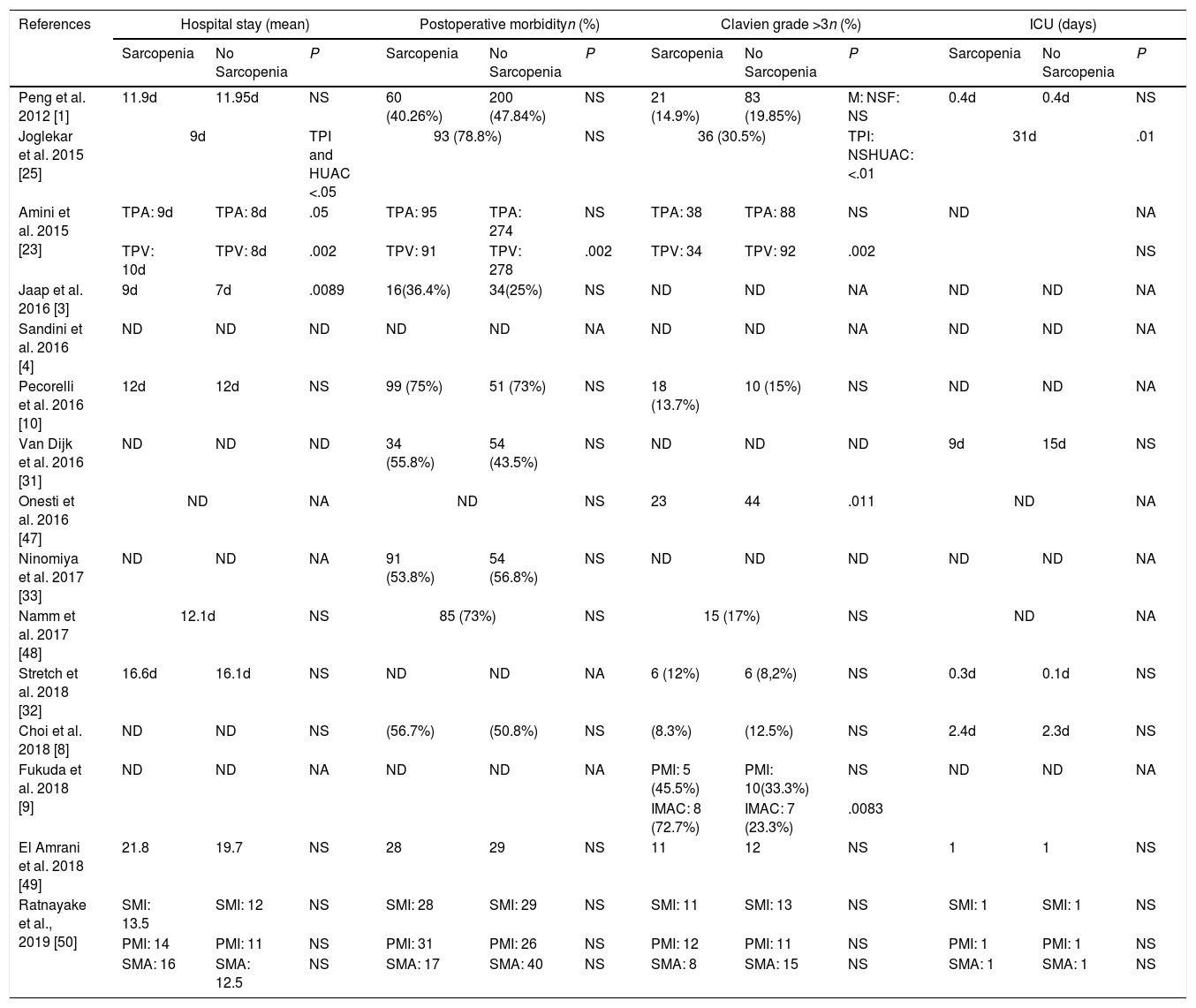
Impact of sarcopenia on postoperative outcomesPatients with sarcopenia had an increased length of hospital stay with statistical signification in 3 of the studies analyzed.3,23,25 Only Joglekar et al. report sarcopenia patients to have longer ICU stay25 (Table 2).
Impact of sarcopenia on hospital stay, ICU stay and general morbidity.
| References | Hospital stay (mean) | Postoperative morbidityn (%) | Clavien grade >3n (%) | ICU (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia | No Sarcopenia | P | Sarcopenia | No Sarcopenia | P | Sarcopenia | No Sarcopenia | P | Sarcopenia | No Sarcopenia | P | |
| Peng et al. 2012 [1] | 11.9d | 11.95d | NS | 60 (40.26%) | 200 (47.84%) | NS | 21 (14.9%) | 83 (19.85%) | M: NSF: NS | 0.4d | 0.4d | NS |
| Joglekar et al. 2015 [25] | 9d | TPI and HUAC <.05 | 93 (78.8%) | NS | 36 (30.5%) | TPI: NSHUAC: <.01 | 31d | .01 | ||||
| Amini et al. 2015 [23] | TPA: 9d | TPA: 8d | .05 | TPA: 95 | TPA: 274 | NS | TPA: 38 | TPA: 88 | NS | ND | NA | |
| TPV: 10d | TPV: 8d | .002 | TPV: 91 | TPV: 278 | .002 | TPV: 34 | TPV: 92 | .002 | NS | |||
| Jaap et al. 2016 [3] | 9d | 7d | .0089 | 16(36.4%) | 34(25%) | NS | ND | ND | NA | ND | ND | NA |
| Sandini et al. 2016 [4] | ND | ND | ND | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Pecorelli et al. 2016 [10] | 12d | 12d | NS | 99 (75%) | 51 (73%) | NS | 18 (13.7%) | 10 (15%) | NS | ND | ND | NA |
| Van Dijk et al. 2016 [31] | ND | ND | ND | 34 (55.8%) | 54 (43.5%) | NS | ND | ND | ND | 9d | 15d | NS |
| Onesti et al. 2016 [47] | ND | NA | ND | NS | 23 | 44 | .011 | ND | NA | |||
| Ninomiya et al. 2017 [33] | ND | ND | NA | 91 (53.8%) | 54 (56.8%) | NS | ND | ND | ND | ND | ND | NA |
| Namm et al. 2017 [48] | 12.1d | NS | 85 (73%) | NS | 15 (17%) | NS | ND | NA | ||||
| Stretch et al. 2018 [32] | 16.6d | 16.1d | NS | ND | ND | NA | 6 (12%) | 6 (8,2%) | NS | 0.3d | 0.1d | NS |
| Choi et al. 2018 [8] | ND | ND | NS | (56.7%) | (50.8%) | NS | (8.3%) | (12.5%) | NS | 2.4d | 2.3d | NS |
| Fukuda et al. 2018 [9] | ND | ND | NA | ND | ND | NA | PMI: 5 (45.5%) | PMI: 10(33.3%) | NS | ND | ND | NA |
| IMAC: 8 (72.7%) | IMAC: 7 (23.3%) | .0083 | ||||||||||
| El Amrani et al. 2018 [49] | 21.8 | 19.7 | NS | 28 | 29 | NS | 11 | 12 | NS | 1 | 1 | NS |
| Ratnayake et al., 2019 [50] | SMI: 13.5 | SMI: 12 | NS | SMI: 28 | SMI: 29 | NS | SMI: 11 | SMI: 13 | NS | SMI: 1 | SMI: 1 | NS |
| PMI: 14 | PMI: 11 | NS | PMI: 31 | PMI: 26 | NS | PMI: 12 | PMI: 11 | NS | PMI: 1 | PMI: 1 | NS | |
| SMA: 16 | SMA: 12.5 | NS | SMA: 17 | SMA: 40 | NS | SMA: 8 | SMA: 15 | NS | SMA: 1 | SMA: 1 | NS | |
NA: not applicable; ND: not described; NS: not significant. TPA: total psoas muscle area; TPI: total psoas index; TPV: total psoas volume; PMI psoas muscle index; IMAC: intramuscular adipose tissue content; SMI: skeletal muscle index; SMA: skeletal muscle attenuation.
Only one series reported a higher postoperative morbidity with statistical signification.14 Though in the study presented by Amini et al.23 this significance is only achieve when TPV is used to define sarcopenia, when sarcopenia is define by TPA, there are no difference in general morbidity. Surprisingly, van Dijk et al.31 found that patients with sarcopenia had a reduced risk of post-operative complications. Eight of the studies1,3,8,10,25,33,49 did not show a benefit for non sarcopenic patients with respect to complication risk (Table 2).
Referring to major complications, patients presenting with sarcopenia were more likely to have a Clavien score >3 in one study.53 Joglekar et al.25 when defining sarcopenia using HUAC; Amini et al.23 in the TPV sarcopenia group and Fukuda et al.9 in the IMAC group also report a higher risk of Clavien >3 postoperative morbidity (Table 2).
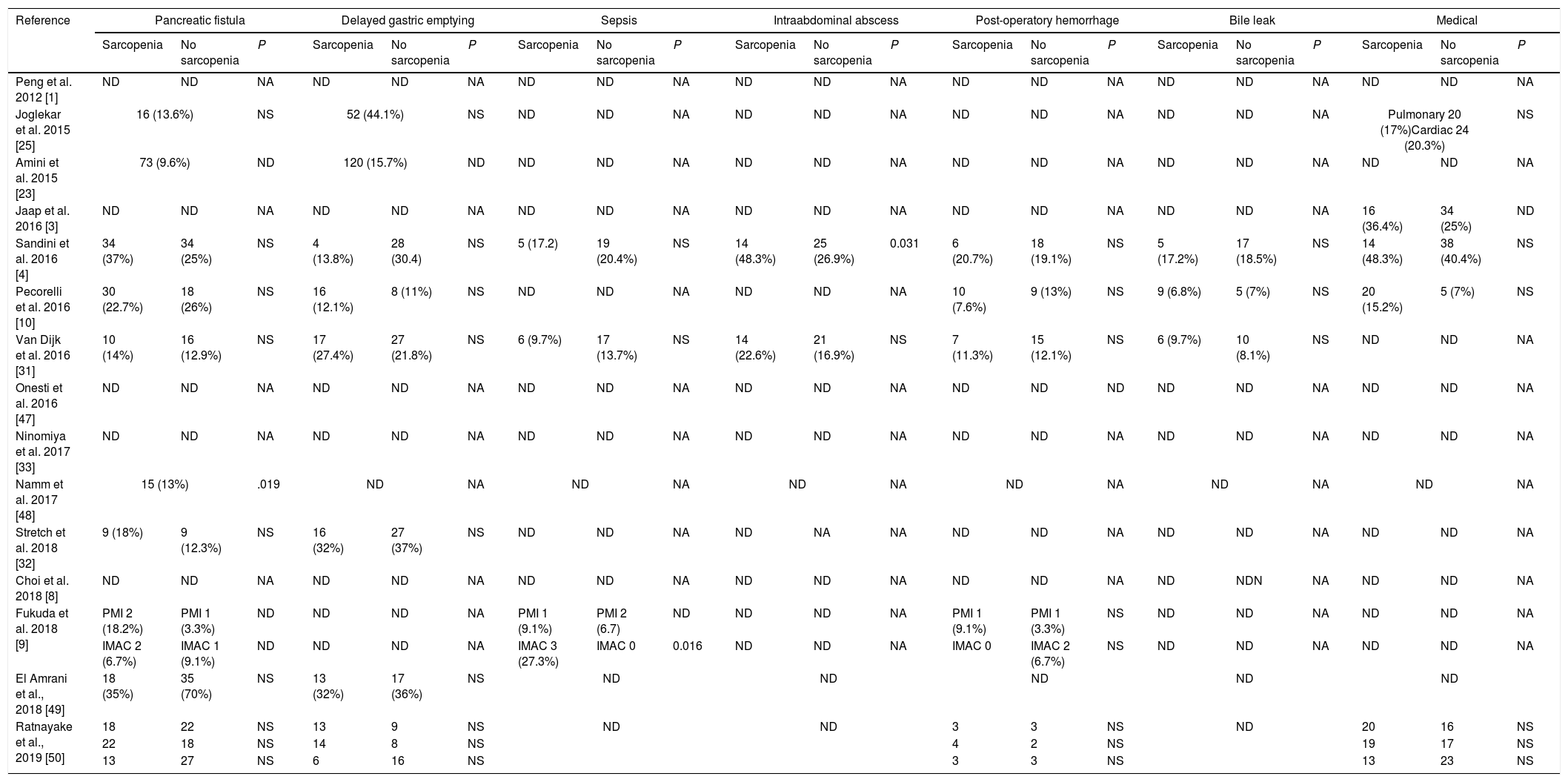
Table 3 describes surgical and medical complications in sarcopenic and non-sarcopenic patients. Only 1 study find a higher risk of pancreatic fistula in patients with sarcopenia.49 Sandini et al.4 reports a higher trend of abdominal abscesses in sarcopenic patients. No difference where observed according to other morbidities.
Morbidity.
| Reference | Pancreatic fistula | Delayed gastric emptying | Sepsis | Intraabdominal abscess | Post-operatory hemorrhage | Bile leak | Medical | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | |
| Peng et al. 2012 [1] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Joglekar et al. 2015 [25] | 16 (13.6%) | NS | 52 (44.1%) | NS | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | Pulmonary 20 (17%)Cardiac 24 (20.3%) | NS | |||
| Amini et al. 2015 [23] | 73 (9.6%) | ND | 120 (15.7%) | ND | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ||
| Jaap et al. 2016 [3] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | 16 (36.4%) | 34 (25%) | ND |
| Sandini et al. 2016 [4] | 34 (37%) | 34 (25%) | NS | 4 (13.8%) | 28 (30.4) | NS | 5 (17.2) | 19 (20.4%) | NS | 14 (48.3%) | 25 (26.9%) | 0.031 | 6 (20.7%) | 18 (19.1%) | NS | 5 (17.2%) | 17 (18.5%) | NS | 14 (48.3%) | 38 (40.4%) | NS |
| Pecorelli et al. 2016 [10] | 30 (22.7%) | 18 (26%) | NS | 16 (12.1%) | 8 (11%) | NS | ND | ND | NA | ND | ND | NA | 10 (7.6%) | 9 (13%) | NS | 9 (6.8%) | 5 (7%) | NS | 20 (15.2%) | 5 (7%) | NS |
| Van Dijk et al. 2016 [31] | 10 (14%) | 16 (12.9%) | NS | 17 (27.4%) | 27 (21.8%) | NS | 6 (9.7%) | 17 (13.7%) | NS | 14 (22.6%) | 21 (16.9%) | NS | 7 (11.3%) | 15 (12.1%) | NS | 6 (9.7%) | 10 (8.1%) | NS | ND | ND | NA |
| Onesti et al. 2016 [47] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | ND | ND | ND | NA | ND | ND | NA |
| Ninomiya et al. 2017 [33] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Namm et al. 2017 [48] | 15 (13%) | .019 | ND | NA | ND | NA | ND | NA | ND | NA | ND | NA | ND | NA | |||||||
| Stretch et al. 2018 [32] | 9 (18%) | 9 (12.3%) | NS | 16 (32%) | 27 (37%) | NS | ND | ND | NA | ND | NA | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Choi et al. 2018 [8] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | NDN | NA | ND | ND | NA |
| Fukuda et al. 2018 [9] | PMI 2 (18.2%) | PMI 1 (3.3%) | ND | ND | ND | NA | PMI 1 (9.1%) | PMI 2 (6.7) | ND | ND | ND | NA | PMI 1 (9.1%) | PMI 1 (3.3%) | NS | ND | ND | NA | ND | ND | NA |
| IMAC 2 (6.7%) | IMAC 1 (9.1%) | ND | ND | ND | NA | IMAC 3 (27.3%) | IMAC 0 | 0.016 | ND | ND | NA | IMAC 0 | IMAC 2 (6.7%) | NS | ND | ND | NA | ND | ND | NA | |
| El Amrani et al., 2018 [49] | 18 (35%) | 35 (70%) | NS | 13 (32%) | 17 (36%) | NS | ND | ND | ND | ND | ND | ||||||||||
| Ratnayake et al., 2019 [50] | 18 | 22 | NS | 13 | 9 | NS | ND | ND | 3 | 3 | NS | ND | 20 | 16 | NS | ||||||
| 22 | 18 | NS | 14 | 8 | NS | 4 | 2 | NS | 19 | 17 | NS | ||||||||||
| 13 | 27 | NS | 6 | 16 | NS | 3 | 3 | NS | 13 | 23 | NS | ||||||||||
NA: not applicable; ND: not described; NS: not significant; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; SMI: skeletal muscle index; SMA: skeletal muscle attenuation.
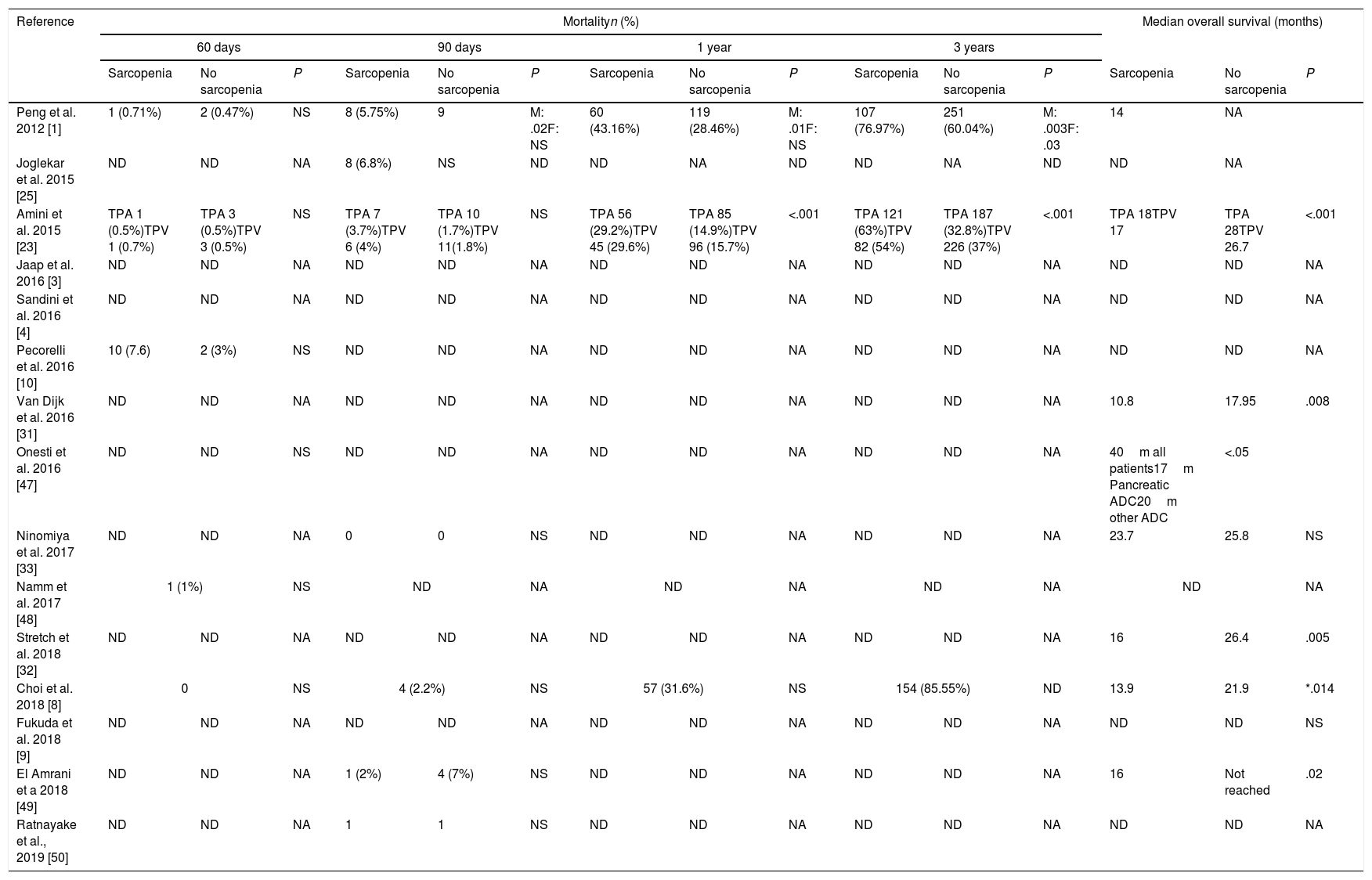
No significant difference was found among the studies that presented 60 days mortality.
Impact of sarcopenia in mortality and overall survival.
| Reference | Mortalityn (%) | Median overall survival (months) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 days | 90 days | 1 year | 3 years | ||||||||||||
| Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | Sarcopenia | No sarcopenia | P | |
| Peng et al. 2012 [1] | 1 (0.71%) | 2 (0.47%) | NS | 8 (5.75%) | 9 | M: .02F: NS | 60 (43.16%) | 119 (28.46%) | M: .01F: NS | 107 (76.97%) | 251 (60.04%) | M: .003F: .03 | 14 | NA | |
| Joglekar et al. 2015 [25] | ND | ND | NA | 8 (6.8%) | NS | ND | ND | NA | ND | ND | NA | ND | ND | NA | |
| Amini et al. 2015 [23] | TPA 1 (0.5%)TPV 1 (0.7%) | TPA 3 (0.5%)TPV 3 (0.5%) | NS | TPA 7 (3.7%)TPV 6 (4%) | TPA 10 (1.7%)TPV 11(1.8%) | NS | TPA 56 (29.2%)TPV 45 (29.6%) | TPA 85 (14.9%)TPV 96 (15.7%) | <.001 | TPA 121 (63%)TPV 82 (54%) | TPA 187 (32.8%)TPV 226 (37%) | <.001 | TPA 18TPV 17 | TPA 28TPV 26.7 | <.001 |
| Jaap et al. 2016 [3] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Sandini et al. 2016 [4] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Pecorelli et al. 2016 [10] | 10 (7.6) | 2 (3%) | NS | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA |
| Van Dijk et al. 2016 [31] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | 10.8 | 17.95 | .008 |
| Onesti et al. 2016 [47] | ND | ND | NS | ND | ND | NA | ND | ND | NA | ND | ND | NA | 40m all patients17m Pancreatic ADC20m other ADC | <.05 | |
| Ninomiya et al. 2017 [33] | ND | ND | NA | 0 | 0 | NS | ND | ND | NA | ND | ND | NA | 23.7 | 25.8 | NS |
| Namm et al. 2017 [48] | 1 (1%) | NS | ND | NA | ND | NA | ND | NA | ND | NA | |||||
| Stretch et al. 2018 [32] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | 16 | 26.4 | .005 |
| Choi et al. 2018 [8] | 0 | NS | 4 (2.2%) | NS | 57 (31.6%) | NS | 154 (85.55%) | ND | 13.9 | 21.9 | *.014 | ||||
| Fukuda et al. 2018 [9] | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NA | ND | ND | NS |
| El Amrani et a 2018 [49] | ND | ND | NA | 1 (2%) | 4 (7%) | NS | ND | ND | NA | ND | ND | NA | 16 | Not reached | .02 |
| Ratnayake et al., 2019 [50] | ND | ND | NA | 1 | 1 | NS | ND | ND | NA | ND | ND | NA | ND | ND | NA |
NA: not applicable; ND: not described; NS: not significant; M: male; F: female; TPA: total psoas muscle area; TPI: total psoas index; TPV: total psoas volume; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content.
60-days mortality was described in 5 of the included studies.1,8,10,23,48 Choi et al.8 report none mortality and Pecorilli et al.10 present a 7.6% mortality rate which is the highest rate in this review.
90-days mortality that can be consider like perioperative mortality54 is described in 7 of the studies. The higher rate, a 6.8%, is reported by Joglekar et al.25 Only one study find a statistical signification between sarcopenic men and 90-days mortality, which does not happen in sarcopenic women.1
Regarding 3-years mortality and overall survival, the three studies1,8,23 that analyzed them find a significant relation between sarcopenia and 3-year mortality but only 2 of them1,23 are significant. Overall survival is reported in 8 studies1,8,23,31–33,47,49 but only 6 have statistical signifition.6,8,23,31,32,49 Choi et al. emphasizes that only when patients with PD were analyzed separately to other pancreatic resection, patients with sarcopenia showed poorer overall survival than those without sarcopenia (P=0.014). Meanwhile, in patients with distal pancreatectomy, there were no difference in survival rates between the two groups (P=0.721).
DiscussionThe prevalence of sarcopenia in pancreatic cancer patients ranges from 20% to 65%.10,23 This is likely due to the heterogeneous groups of patients, difference in disease stage, and different methods of measuring sarcopenia
Despite these variations, it has been repeatedly shown that sarcopenia patients are more likely to have poorer outcomes. In the present review, sarcopenia was not associated with an increased incidence of the specific postoperative complications, such as pancreatic fistula, delayed gastric emptying, sepsis, postoperatory hemorrhage or mortality. Surprisingly, El Amrani49 found that patients with sarcopenia had a reduced risk of post-operative pancreatic fistula. Although eight of the studies1,3,8,10,25,33,48 did not show a benefit for non sarcopenic patients with respect to complication risk, the apparent benefit of sarcopenia patients is unexpected and contrary to what was found in the vast majority of studies published in the field. Therefore, this finding should be interpreted with caution because it may have been the result of uncontrolled bias or statistical error.
Sarcopenia is known to be associated with higher mortality and functional disability.6 In the current literature, it is becoming increasingly evident that concurrent sarcopenia and high fat mass is the worst-case scenario. In Ratnayake et al. study, post-operative morbidity did not differ between SARC and non-SARC (NSARC) patients using all three preoperative computed tomography measures (skeletal muscle index SARC 64%, 28/44, NSARC 64%, 29/45, P=1.000; psoas muscle index SARC 63%, 31/49, NSARC 65%, 26/40, P=0.810; skeletal muscle attenuation SARC 17/25, NSARC 40/64, P=0.247). However, sarcopenic obesity was a significant independent risk factor for overall post-operative morbidity on multivariate analysis (odds ratio 1.241 (SE 0.608), P=0.041) with the highest specificity (81%).50
Our study has a number of limitations. Firstly, most of the studies collected patients of a wide period of time, with a median of 8 years (range: 3–17 years). This large period of time between the first and the last pancreatic resection could suppose a bias for those studies. Secondly, as we have previously remarked, diagnosis criteria and cut off points in all these series were diverse, this can influence the results as some of the reviewed articles have already shown. None of the studies documented muscle strength or power due to be retrospective studies, although there may be certain limitations in these tests, like in patients with mobility problems due to orthopedic or neurological problems, attempts should be made to include these parameters when discussing sarcopenia. Therefore, the true prevalence of sarcopenia in the study populations may still be unknown. Thirdly, many of the studies were carried out with a mixed cohort including patients with other subtypes of pancreatic cancers such as distal cholangiocarcinoma, ampullary and duodenal carcinoma, or even with CP. Patients data were analyzed together, no matter what kind of cancer, surgery or comorbilities, which might have affected the results.
ConclusionThe impact of sarcopenia on morbidity and perioperative mortality in pancreatic resections is still unclear. Sarcopenia does not appear to be a significant negative predictive factor in postoperative morbidity although study heterogeneity and risk of bias limit the strength of these conclusions.
It is necessary to unify criteria both in the definition and in the cut off values. Many of the studies include in the same group patients undergoing PD and distal pancreatectomy it may be useful to perform subgroup analysis of procedures.
Prospective studies and consensus on sarcopenia diagnosis should be achieved.
Author contributionsStudy conception and design: RA. Latorre Fragua and J.M. Ramia Ángel.
Acquisition of data: RA. Latorre Fragua, A. Manuel Vázquez and C. Ramiro Pérez.
Analysis and interpretation of data: RA. Latorre Fragua, A. Manuel Vázquez.
Drafting of manuscript: RA. Latorre Fragua, A. Manuel Vázquez.
Critical revision of manuscript and final approval: C. Ramiro Pérez, R. De La Plaza and J.M Ramia.
FundingNo funding was use for this study.
Conflict of InterestRaquel Aránzazu Latorre Fragua declares that she has no conflict of interest. Alba Manuel Vázquez declares that she has no conflict of interest. Carmen Ramiro Pérez declares that she has no conflict of interest. Roberto de La Plaza Llamas declares that he has no conflict of interest. Jose Manuel Ramia Ángel declares that he has no conflict of interest.