Data from Japanese series show that surface morphology of laterally spreading tumors (LST) in the colon identifies lesions with different incidence and pattern of submucosal invasion. Such data from western countries are scarce. We compared clinical and histological features of LST in a western country and an eastern country, with special interest on mucosal invasiveness of LST, and investigated the effect of clinical factors on invasiveness in both countries.
Patients and methodsPatients with LST lesions ≥20mm were included from a multicenter prospective registry in Spain and from a retrospective registry from the National Cancer Center Hospital East, Japan. The primary outcome was the presence of submucosal invasion in LST. The secondary outcome was the presence of high-risk histology, defined as high-grade dysplasia or submucosal invasion.
ResultsWe evaluated 1102 patients in Spain and 663 in Japan. Morphological and histological characteristics differed. The prevalence of submucosal invasion in Japan was six-fold the prevalence in Spain (Prevalence Ratio PR=5.66; 95%CI: 3.96, 8.08), and the prevalence of high-risk histology was 1.5 higher (PR=1.44; 95%CI: 1.31, 1.58). Compared to the granular homogeneous type and adjusted by clinical features, granular mixed, flat elevated, and pseudo-depressed types were associated with higher odds of submucosal invasion in Japan, whereas only the pseudo-depressed type showed higher risk in Spain. Regarding high-risk histology, both granular mixed and pseudo-depressed were associated with higher odds in Japan, compared with only the granular mixed type in Spain.
ConclusionThis study reveals differences in location, morphology and invasiveness of LST in an eastern and a western cohort.
Los datos de series japonesas muestran que la morfología de los tumores de extensión lateral (LST) en el colon identifica lesiones con diferente incidencia y patrón de invasión submucosa. Esta información es escasa en series de países occidentales. Comparamos las características clínicas e histológicas de LST en un país occidental y un país del este, con especial interés en la infiltración de la lesión, e investigamos el efecto de los factores clínicos sobre esta infiltración en ambos países.
Pacientes y métodosSe incluyeron pacientes con lesiones LST ≥20mm de un registro prospectivo multicéntrico en España y de un registro retrospectivo del National Cancer Center Hospital East, Japón. El objetivo primario fue la presencia de invasión submucosa en los LST. El objetivo secundario fue la presencia de histología de alto riesgo, definida como displasia de alto grado o invasión submucosa.
ResultadosEvaluamos 1.102 pacientes en España y 663 en Japón. Las características morfológicas e histológicas difirieron. La prevalencia de invasión submucosa en Japón fue 6 veces mayor que la prevalencia en España (razón de prevalencia PR=5,66; IC 95%: 3,96, 8,08), y la prevalencia de histología de alto riesgo fue 1,5 mayor (PR=1,44; IC 95%: 1,31, 1,58). En comparación con el tipo granular homogéneo y ajustado por las características clínicas, los tipos granular mixto, plano elevado y pseudodeprimido se asociaron con mayores probabilidades de invasión submucosa en Japón, mientras que solo el tipo pseudodeprimido mostró mayor riesgo en España. Con respecto a la histología de alto riesgo, tanto el granular mixto como el pseudodeprimido se asociaron con mayores probabilidades en Japón, en comparación con solo el tipo granular mixto en España.
ConclusiónEste estudio revela diferencias en la localización, morfología e invasividad de LST en una cohorte oriental y occidental.
Laterally spreading tumors (LST) in the colon are polypoid lesions that precede advanced colorectal cancer (CRC). The term LST was originally proposed by Kudo and is now commonly accepted for relatively flat, large tumors (>10mm) with lateral progression.1,2 LST are more prevalent in the right colon and more frequently overlooked than polypoid lesions, possibly resulting in false-negative explorations. Missed LST lesions are a major factor in colonoscopy failure to prevent cancer in the proximal colon, and evidence suggests they may be responsible for the three-fold incidence of interval cancers found in the right colon as compared to the incidence found in the left colon.3,4
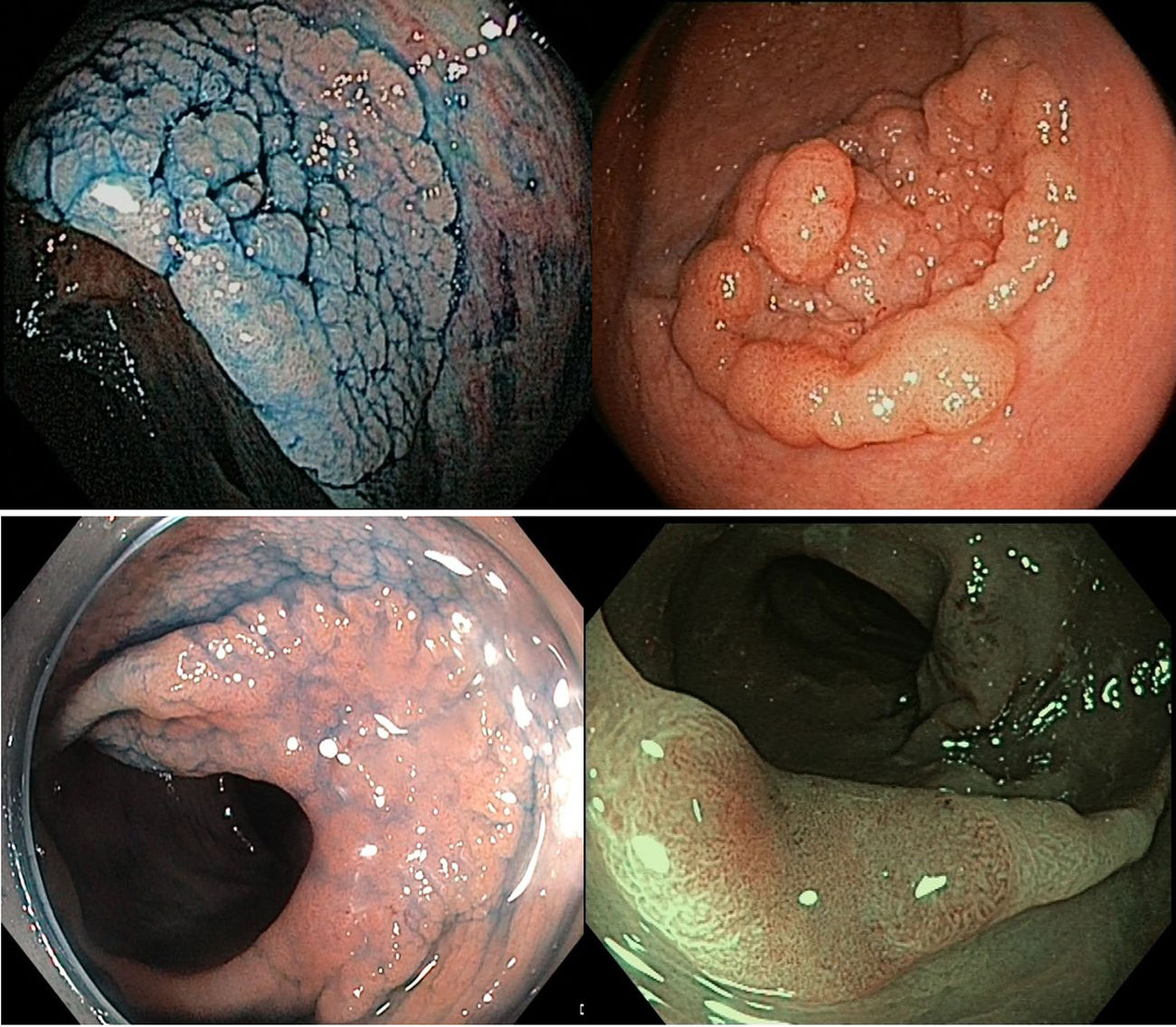
LST correspond to type 0 of the Paris classification. They can be classified into four categories according to their surface morphology: the granular type, with homogeneous or mixed subtypes, and the non-granular type, with flat elevated or pseudo-depressed subtypes.3 These four subtypes, accepted in Japan and in Western countries, define lesions with a different incidence and, more importantly, different patterns of submucosal invasion.5,6 The submucosal invasion is of great relevance because it differentiates lesions that can be treated endoscopically from those requiring surgery. Nevertheless, studies evaluating LST morphology and histology, especially the larger cohorts, have largely been conducted in Japan. Based on these clinicopathological features, Japanese authors have proposed treatment algorithms6,7 that take in account the different morphological LST subtypes and their probability of submucosal invasion. These algorithms focus mainly on endoscopic submucosal dissection (ESD) techniques, although endoscopic resection in western countries is generally performed by endoscopic mucosal resection (EMR). Considering the practical handicaps in applying ESD in western countries, the need for its introduction in daily practice, and the possible differences in LST clinicopathological features in different areas of the world, we felt it would be interesting to compare large series from eastern and western countries in order to determine the appropriateness of currently available algorithms.
The purpose of this multicenter observational study was to compare the clinical and histological findings of LST and their relation to submucosal invasion in two large LST cohorts from different areas of the world.
Patients and methodsWe conducted a multicenter observational study in cohorts collected in a western country (Spain) and in an eastern country (Japan).
PatientsSpanish cohortWe included all consecutive cases recorded in the Multicentre National Prospective Registry of the Endoscopic Resection Group of the Spanish Association of Digestive Endoscopy (GSEED-RE) between February 2013 and January 2015. Inclusion criteria were consecutive patients aged 18 years or older, with colonic LST ≥20mm and indicated for endoscopic resection. All patients provided written informed consent. Patients who did not wish to participate in the study were excluded.
Japanese cohortThis cohort included consecutive cases from the National Cancer Center Hospital East (NCCHE) collected in a retrospective registry between 2006 and 2013. Only lesions ≥2cm were analyzed. Retrospective clinical data from the Japanese cohort were collected from the NCCHE's computerized medical records. All patients signed an informed consent form for the anonymous use of medical data for scientific purposes.
In both cohorts, information included clinical records and endoscopic reports. No data were retrospectively obtained from endoscopic images. The study was approved by the local ethics committees at all participating centers.
VariablesLesions were characterized according to the following features: size, location, LST morphology, and histology. LST morphology was classified into four groups: granular homogeneous type (Paris 0-IIa granular), granular mixed type (Paris 0-IIa+0-Is granular), flat elevated type (Paris 0-IIa non-granular), and pseudo-depressed type (mainly Paris 0-IIa+c non-granular). Fig. 1 includes examples of the different types of lesions. LST histology was grouped into six classes: (1) adenoma; (2) adenoma with high-grade dysplasia (HGD); (3) adenocarcinoma with submucosal invasion; (4) serrated lesion (including hyperplastic, traditional serrated adenoma, and sessile serrated either without dysplasia or with low grade dysplasia); (5) serrated lesion with HGD; and (6) adenocarcinoma with submucosal invasion arising from a serrated lesion. For comparative purposes, we re-categorized this variable into high-risk histology (adenoma or serrated lesion with HGD or submucosal invasion) versus low-risk histology (adenoma or serrated lesion with low-grade dysplasia or without dysplasia).
Gastrointestinal pathologists at each of the Spanish institutions followed the Vienna classification.8 The Japanese series used a different system,9 but as it was more detailed, we were able to adapt it to the Vienna classification. No interobserver agreement tests were performed.
Aim and study outcomesWe aimed to compare the clinical and histological features of LST in a western country with those in an eastern country, with special interest on the prevalence of submucosal invasiveness, and to assess the effect of clinical factors on invasiveness in both countries. The primary outcome was the presence of submucosal invasion in LST, and the secondary outcome the presence of high-risk histology.
Statistical analysisSociodemographic, clinical and histological characteristics of the two cohorts were described using frequency and percentages for categorical variables and mean and standard deviations for continuous variables, together with 95% confidence intervals. These characteristics were statistically compared between cohorts using the parametric T-test, the X2 test, the proportion test for trends, or the Fisher test, depending on the nature of the variables. Prevalence Ratios with 95% Confidence Intervals were estimated for the primary and secondary outcomes. To assess associations between clinical factors and primary and secondary outcomes in each country, we used a two-step procedure. In the first step, we fitted bivariate logistic regression models including the outcome of interest as dependent variable, and each one of the clinical factors as covariates. In the second step, we fitted a multivariate logistic regression model per outcome and country, including sociodemographic variables and relevant clinical factors as covariates. This procedure provided both crude Odds Ratios (OR) and adjusted Odds Ratios (aOR) for each factor and country, together with 95% Confidence Intervals. Stratified models by country were fitted because the effect of some clinical factors on the outcomes significantly differed by country. Statistical analyses were performed using IBM SPSS Statistics 20 and R 3.1.0.
ResultsWe included 1765 lesions: 1102 from the Spanish cohort and 663 from the Japanese cohort. In the Spanish series, all lesions were treated by EMR, while in the Japanese series, 244 were treated by EMR and 419 by ESD. Table 1 shows the demographic and clinicopathological characteristics of each cohort. Patients in the Spanish cohort were older than the patients in the Japanese cohort but there were no differences regarding gender. Both cohorts showed a modest predominance of male patients.
Demographic and clinic-histological characteristics of the LST per cohort.
| Spain (n=1102) | Japan (n=663) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | 95%CI | n | % | 95%CI | p-Value | |
| Sex | |||||||
| Males | 660 | 62 | (59.0, 64.9) | 415 | 62.6 | (58.8, 66.3) | |
| Females | 404 | 38 | (35.0, 41.0) | 248 | 37.4 | (33.7, 41.2) | 0.814 |
| Age Mean(sd) | 68.4 | (10.7) | (67.7, 69.3) | 66.8 | (9.9) | (66.1,67.6) | 0.002 |
| LST morphology | |||||||
| Granular homogeneous | 525 | 47.6 | (44.7,50.6) | 134 | 20.2 | (17.3, 23.5) | <0.001 |
| Granular mixed | 317 | 28.8 | (26.1, 31.6) | 239 | 36.0 | (32.4, 39.9) | |
| Flat elevated | 230 | 20.9 | (18.5, 23.4) | 206 | 31.1 | (27.6, 34.8) | |
| Pseudo-depressed | 30 | 2.7 | (1.9, 3.9) | 84 | 12.7 | (10.3, 15.5) | |
| Size categories | |||||||
| <30mm | 527 | 47.8 | (44.8, 50.8) | 289 | 43.6 | (39.8, 47.5) | 0.130 |
| 30–39mm | 275 | 25.0 | (22.4, 27.6) | 166 | 25.0 | (21.8, 28.6) | |
| ≥40mm | 300 | 27.2 | (24.6, 30.0) | 208 | 31.5 | (27.9, 35.1) | |
| Location | |||||||
| Rectum | 250 | 22.7 | (20.3, 25.3) | 40 | 6.0 | (4.4, 8.2) | <0.001 |
| Left colon | 223 | 20.2 | (17.9, 22.8) | 129 | 19.5 | (16.6, 22.7) | |
| Transverse colon | 125 | 11.3 | (9.6, 13.4) | 150 | 22.6 | (19.5, 26.0) | |
| Ascending | 352 | 31.9 | (29.2, 34.8) | 242 | 36.5 | (32.9, 40.3) | |
| Cecum | 152 | 13.8 | (11.8, 16.0) | 102 | 15.4 | (12.8, 18.4) | |
| Location proximal to transverse | |||||||
| No | 598 | 54.3 | (51.3, 57.2) | 319 | 48.1 | (44.3, 52.0) | <0.001 |
| Yes | 504 | 45.7 | (42.8, 48.7) | 344 | 51.9 | (48.0, 55.7) | |
| Histology | |||||||
| Adenoma LGD | 514 | 48.8 | (45.8, 51.9) | 241 | 36.4 | (32.8, 40.2) | <0.001 |
| Adenoma HGD | 371 | 35.2 | (32.4, 38.2) | 265 | 40.0 | (36.3, 43.9) | |
| Invasive adenocarcinoma | 35 | 3.3 | (2.4, 4.6) | 128 | 19.3 | (16.4, 22.6) | |
| Serrated (no dysplasa or LGD) | 104 | 9.9 | (8.2, 11.9) | 28 | 4.2 | (2.9, 6.1) | |
| Serrated HGD | 28 | 2.7 | (1.8, 3.9) | 0 | 0 | (0.0, 0.7) | |
| Invasive serrated | 1 | 0.1 | (0.0, 0.6) | 0 | 0 | (0.0, 0.7) | |
| Histology by LST morphology | |||||||
| In granular homogeneous | |||||||
| HGD | 176 | 35.2 | (29.5, 37.8) | 45 | 33.6 | (25.8, 42.3) | 0.805 |
| Submucosal invasion | 13 | 2.6 | (1.4, 4.3) | 13 | 9.7 | (5.5, 16.3) | 0.001 |
| In granular mixed | |||||||
| HGD | 155 | 51.8 | (43.3, 54.5) | 123 | 51.5 | (44.9, 57.9) | 1.000 |
| Submucosal invasion | 16 | 5.4 | (3.0, 8.2) | 54 | 22.6 | (17.6, 28.5) | 0.001 |
| In flat elevated | |||||||
| HGD | 61 | 27 | (21.0, 32.8) | 62 | 30.2 | (24.0, 36.9) | 0.522 |
| Submucosal invasion | 3 | 1.3 | (0.3, 4.1) | 37 | 18.0 | (13.1, 24.0) | <0.001 |
| In pseudo-depressed | |||||||
| HGD | 7 | 25 | (10.6, 42.7) | 35 | 41.7 | (31.2, 52.9) | 0.176 |
| Submucosal invasion | 4 | 14.3 | (4.4, 31.6) | 24 | 28.6 | (19.5, 39.6) | 0.131 |
Regarding morphology, we found statistically significant differences, as 47.6% (95%CI: 44.7, 50.6) of LST lesions in the Spanish cohort were granular homogeneous lesions compared to 20.2% (95%CI: 17.2, 23.5) in the Japanese cohort. The most commonly described LST type in the Japanese cohort was granular mixed (36%; 95%CI: 32.4, 39.9), followed by the flat elevated (31.1%; 95%CI: 27.6, 34.8). The proportion of flat elevated LST was significantly lower in the Spanish cohort (20.9%; 95%CI: 18.5, 23.4 vs 31.1%; 95%CI: 27.6, 34.8) and the same occurred for the pseudo-depressed lesions (2.7%; 95%CI: 1.9, 2.9 vs 12.7%; 95%CI: 10.3, 15.5).
Regarding polyp location, the Spanish cohort had a higher percentage of rectal polyps. In contrast, the Japanese cohort had a significantly higher proportion of lesions proximal to the splenic flexure, mainly due to a higher number of lesions located in the transverse colon.
Prevalence of submucosal invasion and high-risk histology by countryRegarding histology, adenomatous LST with low-grade dysplasia were the most common lesions in both cohorts (48.8%; 95%CI: 45.8, 51.9 in Spain and 36.4%; 95%CI: 32.8, 40.2 in Japan). In the Japanese cohort, the proportion of invasive adenoma was significantly higher than that in the Spanish cohort for all types of LST morphology (3.3%; 95%CI: 2.4, 4.6 vs 19.3%; 95%CI: 16.4, 22.6), leading to a Prevalence Ratio (PR) of submucosal invasion equal to 5.66 (95%CI: 3.96, 8.08). In relation to the LST morphology type, the percentage of submucosal invasion in Spain was under 6% for all LST types except for pseudo-depressed (14.3%; 95%CI: 4.4, 31.6), while in Japan it was over 10% for all LST types except for granular homogeneous. Globally, the prevalence of high-risk histology in the Japanese cohort is 1.5 higher than in the Spanish cohort (PR=1.44; 95%CI: 1.31, 1.58).
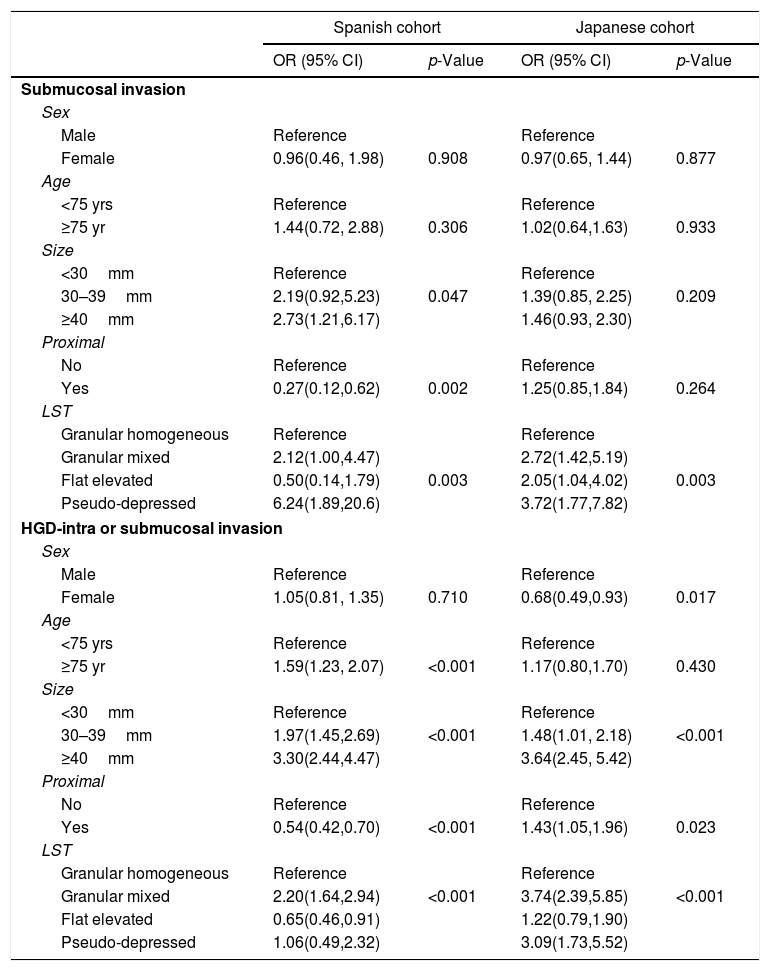
Clinical and lesion factors associated with submucosal invasion by countryTable 2 shows the bivariate logistic regression models fitted to evaluate factors associated with submucosal invasion by country. The effect of sex and age was not statistically significant in any of the cohorts. However, the. effect of lesion size was significant in the Spanish cohort (p=0.047, showing an increasing gradient from smaller to higher size, but was not significant in the Japanese cohort. The effect of location proximal to transverse was protective in Spain and not statistically significant in Japan. Regarding LST morphology, the odds of submucosal invasion increased significantly for granular mixed morphology and for pseudo-depressed types in both cohorts compared to granular homogeneous morphology. The odds were also increased for flat elevated type in the Japanese cohort.
Bivariate logistic regression models for factors associated with submucosal invasion and high-risk histology stratified by country.
| Spanish cohort | Japanese cohort | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Submucosal invasion | ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.96(0.46, 1.98) | 0.908 | 0.97(0.65, 1.44) | 0.877 |
| Age | ||||
| <75 yrs | Reference | Reference | ||
| ≥75 yr | 1.44(0.72, 2.88) | 0.306 | 1.02(0.64,1.63) | 0.933 |
| Size | ||||
| <30mm | Reference | Reference | ||
| 30–39mm | 2.19(0.92,5.23) | 0.047 | 1.39(0.85, 2.25) | 0.209 |
| ≥40mm | 2.73(1.21,6.17) | 1.46(0.93, 2.30) | ||
| Proximal | ||||
| No | Reference | Reference | ||
| Yes | 0.27(0.12,0.62) | 0.002 | 1.25(0.85,1.84) | 0.264 |
| LST | ||||
| Granular homogeneous | Reference | Reference | ||
| Granular mixed | 2.12(1.00,4.47) | 2.72(1.42,5.19) | ||
| Flat elevated | 0.50(0.14,1.79) | 0.003 | 2.05(1.04,4.02) | 0.003 |
| Pseudo-depressed | 6.24(1.89,20.6) | 3.72(1.77,7.82) | ||
| HGD-intra or submucosal invasion | ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.05(0.81, 1.35) | 0.710 | 0.68(0.49,0.93) | 0.017 |
| Age | ||||
| <75 yrs | Reference | Reference | ||
| ≥75 yr | 1.59(1.23, 2.07) | <0.001 | 1.17(0.80,1.70) | 0.430 |
| Size | ||||
| <30mm | Reference | Reference | ||
| 30–39mm | 1.97(1.45,2.69) | <0.001 | 1.48(1.01, 2.18) | <0.001 |
| ≥40mm | 3.30(2.44,4.47) | 3.64(2.45, 5.42) | ||
| Proximal | ||||
| No | Reference | Reference | ||
| Yes | 0.54(0.42,0.70) | <0.001 | 1.43(1.05,1.96) | 0.023 |
| LST | ||||
| Granular homogeneous | Reference | Reference | ||
| Granular mixed | 2.20(1.64,2.94) | <0.001 | 3.74(2.39,5.85) | <0.001 |
| Flat elevated | 0.65(0.46,0.91) | 1.22(0.79,1.90) | ||
| Pseudo-depressed | 1.06(0.49,2.32) | 3.09(1.73,5.52) | ||
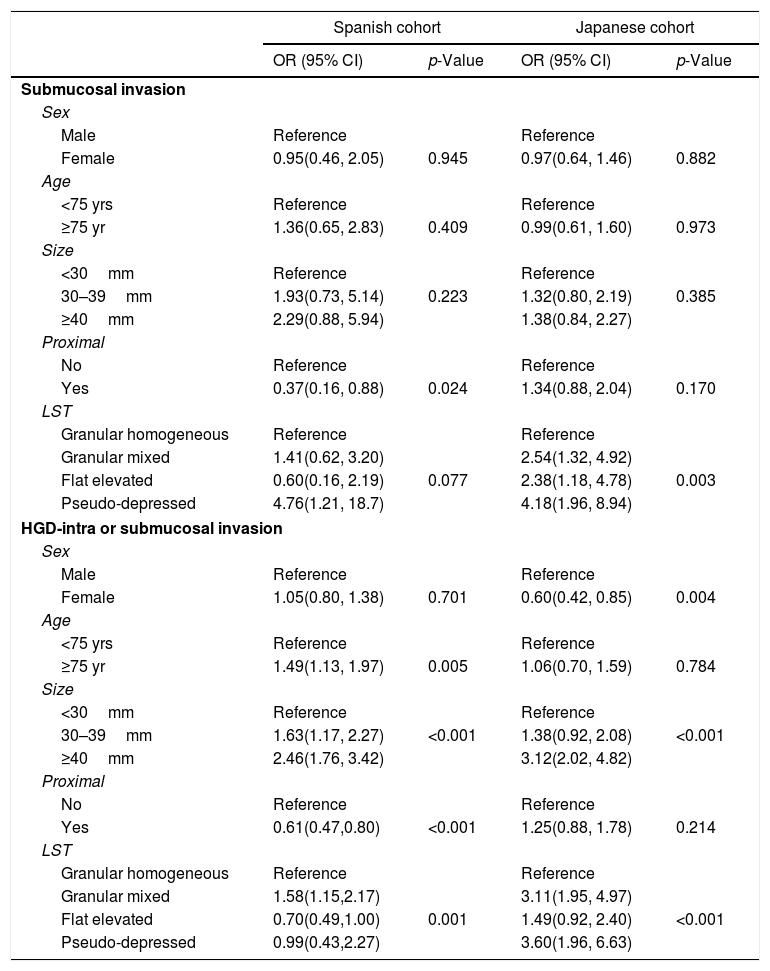
Table 3 details the multivariate models fitted for submucosal invasion stratified by country. They suggest that proximal location and LST morphology were the most related factors. Proximal polyp location, after adjusting by age, sex and size, determined a protective effect in Spain (aOR=0.37, 95%CI: 0.16, 0.88) that is not observed in Japan (aOR=1.34; 95%CI: 0.88, 2.04). Regarding LST morphology, granular mixed and flat elevated types duplicated the odds of submucosal invasion compared to granular homogeneous in Japan but not in Spain, and this risk was multiplied by four in pseudo-depressed lesions, similarly in both regions.
Multivariate logistic regression models for factors associated with high-risk histology and submucosal invasion stratified by country.
| Spanish cohort | Japanese cohort | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Submucosal invasion | ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.95(0.46, 2.05) | 0.945 | 0.97(0.64, 1.46) | 0.882 |
| Age | ||||
| <75 yrs | Reference | Reference | ||
| ≥75 yr | 1.36(0.65, 2.83) | 0.409 | 0.99(0.61, 1.60) | 0.973 |
| Size | ||||
| <30mm | Reference | Reference | ||
| 30–39mm | 1.93(0.73, 5.14) | 0.223 | 1.32(0.80, 2.19) | 0.385 |
| ≥40mm | 2.29(0.88, 5.94) | 1.38(0.84, 2.27) | ||
| Proximal | ||||
| No | Reference | Reference | ||
| Yes | 0.37(0.16, 0.88) | 0.024 | 1.34(0.88, 2.04) | 0.170 |
| LST | ||||
| Granular homogeneous | Reference | Reference | ||
| Granular mixed | 1.41(0.62, 3.20) | 2.54(1.32, 4.92) | ||
| Flat elevated | 0.60(0.16, 2.19) | 0.077 | 2.38(1.18, 4.78) | 0.003 |
| Pseudo-depressed | 4.76(1.21, 18.7) | 4.18(1.96, 8.94) | ||
| HGD-intra or submucosal invasion | ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.05(0.80, 1.38) | 0.701 | 0.60(0.42, 0.85) | 0.004 |
| Age | ||||
| <75 yrs | Reference | Reference | ||
| ≥75 yr | 1.49(1.13, 1.97) | 0.005 | 1.06(0.70, 1.59) | 0.784 |
| Size | ||||
| <30mm | Reference | Reference | ||
| 30–39mm | 1.63(1.17, 2.27) | <0.001 | 1.38(0.92, 2.08) | <0.001 |
| ≥40mm | 2.46(1.76, 3.42) | 3.12(2.02, 4.82) | ||
| Proximal | ||||
| No | Reference | Reference | ||
| Yes | 0.61(0.47,0.80) | <0.001 | 1.25(0.88, 1.78) | 0.214 |
| LST | ||||
| Granular homogeneous | Reference | Reference | ||
| Granular mixed | 1.58(1.15,2.17) | 3.11(1.95, 4.97) | ||
| Flat elevated | 0.70(0.49,1.00) | 0.001 | 1.49(0.92, 2.40) | <0.001 |
| Pseudo-depressed | 0.99(0.43,2.27) | 3.60(1.96, 6.63) | ||
Table 2 shows the results for the bivariate logistic regression models used to evaluate factors associated with high-risk histology. Higher patient age was associated with high-risk histology in Spain, female sex with lower odds in Japan, and bigger lesion size with high-risk in both countries. Left side location was associated with a lower odds in Spain, but it was associated with a higher odds in Japan. Regarding LST morphology, suggest that only the granular mixed type was associated with high-risk histology in Spain, whereas both the granular mixed and pseudo-depressed types were associated with high-risk histology in Japan.
Table 3 details the multivariate models fitted for high-risk histology in both countries. The models identified age ≥75 years as a risk factor in Spain (aOR=1.49; 95%CI: 1.13, 1.97) and female sex as a protective factor in Japan (aOR=0.60; 95%CI: 0.42, 0.85). The size of the lesion was a risk factor with a clear increasing gradient in both countries, with adjusted Odds Ratios close to 1.50 for lesions of size 30–39mm, and close to 3.00 for lesions of size ≥40mm compared to lesions<30mm Similarly to the bivariate models, a proximal location showed a protective effect in Spain (OR=0.61; 95%CI: 0.47, 0.80) but not in Japan (OR=1.25; 95% CI: 0.88, 1.78). Regarding LST morphology types, in Spain, granular mixed morphology was associated with high-risk histology (OR=1.58; 95%CI: 1.15, 2.17) compared with granular homogeneous, and flat elevated morphology with a lower odds (OR=0.70; 95%CI: 0.49, 1.00). In Japan, granular mixed morphology was also associated with a statistically significant higher odds that is two-fold the OR found in Spain (OR=3.11; 95%CI: 1.95, 4.97), but flat elevated lesions did not show a protective effect (OR=1.49; 95%CI: 0.92, 2.40), and pseudo-depressed type was associated with a high-risk histology (OR=3.60; 95%CI: 1.96, 6.63).
DiscussionMorphological and histological characteristics differed considerably in two large LST cohorts from Spain and Japan. More importantly, characteristics that conferred a high risk of deep invasion varied substantially. According to these findings, current ESD and EMR algorithms based on Japanese morphological and histological data might not be adequate in western countries.
The recognition of lesion characteristics that determine a high risk of submucosal invasion is crucial to prevent and treat colorectal cancer in patients with LST. Nevertheless, large or multicenter series describing the morphology and histology of LST are lacking, especially in western countries. Current treatment algorithms are based on the findings of the most relevant cohorts recorded in Japan.5,6 However, these cohorts might have inherent limitations in other populations. First, some LST morphologic types had a poor representation in those series. Second, even though morphology might entail a certain risk of invasion, no type can exclude malignancy.5,6,10 Our study includes the morphology and histology of 1765 lesions and all LST morphology types are widely represented.
It is important to emphasize that treatment algorithms are mainly based on ESD data. In western countries EMR is often the preferred resection technique, even for large lesions. Nevertheless, based on Japanese data, some groups in the west have accepted similar treatment strategies, while others have suggested that most superficial colonic lesions can be successfully managed by EMR despite their size.11–14 As an example, out of 1000 successfully completed EMRs in the Australian Colonic EMR (ACE) study, surgery was avoided in 98.1% of cases at 16 months after resection.13 However, in this study, lesions referred to surgery due to technical or histological reasons were excluded from this particular analysis.13 This might decrease the incidence of invasive lesions. In contrast, in a metanalysis including 6779 large polyps treated by EMR, 8% underwent surgery due to non-curative endoscopic resection15; these data probably reflect daily western practice outside highly specialized centers.
In our comparative analysis, the Spanish and Japanese series differed in relevant clinicopathological aspects. Most lesions were granular homogeneous in Spain, while the most common types in Japan were granular-mixed and flat elevated. Moreover, lesions were more frequently located in the left colon in the Spanish series, but in the right colon in the Japanese series. Interestingly, proximal lesions presented lower odds of high-risk histology in Spain, a finding that did not occur in Japan. As in the Spanish series, a recent large Australian cohort described a higher prevalence of high-risk histology in distal lesions.16 Lastly, we observed major differences concerning the incidence of invasive histological features. The percentage of HGD was clinically similar, whereas the frequency of submucosal invasion in Spain was six times lower. Although the incidence in Spain was very low and possibly underestimated, the trends are similar to those described in the Australian series16 and other Japanese cohorts.17,18 Even more importantly, the incidence of submucosal invasion according to the LST morphology was lower than 5.5% for granular and flat-elevated lesions in Spain, while it ranged from about 10 to 23% in Japan. Consequently, EMR could be a correct approach to these types of lesions in the West. In contrast, pseudo-depressed lesions showed a high prevalence of submucosal invasion in Spain (14.3%), high enough to consider ESD or surgery in a western setting. These differences, were recently highlighted in a study by Burgess et al.16 Considering their findings, they proposed the use of ESD for distal 0-IIa non-granular lesions without overt evidence of submucosal invasive cancer, and for all distal 0-Is or 0-IIa+Is. Furthermore, selective non-granular mixed lesions with large nodules might also have a higher incidence of invasion, and their management should be individualized. Concerning the histopathology, we emphasize that the percentage of serrated lesions was three times higher in the Spanish series than in the Japanese cohort. This might be explained by a higher interest in serrated lesions in the East in the past. Importantly, serrated lesions presented a lower rate of HGD and invasion, but its percentage in both series was low.
This work has several limitations. First, the prevalence of submucosal invasion may have been underestimated. This is particularly likely in the Spanish series because surgical lesions were not recorded and, due to the lower experience with ESD, surgery may have been indicated rather than ESD in lesions with a high risk of submucosal invasion. Additionally, more non-complex lesions might have been included with the participation of multiple centers in Spain, in comparison to a unique tertiary referral center in Japan. However, each of the Spanish centers included at least 20 cases and all receive patients referred from other institutions. Moreover, the submucosal invasion incidence in the Spanish cohort is comparable or slightly lower than those shown in the largest western series.13,16,19 Second, data come from two independent series not designed for comparative purposes, and patient recruitment and data collection methods differed. The retrospective nature of the Japanese series, the inclusion of only one low volume but referral center, and the study's long inclusion period could affect data quality and reflect a referral bias. Nevertheless, all data had been recorded in highly detailed endoscopy reports and it was not estimated from images. Furthermore, considering that the inclusion period dated back nearly ten years, it is likely that all kind of lesions were included, rather than selected advanced lesions. Third, there are evident differences in the endoscopic resection techniques used in each cohort and the morphological classification and histopathological analysis of the lesions also probably varies. Importantly, the initial pathology evaluation in the Japanese series did not follow the Vienna classification. Nevertheless, data had been extensively recorded in the initial database, and the Japanese pathologist and authors adapted it easily to this classification. We do not know whether the described differences between the two samples are due to real histological variations derived from geographical or ethnical disparities, to lower technological development in the western setting, to the wide use of ESD in Japan, or to a selection bias based on the high specialization of the NCCHE. It could be a combination of all the factors. However, as commented in a recent publication, regardless of the cause of the described differences they imply that the approach to endoscopic resection needs to be different in each area.16 Fourth, there was not an interobserver agreement on morphology assessment. Previous studies revealed significant differences on morphology assessment related to the endoscopists’ experience.20 As our study includes multiple institutions and endoscopists with different levels of expertise, a low interobserver agreement could have an impact to the main objective of the study. While the lack of interobserver agreement is frequent in most multicenter EMR series, it might bias the results and it should be considered in future prospective studies. Finally, well known factors of advanced histology and submucosal invasion such as the vascular and crypt pattern were not evaluated. Nevertheless, these factors require high-definition endoscopic equipment, chromoendoscopy, and endoscopists trained in its use. This study aimed to evaluate simple factors such as the location and macroscopic morphology of the lesion.
In conclusion, this study reveals notable differences in the location, morphology and invasiveness of LST between two large cohorts, one from an eastern country and one from a western country. In view of these differences, we consider that treatment algorithms should be based on local or comparable data, rather than only on Japanese histological studies. A prospective study of incidence with data collected from different continents and with colonoscopies performed at centers with different levels of complexity would be useful.
In memoriam: this work was possible thanks to the dedication and commitment of Professor Kaneko, from whom I learned much not only about medicine but also about humanity.
Conflict of interestThe authors declare that they have no conflict of interest.
We would like to acknowledge Carolyn Newey for help in editing the manuscript and Navarrabiomed-Fundación Miguel Servet for their support with the statistical analysis. Eduardo Albéniz is granted by “La Caixa/Caja Navarra” Foundation (ID 100010434; Project PR15/11100006).