The development of the immune checkpoint inhibitors (ICI) is one of the most remarkable achievements in cancer therapy in recent years. However, their exponential use has led to an increase in immune-related adverse events (irAEs). Gastrointestinal and liver events encompass hepatitis, colitis and upper digestive tract symptoms accounting for the most common irAEs, with incidence rates varying from 2% to 40%, the latter in patients undergoing combined ICIs therapy. Based on the current scientific evidence derived from both randomized clinical trials and real-world studies, this statement document provides recommendations on the diagnosis, treatment and prognosis of the gastrointestinal and hepatic ICI-induced adverse events.
El descubrimiento de los inhibidores de checkpoint inmunológicos (ICI) es uno de los logros más importantes en los últimos años en Oncología. Sin embargo, su uso en aumento ha conlllevado a un incremento de los efectos adversos inmunomediados (irAEs). Los eventos hepáticos y gastrointestinales incluyen la hepatitis, colitis y síntomas de tracto digestivo superior, que son de los irAEs más frecuentes, con incidencias entre el 2 y 40%, ésta última en paciente tratados con combo de ICI. Basados en la evidencia científica tanto de ensayo clínicos randomizados como de estudio de vida real, este documento de consenso aporta recomendaciones sobre el diagnóstico, tratamiento y pronóstico de los efectos adversos hepáticos y gastrointestinales asociados con la inmunoterapia.
The development of immune-checkpoint inhibitors (ICIs), including anti-cytotoxic T lymphocyte antigen 4 (anti-CTLA-4), anti-programmed cell death 1 (anti-PD-1) and anti-programmed death ligand 1 (anti-PD-L1) antibodies has marked a significant milestone in cancer therapy over the past decade. As ICIs indication continue to expand, so does the appearance of specific immune-related adverse events (irAEs), which can sometimes be severe and life-threatening.1
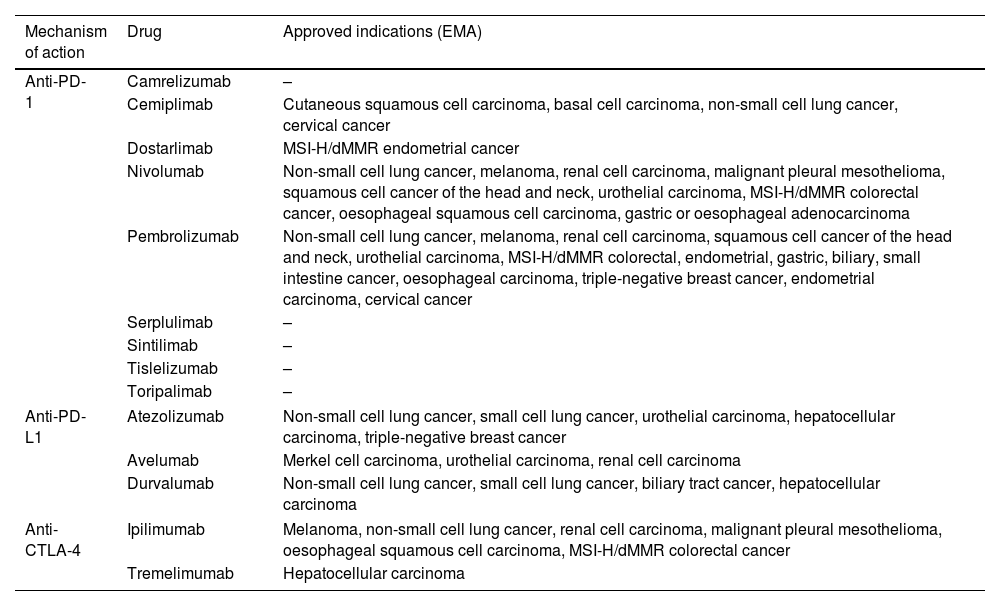
The indications and consequent use of ICIs are constantly growing, either in monotherapy or in combination with chemotherapy or other ICIs. They have approved indications in solid tumors such as melanoma, small and non-small cell lung cancer, breast, colorectal and esophagogastric, liver, renal and bladder, and head and neck cancer, not only for advanced stages but also in certain scenarios of localized disease. Table 1 includes the mechanism of action and names of the ICIs with more advanced clinical development.
Name and mechanism of action of immune checkpoint inhibitors with more advanced clinical development and approved indications (EMA).
| Mechanism of action | Drug | Approved indications (EMA) |
|---|---|---|
| Anti-PD-1 | Camrelizumab | – |
| Cemiplimab | Cutaneous squamous cell carcinoma, basal cell carcinoma, non-small cell lung cancer, cervical cancer | |
| Dostarlimab | MSI-H/dMMR endometrial cancer | |
| Nivolumab | Non-small cell lung cancer, melanoma, renal cell carcinoma, malignant pleural mesothelioma, squamous cell cancer of the head and neck, urothelial carcinoma, MSI-H/dMMR colorectal cancer, oesophageal squamous cell carcinoma, gastric or oesophageal adenocarcinoma | |
| Pembrolizumab | Non-small cell lung cancer, melanoma, renal cell carcinoma, squamous cell cancer of the head and neck, urothelial carcinoma, MSI-H/dMMR colorectal, endometrial, gastric, biliary, small intestine cancer, oesophageal carcinoma, triple-negative breast cancer, endometrial carcinoma, cervical cancer | |
| Serplulimab | – | |
| Sintilimab | – | |
| Tislelizumab | – | |
| Toripalimab | – | |
| Anti-PD-L1 | Atezolizumab | Non-small cell lung cancer, small cell lung cancer, urothelial carcinoma, hepatocellular carcinoma, triple-negative breast cancer |
| Avelumab | Merkel cell carcinoma, urothelial carcinoma, renal cell carcinoma | |
| Durvalumab | Non-small cell lung cancer, small cell lung cancer, biliary tract cancer, hepatocellular carcinoma | |
| Anti-CTLA-4 | Ipilimumab | Melanoma, non-small cell lung cancer, renal cell carcinoma, malignant pleural mesothelioma, oesophageal squamous cell carcinoma, MSI-H/dMMR colorectal cancer |
| Tremelimumab | Hepatocellular carcinoma | |
MSI-H, high microsatellite instability; dMMR, mismatch repair deficient.
The introduction of ICIs has improved the outcome of many different cancers, with the significant improvement in long-term overall survival (OS) often achieved through these therapies being especially important. However, ICIs are not universally effective, making it crucial to explore new treatment approaches and identify predictive factors (for both efficacy and toxicity) to select the best treatment option for each patient.
With the gradual inclusion of ICIs into the cancer treatment armamentarium, it is essential to be familiarized with the potential toxicities they may imply. Early recognition and adequate management of ICIs related toxicities are crucial not only to prevent therapy-related complications but also to ensure convenient treatments are not erroneously interrupted. This decision is even more important given the increasing likelihood of achieving long-term OS with the use of ICIs.
MethodsThis positioning document has been carried out through the collaboration of five Spanish scientific societies, comprising: the Spanish Association for the Study of the Liver (Asociación Española para el Estudio de Hígado; AEEH), the Spanish Association of Gastroenterology (Asociación Española de Gastroenterología; AEG); the Spanish Society of Digestive Pathology (Sociedad Española de Patología Digestiva; SEPD); the Spanish Society of Medical Oncology (Sociedad Española de Oncología Médica; SEOM) and the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa; GETECCU).
The AEEH proposed the elaboration of this document (chair MCL) and contacted the rest of the societies to designate a coordinator for each one. Coordinators (MCL, SC, MM, AD, MRB) invited the listed authors to participate in the project development.
The coordinating team established two task force subgroups (liver-group and gut-group), each with its own leader and divided the key topics among those task forces.
Key questions were discussed and approved by all group members. The process of developing the Guideline included telephone videoconferences and online discussions among the members from March 2021 to February 2023. Searches were performed in MEDLINE, Embase, and the Cochrane Library. Articles were selected through title and abstract screening followed by full-text screening. The results of the search were presented to all group members and statements were created by consensus. Evidence levels and recommendation strengths were assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.2
Once consensus was reached among all group members, the manuscript was reviewed by a member of the AEG Board (JC) and was sent for further comments to national societies and individual members for additional feedback. After this, it was submitted to “Gastroenterología y Hepatología” for publication. This Guideline was issued in 2023 and will be considered for update in 2028.
Hepatitis induced by immune checkpoint inhibitorsEpidemiologyICI-induced hepatitis occurs in 3–20% of patients, generally within the first 4–9 weeks of therapy. In the first trials with nivolumab and pembrolizumab in monotherapy, the incidence of ICI-induced hepatitis ranged between 1% and 4%1 and severe hepatitis was rare.3 The most common manifestation was an isolated increase in transaminase levels (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), which usually normalized after treatment discontinuation, but increased alkaline phosphatase ([ALP) and gamma-glutamyl transpeptidase (GGT) levels have also been observed. Anti-CTLA-4 drugs used in monotherapy have been associated with a higher incidence of ICI-induced hepatitis ranging between 3% and 9% in a dose-dependent manner, with higher rates observed in patients receiving ipilimumab at 10mg/kg vs. 3mg/kg.4 However, approximately 25% of patients treated with the combination of anti-PD-1 or anti-PD-L1 plus anti-CTLA-4 developed ICI-induced hepatitis. In addition, hepatitis was more frequent in patients treated with combinations of ICI with both chemotherapy and targeted therapy, suggesting that perhaps ICIs may sensitize the liver to other forms of drug-induced liver injury (DILI) or the opposite.5 Real-world observational data in ICI-induced hepatotoxicity have emerged and the incidence appears to be comparable to rates reported in clinical trials. The incidence of ICI-induced hepatitis ranges between 1% and 4% in patients treated with monotherapy with anti-PD-1, between 4% and 9% in patients treated with anti-CTLA-4 in monotherapy, and around 18% in those receiving the combination of anti-PD-1 and anti-CTLA-4. In a French series published in 2018, only 3.5% of patients presented acute hepatitis≥grade 3.6
There are two main concerns regarding assessment of the incidence and severity of ICI-related hepatotoxicity. First, hepatic irAEs are probably overrepresented in many reviews of liver toxicity.7–9 A common limitation of these publications is the registry of liver enzyme abnormalities during and/or after the use of ICIs without a formal exclusion of other causes such as hepatic metastasis, thromboembolic disease, biliary compression, perfusion injury, opportunistic infections, or drug reactions. A single center retrospective study conducted in Toronto, included 450 patients treated with anti-PD-1, anti-PD-L1 or anti-CTLA-4 in phase I/II clinical trials between August 2012 and December 2018.10 Liver enzyme elevations≥grade 2 occurred in 120 patients, but this could be attributed to ICI-induced hepatotoxicity in only 17 cases (3.6% of total cohort). The main manifestations of ICI-induced hepatotoxicity were higher ALT values and ALT/AST ratio compared with other causes of liver enzyme abnormalities, such as disease progression or other causes of DILI. In comparison with those treated with ICIs who do not develop liver injury, patients with ICI-induced hepatotoxicity were younger (47.9 years vs. 57 years, p=0.006), had previous exposure to ICI (41.2% vs. 15.9%, p=0.014), and had experienced irAE affecting other organs (76.5% of patients with ICI-induced hepatotoxicity vs. 21.1% without, p<0.001). As described previously, ICI-induced hepatotoxicity was more common in patients receiving an anti-CTLA-4-based treatment (13.9%) compared to those treated with anti-PD-1/PD-L1 (2.8%).10
The second issue is the lack of uniformity of the registries regarding liver irAEs, as shown in the assessment of 32,441 reports of ICI-induced irAEs based on the retrospective pharmacovigilance study of the Food and Drug Administration Adverse Event Reporting System (FAERS) database, from January 2004 to December 2019, which includes more than 50 terms to describe liver events.11
More than 80% of patients with hepatocellular carcinoma (HCC) have an underlying chronic liver disease, which is frequently related to chronic infections such as hepatitis B (HBV) or C virus (HCV), alcohol consumption, or fatty liver disease associated with metabolic syndrome. Most of these patients have abnormal baseline levels of AST and ALT and, therefore, the application of the Common Terminology Criteria for Adverse Events (CTCAE) grading system should be interpreted with caution.12 According to different reports, 22% of patients with HBV/HCV have some grade of liver enzyme abnormalities after ICI therapy and 10.8% present a grade≥3.13 In patients treated for HCC the incidence of liver toxicity varies depending on the type of drug and the dose received (Supplementary Table 1).14–19 According to a recent meta-analysis that included a total of 117 clinical trials with cancer patients treated with anti-PD-1 or anti-PD-L1, the all-grade incidence of immune-related hepatitis was 2.02 (95% CI 1.05–3.88) in patients with primary liver cancer and 1.20 (95% CI 0.87–1.66) for those with other solid tumors. The incidence of ICI-induced hepatitis grade≥3 was 1.27 and 0.96, respectively.9 Biliary tract cancer (BTC) has emerged as another liver cancer that could benefit from ICI treatment.20,21 As in patients with HCC, patients with BTC frequently present with abnormal liver tests complicating the diagnosis of ICI-induced hepatitis. Preliminary data of anti-PD-1 treatment alone or in combination with gemcitabine did not show a significant increase in the rate of liver toxicity between those treated with durvalumab alone vs combined with gemcitabine.22 Similar safety results on pembrolizumab (anti-PD-1) alone vs combined with gemcitabine and cisplatin has been recently been published.23 Yet, in a study combining durvalumab (anti-PD-1) and tremelimumab (anti-CTLA-4), one patient died of DILI-related liver failure.24
Statement 1
Some degree of ICI-induced hepatitis has been observed during ICI treatment.
Quality of evidence: high.
Statement 2
ICI-induced hepatitis is more frequently observed in patients receiving anti-CTLA-4 agents or dual CTLA-4 and PD-1/PD-L1 blockade than in those on PD-1/PD-L1 inhibitors.
Quality of evidence: high.
DiagnosisDefinition of ICI-induced liver toxicityThe diagnosis of ICI-induced hepatitis is challenging, especially because there are no clear and specific diagnostic criteria.
According to the EASL (European Association for the Study of the Liver) clinical practice guidelines the definition of drug-induced liver injury (DILI) includes25: (1) the exclusion of other etiology of liver test abnormality and (2) one of the following criteria, (i) ≥5×upper limit of normal (ULN) elevation in ALT, (ii) ≥2×ULN elevation in ALP or (iii) ≥3×ULN elevation in ALT with a simultaneous elevation of total bilirubin exceeding 2×ULN. In patients with abnormal liver tests prior to starting treatment with the drug involved, ULN is replaced by the mean baseline values obtained prior to DILI onset and increases should be proportionate to this modified baseline.
However, these criteria have not been validated in patients with ICI-induced liver toxicity and milder elevations in transaminases oblige us to closely monitor liver tests and even withhold ICI administration (see below).
The lack of definite diagnostic criteria and the variable setting in which these drugs are used make both diagnosis and differential diagnosis challenging, and thus, a structured evaluation of suspected ICI-induced hepatitis is compulsory. This is especially true for patients with HCC or BTC in whom the tumoral burden, already involving the liver, makes the differential diagnosis of liver test abnormalities detected during ICI treatment difficult. Another important issue is the temporal relationship between the immunotherapy administration and the onset of liver injury, though cases of ICI-induced hepatitis have been described just after the first cycle of immunotherapy until several months after its discontinuation.26,27
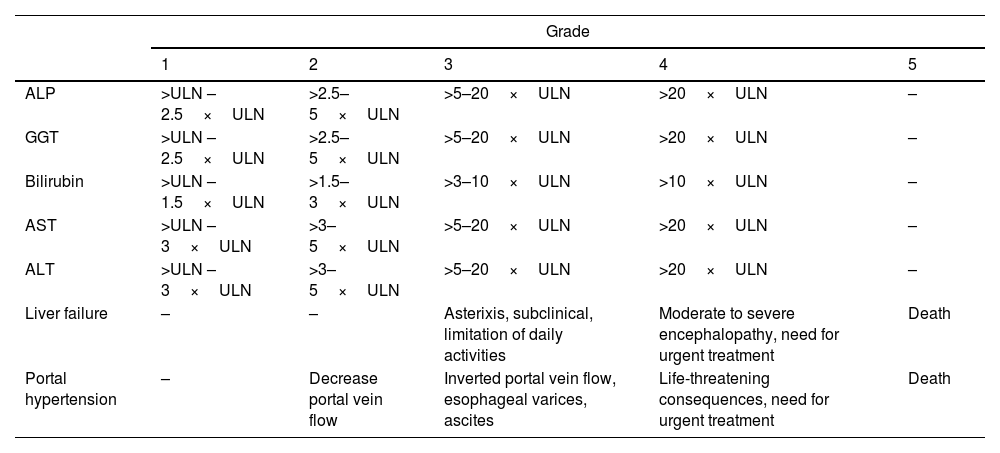
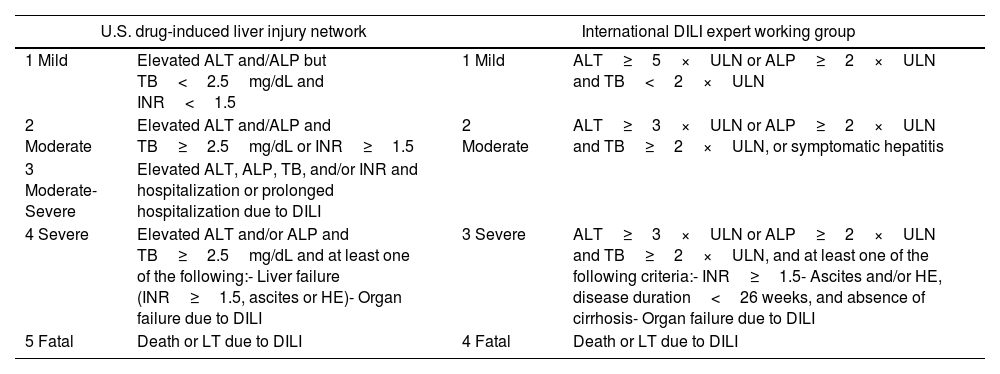
Severity of hepatitisEstablishing the severity of liver damage is a necessary step after the diagnosis of hepatitis of any etiology. In the specific case of ICI-induced hepatic toxicity, the CTCAE grading system (Table 2)6,12,28 is the most accepted. However, it has several limitations: (1) it quantifies the degree of elevation of each liver test separately but there is not a specific scale for hepatitis, and (2) it considers high degree transaminase elevations without a concomitant increase in bilirubin levels as grade 4 hepatotoxicity. This is important because isolated elevation of transaminases is merely an indicator of hepatocellular damage and not liver dysfunction, (3) the International Normalized Ratio (INR), an important parameter to assess liver function and determine the need for treatment, has not been routinely included in oncology guidelines. Therefore, the CTCAE is probably less accurate than Hy's law for reflecting serious hepatotoxicity.25 In this sense, it would be recommendable to incorporate other grading scales (including those from the U.S. Drug Induced Liver Injury Network and the International DILI Expert Working Group or DILI severity index) (Table 3) in the evaluation of patients with suspected ICI-induced liver injury.
Grades of hepatotoxicity according to CTCAE by the National Cancer Institute.12
| Grade | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| ALP | >ULN – 2.5×ULN | >2.5–5×ULN | >5–20×ULN | >20×ULN | – |
| GGT | >ULN – 2.5×ULN | >2.5–5×ULN | >5–20×ULN | >20×ULN | – |
| Bilirubin | >ULN – 1.5×ULN | >1.5–3×ULN | >3–10×ULN | >10×ULN | – |
| AST | >ULN – 3×ULN | >3–5×ULN | >5–20×ULN | >20×ULN | – |
| ALT | >ULN – 3×ULN | >3–5×ULN | >5–20×ULN | >20×ULN | – |
| Liver failure | – | – | Asterixis, subclinical, limitation of daily activities | Moderate to severe encephalopathy, need for urgent treatment | Death |
| Portal hypertension | – | Decrease portal vein flow | Inverted portal vein flow, esophageal varices, ascites | Life-threatening consequences, need for urgent treatment | Death |
ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ULN, upper limit of normal.
Drug-induced liver injury severity scales.
| U.S. drug-induced liver injury network | International DILI expert working group | ||
|---|---|---|---|
| 1 Mild | Elevated ALT and/ALP but TB<2.5mg/dL and INR<1.5 | 1 Mild | ALT≥5×ULN or ALP≥2×ULN and TB<2×ULN |
| 2 Moderate | Elevated ALT and/ALP and TB≥2.5mg/dL or INR≥1.5 | 2 Moderate | ALT≥3×ULN or ALP≥2×ULN and TB≥2×ULN, or symptomatic hepatitis |
| 3 Moderate-Severe | Elevated ALT, ALP, TB, and/or INR and hospitalization or prolonged hospitalization due to DILI | ||
| 4 Severe | Elevated ALT and/or ALP and TB≥2.5mg/dL and at least one of the following:- Liver failure (INR≥1.5, ascites or HE)- Organ failure due to DILI | 3 Severe | ALT≥3×ULN or ALP≥2×ULN and TB≥2×ULN, and at least one of the following criteria:- INR≥1.5- Ascites and/or HE, disease duration<26 weeks, and absence of cirrhosis- Organ failure due to DILI |
| 5 Fatal | Death or LT due to DILI | 4 Fatal | Death or LT due to DILI |
DILI, drug-induced liver injury; ALT, alanine aminotransferase; ALP, alkaline phosphatase; TB, total bilirubin; HE, hepatic encephalopathy; ULN, upper limit of normality.
The diagnosis of DILI is generally made by a combination of clinical suspicion in the appropriate context, and the ruling out of other causes of liver test abnormalities. Nevertheless, several causality scales can aid in the diagnosis and are useful to establish a structured diagnosis, which is particularly appropriate in academic and research contexts. The most widely used scale is the Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM),29 which has been proposed to determine the causal relationship between the drug responsible and liver damage. It entails a scoring system that categorizes suspicion into “definite or highly probable” (score>8), “probable” (score 6–8), “possible” (score 3–5), “unlikely” (score 1–2), and “excluded” (score≤0). The applicability of the CIOMS/RUCAM scale has been hindered by its subjectivity and poor reliability and, therefore, it has recently been updated and computerized into a revised electronic causality assessment method (RECAM) scale using data from the Drug-Induced Liver Injury Network and the Spanish DILI network. The RECAM scale has shown to have higher agreement with expert opinion and higher sensitivity for detecting extreme categories.30 Of course, these scales need to be validated for ICI-induced liver toxicity.
Exclusion of other etiologies- -
Tumor progression
An abdominal ultrasound is indicated to rule-out biliary and/or vascular pathology or hepatic tumoral infiltration. If necessary, an abdominal computerized tomography (CT) scan, liver magnetic resonance (MR) imaging, or magnetic resonance cholangiography should be performed.31 The performance of an imaging study is mandatory since development of liver metastasis has been described as the most common cause of transaminases increase in patients undergoing ICIs.32
- -
Liver toxicity induced by chemotherapy and other concomitant medications
Patients with cancer, and especially those on ICIs, are frequently receiving multiple concomitant medications (and occasionally herbal products) and, therefore it is mandatory to check for potential drug- or herbal-induced liver injury. The risk could be exacerbated by the presence of steatosis or advanced age that could affect the clearance of some drugs.16
Hepatic toxicity secondary to chemotherapy is infrequent. The mechanisms of liver injury are those described for other drugs: (1) idiosyncratic immunological or metabolic reactions, that are not predictable or dose-dependent, and (2) direct liver damage or exacerbation of a pre-existing liver disease. However, chemotherapy can also induce hepatocellular necrosis, hepatic steatosis, hepatic fibrosis, or sinusoidal obstruction.33 The best-known is sinusoidal damage that ranges from sinusoidal dilatation to sinusoidal obstruction syndrome (SOS) causing non-thrombotic obliterations of small intrahepatic veins by fibrin.34 Among the drugs currently used with immunotherapy, oxaliplatin and irinotecan are the most common, and can also be associated with SOS in around 20% of patients.29,30 SOS has also been reported with the use of immunotherapy.35,36
Lastly, liver-directed radiotherapy may cause hepatotoxicity per se, but this can be exacerbated by the use of concomitant chemotherapy.
- -
Viral hepatitis
Screening for the following viral infections is recommended, including:
- •
Hepatitis A, B, C, and E viruses (HAV, HBV, HCV, and HEV): IgM anti-HAV, IgM anti-HEV, anti-HBc, anti-HCV, HEV-RNA, HCV-RNA.
- ∘
In case of previous positive anti-HBc: HBV-DNA.
- ∘
- •
Cytomegalovirus (CMV)
- ∘
If IgM is positive, order CMV-RNA.
- ∘
- •
In selected patients (severe immunosuppression or epidemiological history)
- ∘
Epstein–Barr virus (EBV)
- ∘
Herpes simplex virus (HSV)
- ∘
Human herpes virus 6 and 8 (HHV 6 and HHV8)
- ∘
Screening for viral hepatitis encompassing HBsAg, anti-HBc and anti-HCV is highly recommended prior to beginning ICIs.37 This recommendation is supported by the risk of HBV reactivation associated with ICIs therapy,38 and the increased risk of HCV infection in the oncology setting compared to the general population.39
- -
Alcohol-related liver disease
Alcoholic liver disease (ALD) is the most prevalent cause of liver disease and cirrhosis in Europe, with a prevalence in Spain of 2%. The possibility of alcohol-associated liver disease should be suspected in women with alcohol consumption of >20g per day and in men consuming >40g per day.40 This is especially relevant in patients with acute severe alcohol consumption, with or without prior known liver disease. The diagnosis of cancer may trigger the abuse of toxic substances and/or alcohol in predisposed individuals.
- -
Screening for autoimmune diseases
Autoimmune hepatitis (AIH) is relatively uncommon, with a prevalence of 16–18 cases/100,000 inhabitants in Europe,41 but it must be ruled out in the presence of an increase in transaminase levels, particularly in individuals with prior autoimmune diseases. It is recommended to determine:
- •
Anti-nuclear antibodies (ANA).
- •
Anti-smooth muscle antibodies (ASMA).
- •
Immunoglobulin G (IgG) levels.
However, positivity for these autoantibodies or elevated IgG are not diagnostic of autoimmune liver disease. AIH is an exclusion diagnosis and requires the performance of a liver biopsy. Differentiation between AIH and ICI-induced hepatitis can be difficult as there are no specific diagnostic criteria. The study of the immunophenotype of infiltrating immune cells could be helpful but more data are needed to confirm preliminary results.42
Liver biopsyIn general, a liver biopsy is not mandatory to diagnose ICI-induced liver damage because as in other DILI the clinical diagnosis is appropriate. In addition, no unequivocal histological findings have been established for this entity.17 However, the panel suggest performing a liver biopsy in patients with≥grade 3 hepatitis not improving after ICI withdrawal to evaluate the severity of inflammation. Despite the lack of pathognomonic features, several histological findings have been associated with the use of anti-CTLA-4 or anti-PD-1 treatments. Patients with ipilimumab-related hepatotoxicity may show granulomatous hepatitis with fibrin deposition and those treated with anti-PD-1 may present lobular hepatitis with periportal activity and centrilobular inflammation.1 Interestingly, the inflammatory infiltrate is mainly composed of CD8+ T cells which is completely different from other liver diseases including AIH in which the portal infiltrate predominately contains CD4+ T and B cells.42
Recommendation 1
The presence of HBsAg, and anti-HBc and anti-HCV antibodies must be assessed before starting ICI treatment.
Quality of evidence: moderate
Strength of the recommendation: strong in favor.
Recommendation 2
Patients with anti-HCV antibodies must be tested for HCV-RNA and those with anti-HBc antibodies must be tested for HBV-DNA.
Quality of evidence: moderate.
Strength of the recommendation: strong in favor.
Recommendation 3
The diagnosis of ICI-induced hepatitis requires the exclusion of other causes of liver test abnormalities, especially the development or progression of tumoral liver involvement.
Quality of evidence: high.
Strength of the recommendation: strong in favor.
Recommendation 4
If ICI-induced hepatitis is suspected, it is recommended to perform the following tests: liver ultrasound (and CT or MR if needed), IgM anti-HAV, IgM anti-HEV, anti-HBc, anti-HCV, HEV-RNA, HCV-RNA, IgM anti-CMV, IgM anti-EBV, ANA, ASMA, IgG levels. It is also important to check the potential hepatotoxicity of concomitant medications, and alcohol or substance abuse.
Quality of evidence: moderate.
Strength of the recommendation: strong in favor.
Recommendation 5
The CIOMS/RUCAM scale may help establish the causality of ICI as the hepatotoxic agent.
Quality of evidence: low.
Strength of the recommendation: weak in favor.
Recommendation 6
The severity of an elevation of liver tests should be evaluated with the CTCAE in combination with the U.S Drug Induced Liver Injury Network or the DILI severity index.
Quality of evidence: low.
Strength of the recommendation: strong in favor.
Recommendation 7
In case of ≥grade 3 hepatitis not improving after ICI withdrawal, the panel suggest performing a liver biopsy to rule out other causes of liver injury and to determine the severity of liver inflammation.
Quality of evidence: low.
Strength of the recommendation: weak in favor.
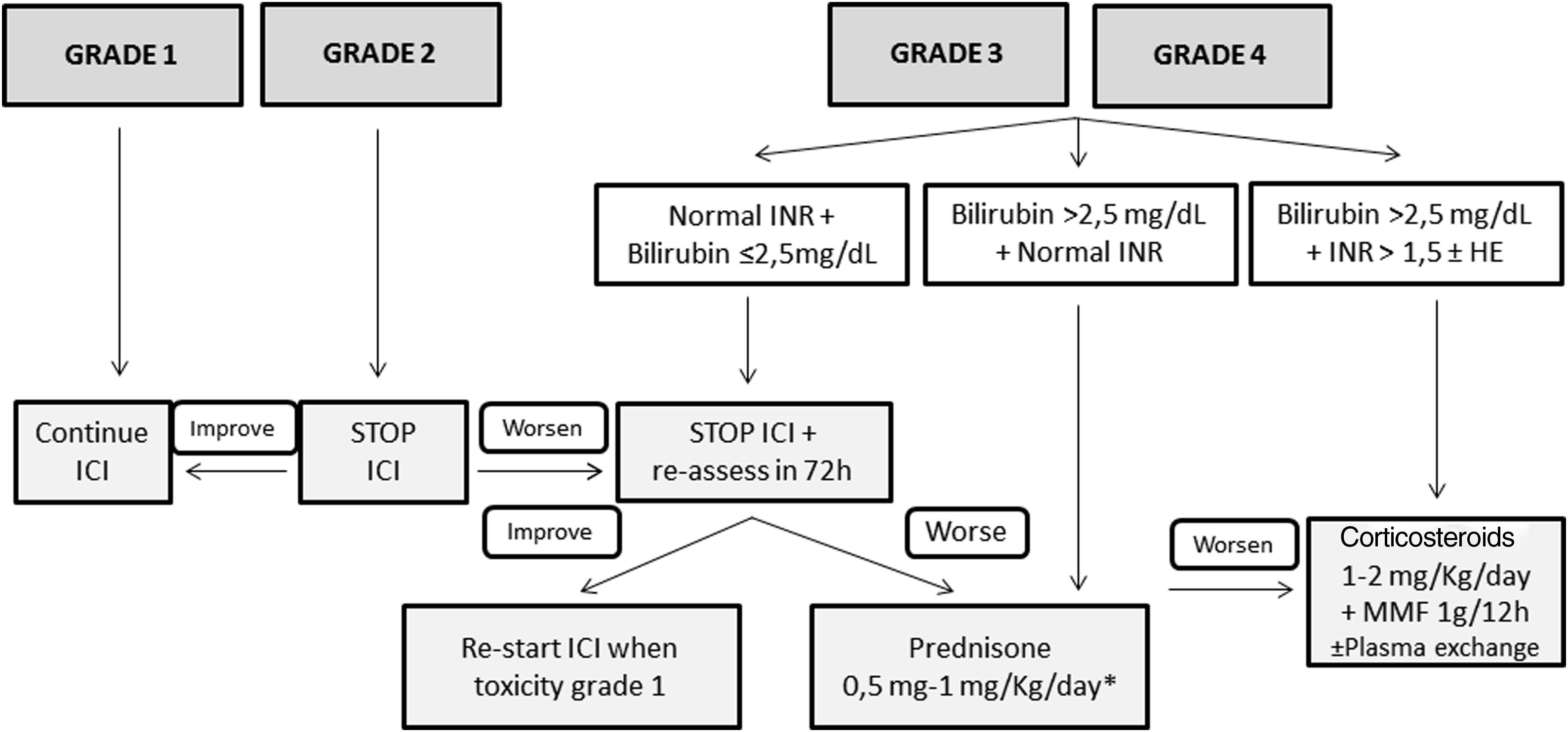
TreatmentFig. 1 shows the management of ICI-induced hepatitis according to the severity. There is wide consensus recommending bi-weekly monitoring without the need for withholding ICI treatment in patients presenting grade 1 hepatotoxicity.5,26–28 However, the paucity of available evidence in terms of management is the reason for the varying recommendations among different guidelines in patients with ICI-induced hepatitis grade 2 or greater. While some position documents and guidelines recommend close monitoring without treatment,3,28 others recommend starting immunosuppressive treatment upon the diagnosis of grade 2 hepatitis.5,26 However, there is increasing information regarding favorable evolutions in untreated patients.3,43 Therefore, in patients with grade 2 hepatitis without liver dysfunction, it is reasonable not to start immunosuppression and weekly monitoring with liver tests. In this specific scenario, Sangro B et al.28 proposed continuing ICI treatment in patients with stable bilirubin values, withholding ICI only in those with rising bilirubin levels with respect to baseline, but less than 3 times the ULN. In patients with an adequate evolution, the resumption of ICI may be considered. However, corticosteroids (CS) must be initiated in patients in whom liver tests steadily worsen or do not improve.
Proposed management of immune checkpoint inhibitors-induced hepatitis according to the CTCAE grading. * Assess the performance of liver biopsy, mainly in case of alternative diagnosis (other drugs, cancer infiltration, etc.). If bilirubin levels>2.5mg/dL, the beginning of steroids should NOT be delayed more than 48h. HE, hepatic encephalopathy; ICI, immune checkpoint inhibitor; MMF, mycophenolate mofetil.
Patients presenting grade 3 or greater represent a challenging scenario. Until recently, most of these patients were invariably treated with different immunosuppressive schemes, all of which were based on corticosteroids (CS).6,43–54 Most of these patients presented a favorable evolution, with only a minority requiring additional immunosuppression. It is of note that some retrospective studies evaluating grade 3 or 4 ICI-induced hepatitis reported a high rate of complete hepatitis resolution in untreated patients. However, many of these studies lack an in-depth evaluation of either the potential risk of selection bias or differences among treated and untreated groups, and thus, the results must be interpreted with caution. In a large retrospective cohort of patients, Miller ED et al.,54 did not identify relevant differences between grade≥3 treated or untreated patients, in the baseline characteristics, evolution, or the rate of ICI rechallenge. Thus, considering the information available, as well as the proposals of different experts, CS may not always be warranted in grade≥3 hepatitis.3,28,44 After ruling out other potential causes of hypertransaminasemia, – withholding ICI and liver test monitoring each 2–3 days is advisable in stable patients with preserved liver function – bilirubin<2.5mg/dL and INR<1.5. If liver tests worsen or do not present a clear decline after 7 days in this subgroup of patients, CS induction therapy is recommended. The performance of a liver biopsy may be helpful for selection of patients who would benefit from CS initiation based on the severity of necroinflammation.55 In patients presenting bilirubin levels≥2.5 and/or an INR≥1.5 at diagnosis, ICI should be withheld, and immunosuppressive treatment must be promptly started.
If treatment is finally indicated, the recommended drug is predniso(lo)ne – or equivalent – with doses ranging from 0.5 to 1mg/kg/d. Nevertheless, there is growing evidence suggesting that doses of prednisone greater than 60mg/d may not add any additional benefits.46 In the absence of response, either because of worsening or stabilization, the panel recommends: (1) hospital admission, (2) rule-out of other tumoral disease progression and other etiologies of liver test abnormalities, (3) individual assessment of the need for liver biopsy, (4) consider the administration of intravenous CS increasing the dose to 2mg/kg/d. Additional immunosuppressive drugs, including mycophenolate mofetil (MMF) 1g every 12h and/or tacrolimus, can be used, but should be retained for refractory cases. In the setting of persistent lack of response or the development of acute liver injury, anti-thymocyte globulin therapy and/or plasma exchange may be an option, but further information is needed to establish any formal recommendation.56,57 Budesonide, a CS with low systemic bioavailability because of the 90% liver first pass clearance, has not been tested as induction therapy in this scenario, and therefore no recommendation regarding its use can be made.58,59 The use of anti-tumor necrosis factor (TNF) therapies is not recommended due to the potential risk of ICI-induced hepatitis reported with these drugs.60
Once improvement is documented, CS tapering can be initiated, reducing predniso(lo)ne by 10mg per week, usually with tapering over 4–8 weeks. Although the time to hepatitis resolution varies widely across series, ranging from 4 days to 4 months,6,43–54 recovery is usually observed within 5–9 weeks.44 Strikingly, two studies have described that the time to recovery is slightly shorter in patients without CS treatment than in those with.43,54
Recommendation 8
In case of grade 1 hepatitis, ICI must be continued, and liver tests should be monitored bi-weekly.
Quality of evidence: high.
Strength of the recommendation: strong in favor.
Recommendation 9
In patients with grade 2 ICI-induced hepatitis, the panel recommends withholding ICI treatment and monitoring liver tests weekly. In case of improvement continue as in grade 1 hepatitis. In case of worsening, perform liver tests and re-test in 72h. If worsening continues, prednisone should be started at 0.5–1mg/kg/d.
Quality of evidence: moderate.
Strength of the recommendation: strong in favor.
Recommendation 10
In patients with grade 3 or 4 ICI-induced hepatitis, the panel suggests withholding ICI treatment and starting prednisone (0.5–1mg/kg/d). The decision to start prednisone could be deferred on an individual basis in patients with TB levels<2.5mg/dL until a new assessment of liver function.
Quality of evidence: moderate.
Strength of the recommendation: weak in favor.
Recommendation 11
In patients with grade 3 or 4 ICI-induced hepatitis with bilirubin levels>2.5mg/dL and an INR>1.5 (with or without HE), the panel recommends withholding ICI treatment and starting prednisone (1–2mg/kg/d) in combination with MMF or tacrolimus.
Quality of evidence: moderate.
Strength of the recommendation: strong in favor.
Recommendation 12
Plasma exchange may be an option in patients with grade 3 or 4 hepatitis and liver dysfunction (TB>2.5mg/dL and INR>1.5, with or without HE), particularly in the absence of response to immunosuppressive treatment.
Quality of evidence: low.
Strength of the recommendation: weak in favor.
RetreatmentHepatitis recurrence after retreatment with ICI is uncommon. In a large recently published retrospective cohort that included 6123 patients treated with ICI who developed irAEs, the rate of recurrence of any-degree of hepatitis was only 29% (95% CI, 16–47%).61
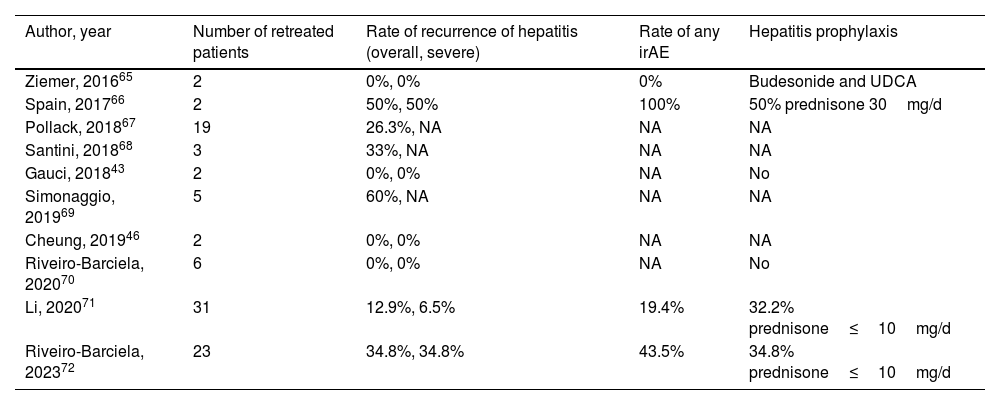
In case of grade 2 ICI-induced hepatitis, the recommendation is to discontinue ICI treatment and restart in the case of spontaneous or CS-induced improvement to either grade 1 hepatitis or complete normalization of transaminases. However, in the case of severe ICI-induced hepatitis (CTCAE grade 3 or 4), international guidelines recommend permanent discontinuation of ICI.26,27 Nevertheless, some of these patients may benefit from restarting ICI in view of increasing data on the better prognosis of cancer associated with severe irAEs, or, in other cases, due to absence of other lines of therapy alternatives to ICI.62–64 Evidence in clinical practice on the safety of rechallenge with ICI after an episode of severe ICI-induced hepatitis is scarce and mainly based on small series of cases or individual reports (Table 4).43,46,65–72 Nonetheless, these preliminary data have pinpointed the fact that recurrence is neither universal nor associated with greater severity of hepatotoxicity. In the studies including the largest number of patients, the risk of relapse ranged from 13 to 35%, with progression of the oncological underlying disease and not toxicity as the most frequent cause for ICI discontinuation.
Series of cases of retreatment with immune-checkpoint inhibitors after an episode of severe immune-related hepatitis.
| Author, year | Number of retreated patients | Rate of recurrence of hepatitis (overall, severe) | Rate of any irAE | Hepatitis prophylaxis |
|---|---|---|---|---|
| Ziemer, 201665 | 2 | 0%, 0% | 0% | Budesonide and UDCA |
| Spain, 201766 | 2 | 50%, 50% | 100% | 50% prednisone 30mg/d |
| Pollack, 201867 | 19 | 26.3%, NA | NA | NA |
| Santini, 201868 | 3 | 33%, NA | NA | NA |
| Gauci, 201843 | 2 | 0%, 0% | NA | No |
| Simonaggio, 201969 | 5 | 60%, NA | NA | NA |
| Cheung, 201946 | 2 | 0%, 0% | NA | NA |
| Riveiro-Barciela, 202070 | 6 | 0%, 0% | NA | No |
| Li, 202071 | 31 | 12.9%, 6.5% | 19.4% | 32.2% prednisone≤10mg/d |
| Riveiro-Barciela, 202372 | 23 | 34.8%, 34.8% | 43.5% | 34.8% prednisone≤10mg/d |
NA, not available; UDCA, ursodeoxycholic acid; irAE, immune-related adverse events.
Therefore, after an episode of severe ICI-induced hepatitis, the possibility of retreatment with ICI may be considered based on the risk-benefit assessment: status of the cancer, options for oncological therapy beyond ICI and the severity of prior immune-related hepatitis (according to the DILI severity score). In case of rechallenge with ICI, it is essential to ensure close analytical and clinical follow-up to achieve early identification and treatment in the case of recurrence of hepatotoxicity.
Recommendation 13
In patients with grade 3 or 4 ICI-induced hepatitis, ICI rechallenge should be considered after appropriate risk-benefit assessment.
Quality of evidence: moderate.
Strength of the recommendation: weak in favor.
Immune checkpoint inhibitor-induced cholangitisInflammation of the biliary tree during treatment with ICIs manifests as two separate entities: (1) small duct cholangitis, and (2) large duct cholangitis affecting the intra- and extra-hepatic bile ducts.73–97 Regardless of its presentation, ICI-induced cholangitis is a very uncommon irAE (0.05–0.7% of treated patients).63 However, the actual prevalence might be underestimated because ALP and GGT determinations are not routinely performed as part of the standard monitoring of liver tests during ICI treatment.34,64 ICI-induced cholangitis has more frequently been reported in patients treated with PD-1/PD-L1 blockers, especially nivolumab and pembrolizumab.75 This is probably explained by the abundant expression of PD-1 ligands (PD-L1 and PD-L2) by the cholangiocytes, which do not express CTLA-4 ligands.98
Small duct cholangitisPatients are frequently asymptomatic and present with mild to moderate elevations of ALP and GGT99 typically after 2–4 cycles of therapy.75 The diagnosis of a small duct cholangitis requires a liver biopsy, in which a peribiliary lymphocytic infiltrate rich in T lymphocytes (mainly CD8+ T cells) is the typical lesion.75,100 Other histological findings described in case reports are small bile duct injuries with irregularity of bile duct epithelium and degeneration of the bile ducts.77
Large duct sclerosing cholangitisThe clinical presentation of this entity is highly variable and ranges from asymptomatic presentation to a clinically apparent cholangitis with jaundice, fever, and abdominal pain. The median number of cycles between the beginning of the treatment and the diagnosis of sclerosing cholangitis is longer than in patients with small duct cholangitis (range 1–27).73,77 The involvement of large bile ducts is detected by magnetic resonance cholangiopancreatography (MRCP). The radiological findings most frequently described are: (1) dilatation of the extra-hepatic bile ducts, and (2) diffuse hypertrophy of the extra-hepatic bile duct wall.101 Histological findings of the bile ducts biopsies are similar to those described for small duct cholangitis with CD8+ T cell infiltration.102 A subgroup of patients also present features of sclerosing cholangitis with strictures and dilatations of the biliary tree. As in other forms of ICI-related hepatotoxicity, it is mandatory to rule-out other causes of biliary tree abnormalities, specifically the presence of tumoral infiltration of the bile duct.98
Peroral cholangioscopy has also helped to describe the characteristics of the bile duct lesions in large duct cholangitis showing thickening of the extra-hepatic bile ducts, band-like narrowing, and diverticulum-like outpouching of the wall.66 Ulceration of the biliary epithelium has also been described indicating a more severe form of the disease.
Vanishing bile duct syndromeThis is a rare entity that consists of the destruction of intrahepatic bile ducts leading to cholestasis and complete ductal loss. Over time patients can develop cirrhosis and liver failure. There are very few case reports of vanishing bile duct syndrome in the context of ICI treatment and the prognosis is poor.103–105
TreatmentMild forms of small duct ICI-induced cholangitis improve after ICI withdrawal and do not require specific treatment. The remaining patients, including those with large duct cholangitis, likely require treatment with CS and ursodeoxycholic acid (UDCA). However, the appropriate dose, treatment duration, and response criteria are currently unknown. In patients with large duct cholangitis, treatment response is unpredictable and some patients present liver enzyme elevations while tapering steroid doses. The results of a systematic literature review comprising 53 cases of ICI-induced cholangitis indicated that after CS, liver enzymes took a long time (longer than cases of ICI-induced hepatitis) to decrease, and frequently did not completely normalize.77 In cases showing a lack of response to CS, other immunosuppressive drugs have been used (azathioprine, MMF,88 or tocilizumab106) with variable results. UDCA has cytoprotective, antiapoptotic, and immunomodulatory effects. In the reported cases, UDCA was continued for a long time after CS withdrawal to promote recovery of the bile ducts.77
Rechallenge after an episode of ICI-induced cholangitis has been reported in only one case in which cholangitis did not recur after 30 months of ICI treatment.107
Recommendation 14
We suggest performing an MRCP to rule-out ICI-induced cholangitis in patients with significant ALP and GGT elevations and/or bile duct dilation in liver ultrasound.
Quality of evidence: low.
Strength of the recommendation: strong in favor.
Recommendation 15
We suggest CS therapy with or without UDCA for management of ICI-induced cholangitis.
Quality of evidence: low.
Strength of the recommendation: weak in favor.
Gastrointestinal toxicity induced by ICIsGastrointestinal is the second most common irAE after skin involvement, and is at the top as the most common cause for immunotherapy discontinuation.26,27
Clinical manifestationsSymptoms of gastrointestinal (GI) toxicity secondary to ICIsConsidering that ICIs can produce irAEs in any section of the digestive tract, the symptoms that they can cause can be varied and nonspecific depending on the location and/or extension of digestive affectation. The predominant symptom recorded in all clinical trials and in the different studies in real practice (most of them retrospective) is diarrhea (defined as the presence of more than 3 daily stools together with a low stool consistency).108 Diarrhea may be accompanied by other symptoms, such as abdominal pain or bloating (up to 53% of cases), rectal bleeding or mucus in stools (up to 26% of cases), fecal urgency or fever (up to 15% of cases).108–120 These latter symptoms may refer to another term that appears repeatedly in the literature such as colitis and is usually derived from the presence (detected by endoscopy and/or radiology) of an inflammatory involvement of the colon.112,113,117,120 Other symptoms described in some series (some of which show upper digestive involvement) are nausea/vomiting (up to 22% of cases) or epigastric pain (see gastric toxicity section).111–115,121
Clinicians should have clinical suspicion for other infrequent but serious manifestations that patients receiving ICIs can present, especially, intestinal perforation (mainly of the colon, although there are cases described in the small intestine), reported in up to 1.5% of cases of colitis or ileitis.122,123 The symptoms do not differ from those caused by other etiologies (acute abdominal pain, fever, deterioration of the general condition) but it is usually accompanied by a picture of previous digestive toxicity that tends to worse over time despite specific treatment. Apart from this complication, there is a published case of massive gastrointestinal bleeding and another of intestinal obstruction due to marked inflammation of the terminal ileum.124,125 Patients with GI toxicity secondary to ICI may present a greater sensitivity to present toxicities in other organs and systems and the concurrence of more than two of them may be a risk factor for new irAEs.126 A multicenter study in 1281 patients treated with anti-PD-1, 191 cases of GI toxicity in the form of diarrhea/colitis were identified. In this subgroup of patients, a second toxicity (skin, liver or endocrine among the most frequent) was detected in 45.5% of them (and up to 60% in those with two drugs in combination). This second toxicity was detected prior to, subsequent, or concomitant to GI toxicity. Although the pathogenesis of this cascade phenomenon is unclear, it is important for the clinician to recognize and interpret each and every one of the symptoms that these patients may present during treatment and after discontinuation.127
Hospitalization could be other severity clinical presentation form. In a retrospective multicenter study, hospital admissions for one year were analyzed in a cohort of patients receiving ICI. Of the 99 patients included, there was a total of 202 admissions, 33 (16%) motivated by an irAE, being hepatoxicity and colitis the most frequent.128 Another study found that up to 33% of the reasons for consultation in the emergency room among patients receiving ICI were toxicities that required hospital admission; with colitis (39%) being the most frequent cause of admission.129 Finally, in a systematic review and meta-analysis, fatal adverse events produced by immunotherapy between 2009 and 2018 were analyzed through the WHO pharmacovigilance registry on the notifications of all clinical trials published to date. In this meta-analysis, 613 irAEs were recorded: 70% and 37% of fatal events secondary to ipilimumab use and anti-PD-1 combination therapy, respectively, were in the form of colitis.7
Statement 3
Diarrhea is the main clinical manifestation of GI toxicity secondary to ICIs. Symptoms are nonspecific, and clinicians should carry out a differential diagnosis with other processes. Colitis is an endoscopic and/or radiological entity.
Quality of evidence: high.
Time of onset of GI toxicity associated to ICIsThe time of onset of GI toxicity (diarrhea/colitis) is unpredictable. A systematic review in which 50 studies were included, only 14% documented the moment of appearance of the different irAEs.130 In a pooled analysis conducted on 23 clinical trials with 8436 patients who had received ipilimumab, nivolumab, or combined therapy, the median time to onset of any kind of toxicity was established between 2 and 15 weeks. Diarrhea/colitis appeared after a mean time of 5, 9 and 5 weeks for ipilimumab, nivolumab and combined treatment respectively.131 In this study, it was demonstrated that GI toxicity was one of the earliest manifestations along with the skin or liver. Similarly, another meta-analysis showed median time to onset of any symptom was 10 weeks (range 6–20).132
These data do not differ substantially from those reported in real practice. The FDA spontaneous notification registry published in 2020, in which 3786 cases of colitis were identified (between 2004 and 2019), showed that >70% of the events occurred in the first 90 days from the administration of the first dose.133
Nevertheless, there are patients who develop late-onset toxicity or even after months of drug discontinuation.134 In a retrospective study that evaluated the characteristics of late irAEs (more than a year from the start of treatment) presented in 144 patients with melanoma, it was found that colitis was found to be the most common late manifestation (22% cases) and up to 58% of cases were serious. Most of these patients were receiving treatment, but up to 26% had stopped it for more than 3 months.135 It is important, therefore, that the clinician and the patient are aware of this late toxicity profile to identify and treat these forms of presentation as soon as possible.
Although variability has been mentioned concerning the time of onset of diarrhea/colitis, there are some factors associated with the earlier appearance of these events. The most relevant is the use of combination therapies (ipilimumab plus nivolumab or ipilimumab plus pembrolizumab).133 In a multicenter study in which 1261 patients were included, colitis was more frequent and appeared earlier in patients receiving combined treatment (7 vs 25 weeks).126 Similarly, the toxicity produced by anti-CTLA-4 tends to appear earlier than anti-PD-1 or anti-PD-L1 drugs.130,133 Finally, a French study that analyzed the toxicity profile in 356 patients treated with ICIs found that those with severe forms presented earlier than mild presentations (47 vs 91 days, p=0.021).136
Statement 4
Gastrointestinal toxicity can appear at any time after starting ICIs, but generally occurs between 2–15 weeks after therapy initiation.
Quality of evidence: high.
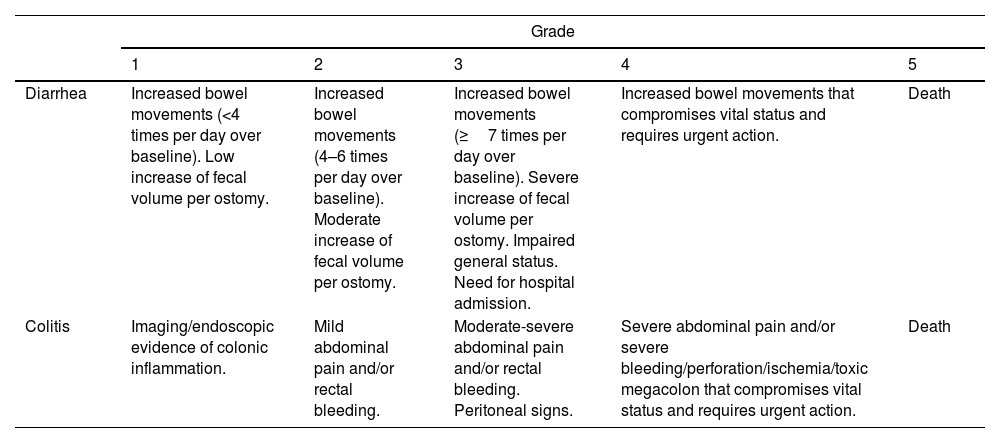
Classification of GI toxicity due to ICIsIn oncology clinical trials, AEs are recorded using the CTCAE. The severity of these events is classified into 5 grades ranging from mild to severe and fatal and are specified for each symptom or manifestation (Table 5).12,137 The different guidelines and recommendations of the scientific oncological societies recommend identifying, classifying and managing the toxicity produced by ICI based on this classification.26,27,138 However, CTCAE are based exclusively on clinical (subjective) parameters that have not been correlated with prognostic factors such as the need for immunosuppressive treatment, CS-resistance rates or colectomy. A British study analyzed the association between CTCAE grade, biochemical parameters, endoscopic activity and histological findings with prognostic factors such as need for prolonged CS and/or infliximab (IFX). However, CTCAE classification (grades 2, 3 and 4) did not correlate with the duration of CS treatment and need for IFX (p=0.18). Conversely, there was a good correlation between endoscopic activity index and histological activity with these prognostic factors. The authors concluded that endoscopic and histological data could be the most accurate factors to determine severity and need for salvage treatments.118 Several studies (most of them retrospective) have reported the presence of diarrhea/colitis according to grade severity, observing different rates.
Grades of ICI-induced gastrointestinal adverse events according to CTCAE by the National Cancer Institute (version 5).137
| Grade | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Diarrhea | Increased bowel movements (<4 times per day over baseline). Low increase of fecal volume per ostomy. | Increased bowel movements (4–6 times per day over baseline). Moderate increase of fecal volume per ostomy. | Increased bowel movements (≥7 times per day over baseline). Severe increase of fecal volume per ostomy. Impaired general status. Need for hospital admission. | Increased bowel movements that compromises vital status and requires urgent action. | Death |
| Colitis | Imaging/endoscopic evidence of colonic inflammation. | Mild abdominal pain and/or rectal bleeding. | Moderate-severe abdominal pain and/or rectal bleeding. Peritoneal signs. | Severe abdominal pain and/or severe bleeding/perforation/ischemia/toxic megacolon that compromises vital status and requires urgent action. | Death |
Recommendation 16
We recommend the use of CTCAE classification to assess the degree of severity of diarrhea and/or colitis.
Quality of evidence: high.
Recommendation: strong in favor.
EpidemiologyThe incidence of ICI-induced colitis among patients included in clinical trials and observational studies ranges between 0.7% and 13.6%109,126,139–142; nevertheless, its real incidence is unknown since endoscopy or even fecal inflammatory markers determination are not systematically performed. In fact, most studies present the percentage of diarrhea or GI adverse events which are higher than those reported for colitis.
Diarrhea/colitis secondary to anti-CTLA-4The first irAEs reported due to the use of anti CTLA-4 come from clinical trials in metastatic melanoma and renal cell carcinoma.115 In the first meta-analysis published in 2015 that included 22 clinical trials (1265 patients) of patients treated with ipilimumab (mainly) or tremelimumab, the rate of GI irAEs was 35% (95% CI: 29–41%), being the predominant toxicities together with the cutaneous ones. Of these events, 11% (95% CI: 8–13.5%) were categorized as severe (grades 3–5). This meta-analysis also demonstrated that toxicity increased in the subgroup of patients treated with high doses of ipilimumab (10mg/kg vs. 3mg/kg), especially in cases of GI toxicity (RR 1.43, p=0.03).143 Another subsequent meta-analysis that included 5 new clinical trials showed the highest percentage of diarrhea secondary to ipilimumab (up to 47.8% for all grades) and a 6.4% occurrence of colitis.144
Real practice data could differ with respect to clinical trials and may be related to certain limitations such as study design, underdiagnosis, misconception between diarrhea/colitis or limited follow-up data coming from spontaneous notifications. In the systematic review and meta-analysis published in 2020 with 34 observational studies and 3699 patients, the rate of severe colitis (grades 3–5) was 4% (95% CI: 3–7%); however, the mean follow-up time was very short (3 months).145Supplementary Table 2 summarizes global incidence of diarrhea/colitis due to anti-CTLA-4 therapy in several published studies.115,118,119,143–147
Statement 5
Anti-CTLA-4 therapy (mainly ipilimumab) may be associated with diarrhea and/or colitis as adverse event for up to one in three patients, being a serious event in 11% of these.
Quality of evidence: high.
Diarrhea/colitis secondary to anti-PD-1 or anti-PD-L1A meta-analysis published in 2017 that analyzes the incidence of adverse events produced by the different antibodies against the PD1 protein and its ligand found a global rate of irAEs of 26.8% (6% serious). The incidence rate for diarrhea of any grade and for severe rate varied between 4 and 13% (the highest percentage corresponding to patients receiving nivolumab treatment) and 0.5–1.9%, respectively, while, colitis rate was <1% for all lines of treatment.148 These rates were similar in the individual analysis for several drugs.139,149 However, Sonpavde GP et al. meta-analyzed 35 clinical trials (phase I–IV) comparing the rate of different serious irAEs in patients treated with anti-PD-1 vs. anti-PD-L1 and found an increased rate of colitis (Odds Ratio [OR] 2.53) and severe colitis (OR 3.79) for patients treated with anti-PD-1 (nivolumab, pembrolizumab), concluding that the toxicity profile could vary between both drugs.150 Two recent metanalysis suggest that the risk of colitis is also higher with anti-PD-1 treatment compared to anti-PD-L1 therapy.151,152 Cases of diarrhea and colitis reported in real practice are scarce.151,153,154 On the other hand, some cases of microscopic colitis in relation to anti-PD-1 have been reported, although the real prevalence is difficult to establish. For this reason, it is important to perform colonic random biopsies in cases of immune checkpoint-associated unexplained diarrhea, even when colonoscopy shows macroscopically normal colonic mucosa.155Supplementary table 3 summarizes studies that have evaluated incidence of gastrointestinal toxicity secondary to anti-PD-1/PD-L1.118,119,126,134,135,139,146–150,153,154,156,157
Statement 6
Anti-PD-1 or anti-PD-L1 therapy may be associated with diarrhea and/or colitis as adverse event in 4–13% of cases. This risk seems to be lower for anti-PD-L1 treatment.
Quality of evidence: high.
Risk of diarrhea/colitis comparing anti-CTLA-4 vs. anti-PD-1(PD-L1)Based on the data previously mentioned, a low risk of GI toxicity (diarrhea/colitis) is expected in patients treated with anti-PD-1/anti-PD-L1 drugs. In the meta-analysis with the largest number of studies and patients included (145 trials, 21,786 patients) recently published by Ouyang T et al. the comparative risk of severe toxicity in patients treated with several lines of ICI were specifically evaluated.158 The incidence of severe irAEs was significantly higher for anti-CTLA-4 than anti-PD-1 or anti-PD-L1 (21.7% vs 3.2%, p<0.001, 21.7% vs 2.4%, p<0.001, respectively). These differences were also maintained when the different irAEs were analyzed; thus, diarrhea and colitis were significantly more common in patients treated with anti-CTLA-4 vs. anti-PD-1/PD-L1 group [OR 8.1, 95% CI: 6.4–10.3 (p<0.001) and OR 12.2, 95% CI: 8.7–17.1 (p<0.001); respectively]. In fact, the immunological profile of colitis associated with anti-CTLA-4 seems to be different from colitis induced by anti-PD-1 therapy.159
Despite these differences in the GI toxicity profile and although safety may be a limitation when selecting a treatment, in many cases the decision to start an immunotherapy drug is closely linked to the tumor lineage and the clinician cannot choose between different drugs.
Statement 7
The risk of diarrhea/colitis seems to be higher for anti-CTLA-4 therapy than anti-PD-1 or anti-PD-L1, even for serious events (RR 8–12).
Quality of evidence: high.
Risk of diarrhea/colitis with the combination of anti-CTLA-4 and anti-PD-L1The combination of two immunotherapeutic drugs (anti-CTLA-4 plus anti-PD-1 or anti-PD-L1) is used as a therapeutic strategy in some neoplasms that show resistance to monotherapy treatment. Considering the risk of toxicity in monotherapy presented by these treatments, we could expect an increase in irAEs with this therapeutic approach. CheckMate clinical trials evaluated the efficacy and safety of ipilimumab plus nivolumab in patients with advanced melanoma; of the 945 patients included, 314 received combination therapy. At 4 years of follow-up, 59% of the patients in combination therapy presented some serious adverse event (grades 3–4) compared with 22 and 28% of the patients treated with nivolumab and ipilimumab monotherapy, respectively. The most frequently reported event was diarrhea. Up to 40% of the patients in combination therapy needed to stop treatment, mainly due to the appearance of colitis (30 patients, 10%).160 The most robust subsequent evidence comes from different meta-analysis showing an increased risk compared to monotherapy, especially with anti-CTLA-4.158,161–164 A recent meta-analysis that evaluates 18 studies (2767 patients) included patients treated with combined therapy (mainly ipilimumab plus nivolumab) vs monotherapy and showed an increase in serious adverse events with a RR 2.21 (95% CI: 1.57–3.10).165 The most reported events were diarrhea and colitis (for all grades) with an accumulated incidence of 3 out of 10 patients. Finally, retrospective studies and the pharmacovigilance data from the FAERS show data along the same lines.11,146,166,167
Statement 8
The risk of diarrhea and/or colitis is higher with combination of ICI (anti-CTLA-4 plus anti-PD-1 or anti-PD-L1) compared to ICI in monotherapy (RR 2).
Quality of evidence: high.
Risk factors for GI toxicity induced by ICIsMany risk factors for severe diarrhea and endoscopically proven colitis have been suggested; however, strong evidence is lacking and, to date, there are no reliable baseline biomarkers that can predict the development of ICIs-induced colitis in the clinical practice.
Risk of GI toxicity depending on the type of ICIMain risk factor for ICI-induced colitis, as a group, seems to be the class of drug with a higher risk with anti-CTLA-4 and the combination of two ICIs.118,126,139,146,168–170 Moreover, the occurrence of colitis in patients under ipilimumab has been shown to be dose-dependent,171–173 although some studies did not show differences between lower and higher doses.115,174 On the other hand, the recurrence rate of colitis in those patients who discontinued ICIs is high after patients received a rechallenge with the same drug,61 suggesting that a previous episode of ICIs-induced colitis is a significant risk factor for further development of new flares of this adverse event.
Risk of GI toxicity in patients treated with combination of ICIs and oncology therapiesChemotherapyChemotherapy and immunotherapy have different safety profiles. Shao J et al. demonstrated in a meta-analysis that the risk of presenting classic adverse events (such as cytopenia, nausea, asthenia, etc.) in patients with lung cancer treated with ICIs were lower than those treated with CT (risk ratio [RR]: 0.9, 95% CI: 0.84–0.95, p 0.001). Nevertheless, when the rate of irAEs in different organs was compared, this risk was higher for ICIs (colitis RR 5.54, hepatitis RR 2.49, hypophysitis RR 3.91, or pneumonitis RR 2.57). Therefore, with the advent of immunotherapy, the safety profile of treatments used in oncology has changed.175
Considering the therapeutic resistance of different tumors to chemotherapy or immunotherapy used in monotherapy, different therapeutic strategies that combine both drugs have been evaluated. A meta-analysis published in 2020 analyzed the risk of GI toxicity comparing combined ICIs and chemotherapy regimens and it was demonstrated that chemotherapy plus ICIs (both anti-CTLA-4 and anti-PD-1/PD-L1) vs. chemotherapy alone significantly increased the risk of diarrhea (RR 2.23 and RR 1.38, respectively) and colitis (RR 28.39 and RR 2.90, respectively).176 These results were confirmed in other meta-analysis.177
Considering the essence of this guide, it would be interesting to know if there is an increased risk between associating chemotherapy with immunotherapy vs. isolated immunotherapy; however, scientific evidence regarding this topic is still scarce. A meta-analysis of 16,409 patients included in 26 randomized controlled trials (RCT), of which five studies included ICIs (atezolizumab or pembrolizumab) plus chemotherapy regimens showed a higher rate of colitis of any grade with the combination of ICIs and chemotherapy (RR 2.62; 95% CI, 1.25–5.48, p=0.01), with no statistically significant differences in severe colitis (RR 2.27; 95% CI: 0.93–5.53, p=0.07).177
Targeted therapiesAnother therapeutic approach includes the use of targeted therapies [antiangiogenic monoclonal antibodies (mAbs) and tyrosine Kinase inhibitors (TKIs)] together with immunotherapy as a synergistic effect of two different mechanisms of action assumed. A systematic review analyzed the safety profile of a combination of ICIs and antiangiogenic drugs (14 with bevacizumab and 1 with ramucirumab) or TKIs (5 with axitinib, 4 with pazopanib, 2 with sorafenib, 2 with lenvatinib and 1 each with sunitinib, cabozantinib, apatinib and cediranib). The rate of serious adverse events (AEs) with ICIs plus TKI was higher than that of ICI plus mAbs (60.1% vs 44.5%). The incidence of severe colitis was less than 1% for both strategies.178 The data available with the different immunotherapies and targeted therapies are scarce and many of them are poor quality and without a control group.179–181 The largest extractable evidence shows that atezolizumab plus bevacizumab could increase the rate of AEs but not the rate of irAEs.182 In summary, the available data on the safety of combining immunotherapy with targeted therapies makes it difficult to draw conclusions about its safety at present.
On the other hand, two retrospective studies showed that patients treated with IPCIs and concomitant radiotherapy – particularly those who received a higher dose – had a greater rate of irAEs than patients treated with ICIs alone183,184; however, these studies did not find a significant association between site irradiated and organ-specific adverse events, probably due to their small sample size.
Statement 9
The risk of diarrhea/colitis due to ICIs plus chemotherapy has not been properly addressed and does not appear to be increased.
Quality of evidence: moderate.
Statement 10
Combination therapy with ICIs plus target therapy may increase the risk of diarrhea/colitis (especially for TKIs).
Quality of evidence: low.
Patient-related risk factors for developing ICI-induced GI-toxicityA retrospective analysis of a cohort of 327 patients from MD Anderson Cancer Center174 and a nationwide, population-based study including more than 13,000 patients treated with ICIs185 showed that caucasians had higher odds of developing diarrhea or colitis. Thus, it is tempting to hypothesize that genetic factors might play a role in the development of ICI-induced colitis; but, few studies have investigated this issue demonstrating association with some polymorphisms and the development of irAEs.186–189 However, to date, all these data have not been validated.
Compared to patients diagnosed with other malignancies, melanoma patients seem to have a higher risk for ICIs-induced colitis development.109,174,190 The reason for this association is unknown, but it should be considered that ICIs, and particularly ipilimumab, were initially tested on melanoma patients, and the majority of trials on lung cancer patients evaluate anti-PD-1/PD-L1 therapy. In fact, multivariate logistic regression model for the risk of colitis depending on tumor type carried out from a systematic review including around 7000 patients did not confirm this relationship.140
It is well known that body composition is related with drug toxicity: in this sense, sarcopenia and low muscle attenuation – assessed before treatment by computed tomography – were significantly associated with the development of high-grade irAEs, including colitis, in a study that included 84 patients diagnosed with melanoma and treated with ipilimumab.191 In addition, it has been shown that obese individuals had a significantly higher risk for ICI-induced colitis.192 On the other hand, gut microbial composition – which is influenced by the body mass index – might also be a risk factor for ICIs-induced colitis: in 2016 Dubin K et al. demonstrated, analysing fecal samples from a cohort of patients treated with ipilimumab, that an increased representation of bacteria belonging to the Bacteroidetes phylum was associated with a lower rate of development of ICI-induced colitis, consistent with a previously suggested immunomodulatory role of these commensal bacteria. They also showed that a paucity of genetic pathways involved in polyamine transport and vitamin B biosynthesis was associated with an increased risk of colitis.193 A second study that analyzed fecal samples from 26 melanoma patients treated with ipilimumab confirmed those findings,194 showing that individuals with a baseline gut microbiota enriched with Faecalibacterium and other Firmicutes had a higher rate of ipilimumab-induced colitis in comparison with those whose baseline microbiota was driven by Bacteroides. Therefore, a high Firmicutes to Bacteroidetes ratio in fecal samples at baseline may predict a higher risk of ICI-induced colitis.195 Moreover, baseline levels of antibodies directed to microbial antigens such as Escherichia coli outer membrane porin (anti-OmpC) could also be related with a higher risk for ICI-induced colitis.196 Additional evidence on the role of microbiota in the pathogenesis of ICI-induced colitis comes from a retrospective study including more than 800 patients that showed antibiotic use at any time was associated with a reduced ICI-induced colitis incidence but a more frequent hospital and intensive care unit admission due to more severe forms of intestinal inflammation. Furthermore, those receiving antibiotics after ICIs therapy start, and those treated with antibiotics with anaerobic activity had a higher rate and severity of colitis.197
Certain cellular and molecular features suggestive of immune dysregulation at baseline or immediately after ICIs treatment initiation have been proposed as risk factors for immune-related – including gastrointestinal – adverse events and could predict their development.198,199 Neutrophil to lymphocyte ratio – an increasingly used biomarker of systemic inflammation – before ICIs treatment has been shown to be significantly lower in patients with irAEs,200–202 including colitis.140,170 It has been reported that patients with ICI-induced colitis tend to have higher absolute CD4+ T-cell numbers and lower percentage of regulatory T cells in peripheral blood at baseline compared to patients who did not develop such AE.194 Early changes in circulating B cells subpopulations following combination of ICIs may also identify patients at risk of irAEs, including colitis.203 Moreover, gene expression profiling of peripheral blood, sampled before or early after ipilimumab treatment start, resulted in the identification of a set of potential biomarkers – notably increases of the gene expression of neutrophil-activation markers CD177 and CEACAM1 – that were associated with the subsequent development of GI AEs.204 In a more recent study on melanoma patients included in two clinical trials evaluating the anti-CTLA-4 antibody tremelimumab, an RNA transcript-based gene signature (including 16 immune response-related genes) in peripheral blood obtained after treatment initiation have been shown to discriminated patients developing grades 0–1 from grades 2 to 4 diarrhea or colitis.205 On the other hand, a positive correlation between elevated baseline serum IL-17 levels and the risk of diarrhea and severe colitis was found in a phase 1 trial of ipilimumab.206 Finally, vitamin D intake – that has been associated with immunomodulatory effects – was correlated with a reduced risk for ICI-induced colitis in a retrospective analysis.170 In any case, this interesting finding should be confirmed in future RCTs. Risk factors associated with the development of diarrhea and/or colitis are summarized in the supplementary table 4.
IBD as a risk factor for the subsequent development of ICI-induced enterocolitisCancer patients with a previous diagnosis of an immune-mediated (IM) disease, including inflammatory bowel disease (IBD), were mostly excluded from ICI clinical trials.5 But real-world studies have suggested that patients diagnosed with pre-existing IBD before ICI treatment seems to have an increased risk of severe diarrhea and colitis after treatment with both anti-CTLA-4 and anti-PD-1/PD-L1 therapy.207–210 In patients with pre-existing IBD, the risk of flare after ICIs treatment seems to be higher in patients treated with ipilimumab (vs anti-PD-1/anti-PD-L1),207 in younger patients and, probably, in those previously diagnosed with microscopic colitis (vs. ulcerative colitis or Crohn's disease).208
Statement 11
Many patient-related factors, such as race, body mass index, genetic or immunogenic profile, antibiotic use, gut microbiome composition, or vitamin D intake, have been proposed as risk modifiers for developing ICI-induced GI toxicity. Patients with pre-existing IBD seems to have an increased risk of severe diarrhea and colitis.
Quality of evidence: low.
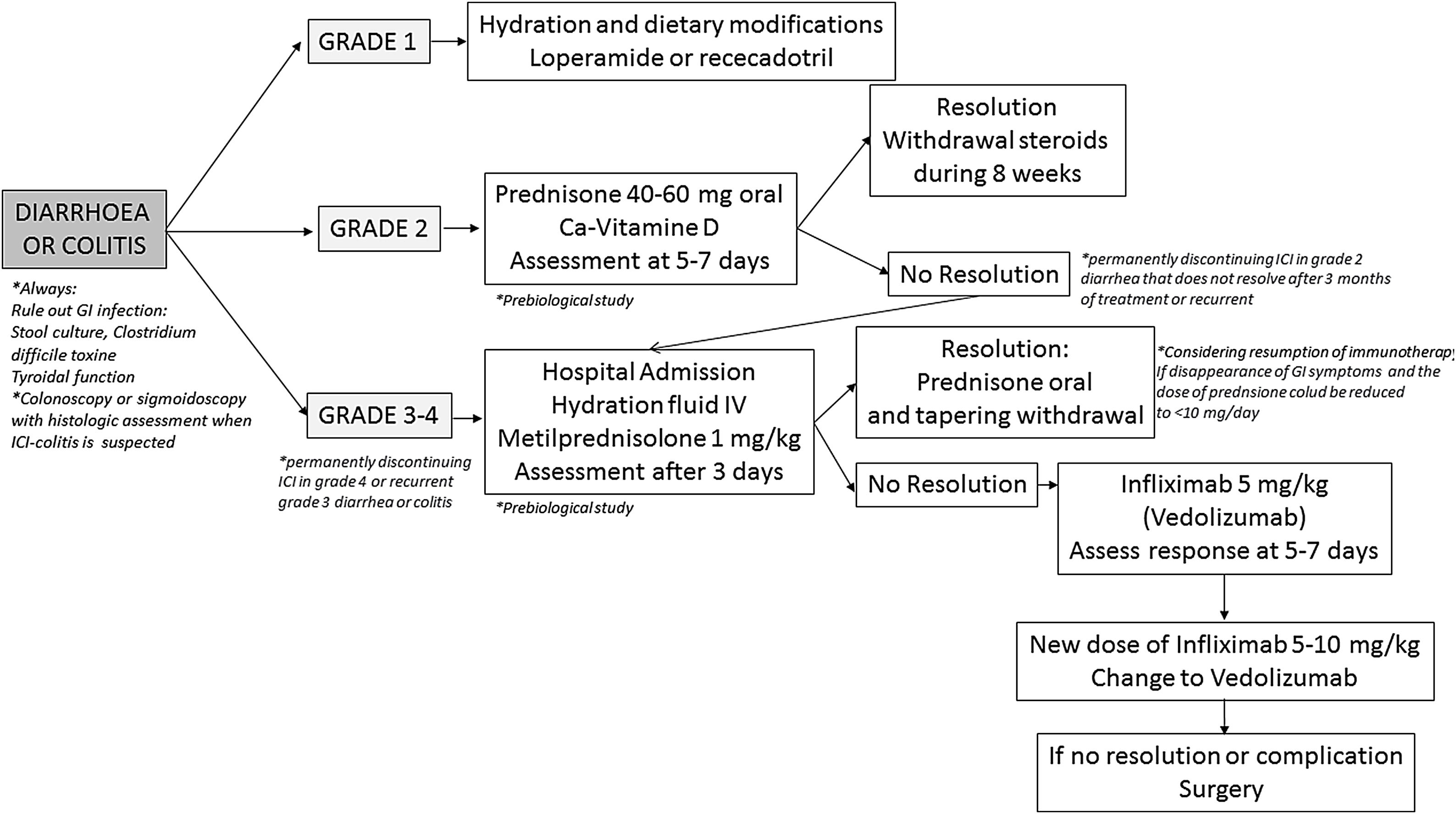
Diagnosis and differential diagnosisDiagnosis toolsColonoscopy with biopsies has been proposed as the gold standard diagnostic tool for patients with suspicion of ICI-induced colitis.26,138,207 In the majority of patients, the inflammatory process affects exclusively the colon, more than 40% of them having pancolitis, and approximately half of cases show continuous involvement; less than 20% show both ileal and colonic involvement, whereas isolated ileal involvement is anecdotic.109,112,114–116,146,151,196,211–213 Mucosal erythema, absence of vascular pattern, granularity and friability seems to be the most frequent endoscopic findings, but in up to one to two thirds of cases, ulcers are present at endoscopic examination.112,116,212,213 Ulcers are associated with a worse prognosis in terms of need for hospital admission, CS-refractoriness and need for biologic treatment117,118,120,211; therefore, early endoscopic evaluation of patients with ICIs-associated diarrhea is mandatory, it being associated with better prognosis.117 It is noteworthy to underline that the vast majority of patients with ICI-induced colitis have recto-sigmoid involvement; thus, flexible sigmoidoscopy might represent a safer and more affordable alternative to complete colonoscopy,214 and recent studies suggested that sigmoidoscopy with biopsies may be sufficient for the initial evaluation of suspected ICI-induced colitis.215–217Histological features of ICIs-induced enterocolitis are non-specific, and include lymphoplasmocytic infiltration of the lamina propria, increased intraepithelial lymphocytes and/or neutrophils, cryptitis and crypt distortion – usually mild – and, characteristically, augmented epithelial apoptosis.109,218,219 Moreover, granulomas and thickening of the subepithelial collagen band have been described.112,114–116,146,196,211,213,220–223 Immunohistochemical analysis shows an increase of all T-cell subsets (CD3+, CD4+, and CD8+) and of CD4+CD25+ regulatory T cells.220,222 A recent, comprehensive single-cell analysis of mucosal immune cell populations in ICI-induced colitis showed a predominant accumulation of CD8+ T cells with highly cytotoxic and proliferative states – without evidence of regulatory T cells depletion – as well as an increase in myeloid-lineage cells.224 In the same line, another recent study has elegantly demonstrated that the predominant activated T cell subset in this condition is a population of interferon gamma-producing CD8+ tissue-resident memory T cells.225 In those cases of recurrent ICIs-induced colitis, histological features are similar to the initial episode but, in addition, features of chronicity develop – basal plasmocytosis, prominent crypt architectural irregularity and Paneth cell metaplasia – mimicking classical IBD.221 Few studies have reported ICI-induced gastrointestinal pathological findings outside the colon, such as lamina propria expansion and intraepithelial neutrophils in the stomach, or lamina propria expansion by lymphoplasmocytic infiltrates and eosinophils, villous blunting, intraepithelial lymphocytosis and neutrophilic villitis in the duodenum and ileum.115,213,220,226
Nevertheless, it should be considered that gastrointestinal clinical symptoms do not properly correlate with ICIs-induced histological or endoscopic inflammation.120,211 In this sense, non-invasive, fecal markers could represent a first screening test in this clinical scenario, to identify those patients at risk of colitis.5,26,138 It is well known that fecal calprotectin and lactoferrin correlates with endoscopic inflammation in patients diagnosed with IBD, but these markers also have an adequate sensitivity detecting endoscopic and even histological inflammation in patients with ICI-induced diarrhea, and can predict endoscopic and histological remission in patients receiving treatment for this condition.117,196,227 However, we must keep in mind that calprotectin may be elevated by concomitant treatment with either non-steroidal anti-inflammatory drugs (NSAIDs) or proton-pump inhibitors (PPIs).228,229
The role of cross-sectional imaging techniques – CT and MR – in the diagnosis of ICI-induced enterocolitis has also been explored. CT might have a role in the diagnosis of ICI-induced colitis, particularly in the emergency room setting,5,230 and is essential for excluding complications such as perforation and abscesses.114,115 Nevertheless, and although positive-predictive value of CT is reported from being high, negative predictive value and correlation with colonoscopy seem far to be optimal.211,231 CT findings in patients with ICI-induced colitis include diffuse or segmental colonic wall thickening with mucosal or mural hyperenhancement, colonic dilation, pericolonic inflammatory changes and mesenteric vascular engorgement.126,231–236Clostridium difficile and CMV superinfections have been described in patients treated with ICIs,112,151,237 and should be ruled out in every patient.5,26,214,238,239 In relation to CMV superinfection, IgM and IgG serology in blood must be first requested to guide whether it is a reactivation or primary infection, and also the presence of inclusion bodies, immunohistochemistry staining or determination of the viral load in the biopsies is recommended. Those patients with CMV superinfection should start treatment with intravenous ganciclovir in severe cases or oral valganciclovir in outpatient cases for 21 days.240
Other enteric infections have been also diagnosed,112,241,242 and it seems reasonable to rule them out by means of stool culture and direct parasite examination in fecal samples in every patient with new onset or recurrent ICI-induced diarrhea.5,26 Other causes of diarrhea within this clinical scenario include pancreatic exocrine insufficiency due to immune-induced pancreatitis243 or pancreatic cancer, de novo coeliac disease,244 and immune hyperthyroidism.245 Finally, radiation proctitis or intestinal metastasis of malignant melanoma, among many other conditions, can cause haematochezia and should be considered in the appropriate clinical setting.
Recommendation 17
We recommend early endoscopic evaluation (colonoscopy or sigmoidoscopy) with histologic assessment as the gold standard diagnostic test for patients with suspicion of ICI-induced enterocolitis.
Quality of evidence: moderate.
Recommendation: strong in favor.
Recommendation 18
We recommend the use of fecal markers such as calprotectin as first screening test in patients treated with ICIs who develop diarrhea.
Quality of evidence: low.
Recommendation: strong in favor.
Recommendation 19
We recommend ruling out bacterial infection (including Clostridiodes difficile), CMV and parasites superinfections in every patient with suspicion of ICI-induced enterocolitis.
Quality of evidence: moderate.
Recommendation: strong in favor.
Differential diagnosisDiarrhea is a common AE with both immunotherapy and chemotherapy, as demonstrated in the phase III RCT KEYNOTE-177, which compared pembrolizumab vs. chemotherapy in advanced microsatellite instability-high colorectal cancer. Diarrhea was the most common toxicity, 44% (6% grades 3–4) with pembrolizumab and 62% (11% grades 3–4) with chemotherapy.246 On the other hand, differentiating between ICI-induced colitis and chemotheraphy-induced colitis can be complicated in patients treated with a combination of both, as evidenced by the diagnosis of up to 15% of GI-AEs with potential immunologic etiology in the chemotherapy and placebo arm of the phase III RCT CHECKMATE-648 in advanced esophageal squamous cell carcinoma.247
Any chemotherapy can cause diarrhea but it is often self-limiting, not usually associated with abdominal pain, and responds to diet and loperamide.248 In contrast, in the presence of events of significant duration or magnitude or associated with abdominal pain, signs of systemic inflammation or fecal calprotectin and lactoferrin increase, ICI-induced colitis should be suspected and sigmoidoscopy with colonic biopsy should be performed to check for inflammation and lymphocytic infiltrates to support the diagnostic suspicion.
When diarrhea is refractory, prolonged, aggravated or associated with unusual symptoms/signs, other etiologies should be ruled out. Common causes include infectious, medicinal (antibiotics, antacids), food intolerances to lactose or gluten (coeliac disease), irritable bowel, inflammatory bowel disease and ischemic colitis.249 Certain neoplasms, complications or treatments are also associated with diarrhea such as neuroendocrine tumors with carcinoid syndrome, and peritoneal mesothelioma,250 oncological surgeries such as wide bowel resections (short-bowel syndrome), partial intestinal obstruction, and radiation toxicity. In addition, immunotherapy may cause diarrhea due to less common causes than colitis such as pancreatic atrophy (and exocrine pancreatic insufficiency),251 interstitial nephritis252 or pseudolipomatosis.253
Treatment and prognosis of ICI-induced colitisAll available recommendations and published guidelines regarding the treatment of ICI-induced colitis are based on the opinion or consensus of experts based on limited data from observational studies and case series. There are no prospective clinical trials to guide ICI-induced colitis treatment. Four consensus guidelines on the management of irAEs have been published.26,27,138,254 Due to the lack of clinical evidence, some criteria used in IBD are assumed. A management algorithm of diarrhea and colitis in patients undergoing ICI therapy is proposed in Fig. 2.
Fig. 2 shows the management of ICI-induced diarrhea. Patients with grade 1 who do not respond to supportive therapy (hydration, fiber, anti-diarrheal drugs) and patients with grade 2 diarrhea should start oral CS (0.5–1mg/kg/d prednisolone).119,142,255–258 CS should be started as soon as possible. Once clinical improvement to grade 1 or less is achieved, CS should be tapered over 4–6 weeks.27,114 In grades 3–4 diarrhea, hospitalization is required for systemic symptoms (fever, hypotension, tachycardia, and/or dehydration) and electrolyte imbalance.142 Patients with grades 3–4 diarrhea should receive intravenously CS (1mg/kg/d methylprednisolone).114,119,128,255–258 Doses higher than 1mg/kg/d do not increase the efficacy and have poor tolerance, moreover, there are more efficient and safer alternatives. Patients who respond to intravenous CS within 3 to 5 days should be switched to the oral form; subsequently, oral CS should be tapered during a period of 8-week by analogy with IBD management.26,27,114,119,128,138,142,254–259 Overall, between 40 and 70% of patients who develop ICI-induced colitis respond to CS.114,116,119,126,128,255–258,260 Data from a retrospective study show that the median duration of CS for grade 3 diarrhea/colitis is 58 days, this long mean time increases the risk of AEs due to CS.260 In fact, around 17% of patients with GI irAEs have adverse events due to CS, such as adrenal insufficiency, diabetes, infections, and volume overload.126
There are no data regarding the usefulness of antibiotics in this scenario although their use could be considered in case of severe colitis. Analogous to other types of severe colitis, morphine derivatives (including loperamide) and NSAIDs are contraindicated for the risk of precipitating a toxic megacolon. In addition, dehydration and hydroelectrolytic disorders need to be corrected as well as an assessment of nutritional status. Patients with ICI-induced colitis have an increased risk of thromboembolic disease due to both the colitis itself and the underlying neoplasm. Therefore, low molecular weight heparin prophylaxis is recommended. In this clinical setting (severe colitis), immunotherapy should be discontinued.
Many patients with ICI-induced colitis are treated without prior endoscopy and histology evaluation. Some patients developed IM-microscopic colitis. The treatment of choice for microscopic colitis is budesonide, however, a case-control study comparing IM-microscopic colitis with microscopic colitis showed that patients with IM-microscopic colitis required significantly more systemic CS or biologic agents.261 Some data demonstrated a rate of 60% of response to CS in IM-microscopic colitis.262 Patients with grade 2 IM-microscopic colitis could be treated with budesonide, but patients who do not improve after 1-week or those with grades 3–4 should be treated with CS.263,264
Recommendation 20
We recommended a management based on supportive therapy in patients with grade 1 diarrhea.
Quality of evidence: low.
Recommendation: strong in favor.
Recommendation 21
We recommend starting oral CS (0.5–1mg/kg/d prednisolone oral route) in patients with grade 1 who do not respond to supportive therapy and those with grade 2 diarrhea. In case of response, CS should be tapered over 4–6 weeks.
Quality of evidence: low.
Recommendation: strong in favor.
Recommendation 22
We recommend starting systemic CS (1mg/kg/d of methylprednisolone intravenously) in patients with grades 3–4 diarrhea-colitis. Patients who respond to intravenous CS within 3–5 days should be switched to the oral form and should be tapered during a period of 8 weeks.
Quality of evidence: low.
Recommendation: strong in favor.
Corticosteroid-refractory ICI-induced colitisCS-refractory ICI-induced colitis is defined as the persistence of symptoms within 3–5 days of high-dose CS intravenously. Around 30–50% of patients develop CS-refractory ICI-induced colitis and 15–50% of patients relapse during CS tapering.114,116,119,126,128,255–258,260,262 These patients could be treated with rescue therapies for avoiding serious complications such as perforation and colectomy. In this scenario, more data are available with IFX.265 IFX use has been associated with a shorter time to resolution and CS titration, without a negative impact on OS or response, although patients who received IFX had more severe enterocolitis than those treated with CS.112,114,126,157,181,224,256–258,265–273 In this sense, a retrospective study showed a shorter duration of CS use and faster symptoms resolution (median 3 days) in IFX-treated patients compared to CS-treated patients (p=0.001).266 No prospective studies are available to guide IFX dosing, but, due to similarities with IBD, IFX should be started at a dose of 5–10mg/kg. Most published cases used IFX at the standard dose of 5mg/kg, except for some cases of partial response in which the subsequent dose was increased to 10mg/kg.274 Around 70% of patients achieved response after the first IFX infusion and in most cases the number of IFX infusions did not exceed 3 doses (baseline, day 14, and day 42).112,115,116,151,211,265–276Some retrospective data have reported the results of vedolizumab therapy in patients with ICI-induced colitis refractory to CS or IFX.260,262,277–285 With vedolizumab, clinical remission was obtained in most patients (80%) and improvement was observed after a median of 5 days and sustained at 15 months. Overall, the median number of vedolizumab infusions was 3, the median interval for symptom resolution was 56 days, with endoscopic and histologic remission achieved in 54% and 29%, respectively.211,260,262,275,277–284 One of the largest series of ICI-induced colitis refractory to CS treated with vedolizumab showed that 67% of patients who failed previously to IFX attained clinical remission compared with 95% of patients who did not receive IFX.277 Vedolizumab was also an effective treatment for ICI-induced colitis with microscopic colitis on histology.262 Vedolizumab is an option in patients who do not respond to IFX or in whom IFX is contraindicated.
Guidelines recommend IFX as first line therapy for patients with ICI-induced colitis and vedolizumab is recommended for IFX refractory patients or when IFX therapy is contraindicated. However, no prospective clinical studies are available to guide the choice of IFX vs. vedolizumab. Based on IBD area, IFX is associated with rapid response, but vedolizumab is linked with a better safety profile.
On the other hand, early introduction of biologic therapy in the treatment algorithm had been associated with a better outcome in a retrospective study of 179 patients with ICI-induced colitis, 84 of them treated with biologics agents. Those patients who received biologic therapy in less than 10 days from colitis onset had fewer hospitalizations (p=0.03), shorter CS taper (p=0.09), and shorter duration of symptoms (p=0.01).275
In patients who have not been tested for viral hepatitis prior to the beginning of ICIs in order to assess the risk of reactivation, the performance of HBsAg, anti-HBc and anti-HCV is highly recommended before the administration of monoclonal antibodies, especially IFX.286,287
Moreover, in all patients treated with CS, screening for latent tuberculosis infection by means of an interferon-gamma release assay is highly recommended for the possibility of later refractoriness and need of biological therapy.
Recommendation 23
We recommend treatment with Infliximab 5mg/kg in patients with CS-refractory ICI-induced colitis. If there is no response at 7 days another infusion at week 1 and week 6 is recommended or increase the dose to 10mg/kg in case of severity.
Quality of evidence: low.
Recommendation: strong in favor.
Recommendation 24
We recommend treatment with vedolizumab (300mg) in those patients who do not respond to infliximab or in whom infliximab are contraindicated.
Quality of evidence: low.
Recommendation: strong in favor.
Recommendation 25
We recommend that, once clinical remission has been achieved, biological treatment can be discontinued, and CS removed slowly. An extended course of 2–3 months of CS is recommended for those patients with initially severe symptoms. The dose of CS could be reduced to ≤10mg per week without the need to add any other maintenance immunosuppressive therapy.
Quality of evidence: low.
Recommendation: strong in favor.
Immune-induced colitis-refractory to biological agentsPatients who do not response to biological agents may develop a toxic megacolon, an intra-abdominal abscess or a colonic perforation. This situation is very severe, nonetheless it is rare. In case of perforation, surgical intervention should be mandatory (usually a subtotal colectomy with temporal ileostomy). If no response to IFX, vedolizumab or other rescue therapies,288–292 surgical interventions are indicated.
Anecdotally, some case reports have shown positive outcomes treating ICI-induced colitis refractory to biological agents with conventional immunosuppressants and selective immunosuppressants such as cyclosporine, mycophenolate, tofacitinib and ustekinumab with good results.260,274,283,284 Fecal microbiota transplantation has been tested in ICI-induced colitis refractory to CS, IFX and vedolizumab. However, further prospective studies are needed to define appropriate candidates and candidate donors.293
Continuation, resumption and withdrawal of immunotherapy in patients with prior ICI-induced colitisThe irAEs that force the withdrawal of ICIs most frequently are diarrhea and colitis.115 ICIs can be maintained in grade 1 diarrhea and may be continued without any interruption or delay in therapy but should be withheld in grade 2 diarrhea. Overall, discontinuation rates of ICI are approximately 7.5% with anti-PD-1 and 14% with anti-CTLA-4.114,142 Management after developing an irAE varies depending on the ICIs received. Guidelines recommend considering permanent discontinuation of anti–CTLA-4 agents, due to high incidence of recurrent GI irAEs (44%).114,142 However, anti-PD-1/PD-L1 drugs can usually be resumed in monotherapy when symptoms are resolved or improve to grade 1 diarrhea, or when CS is tapered to daily doses≤10mg. Biological rescue therapy for initial ICI-induced colitis and a long duration of symptoms was associated with a more likely ICI-induced colitis recurrence after the resumption of ICI therapy.68,238
Resumption of immunotherapy can be considered if the following requirements are met: (1) disappearance of gastrointestinal symptoms, (2) the dose of CS can be reduced to ≤10mg/d without the need of any other maintenance immunosuppressive therapy and, (3) the benefit of immunotherapy outweighs the potential risks.
Re-treatment with immunotherapy after irAEs can be grouped into four scenarios: (1) class switch (anti-CTLA-4 to anti-PD-1)294; (2) resume same agent after recovery from irAE238; (3) drop anti-CTLA-4 after irAE with anti-CTLA-4 and anti-PD-1 combination294; and (4) sample with a mix of the above attitudes.68,238 When sequencing or switching from one class of ICI to another, the relatively long half-life of the agents and the duration of T-cell receptor occupancy of up to 2 months should be considered since early exposure to the second ICI may be equivalent to giving these agents in combination with a higher likelihood of inducing irAEs.295
On the other hand, ICI should be permanently discontinued in the following cases: (1) diarrhea or grade 4 colitis; (2) recurrent grade 3 diarrhea or colitis and, (3) grade 2 diarrhea that does not resolve after 3 months of treatment.
Risk of GI-toxicity with anti-PD-1 after GI-toxicity due to anti-CTLA-4 (or vice versa)The risk of immunotherapy withdrawal due to the development of serious irAEs can reach up to 25% of cases in patients treated with anti-CTLA-4.140 Although it is described that the clinical outcome for these patients could be favorable without treatment (see treatment section), it would be interesting to know the risk of developing recurrence or onset of GI toxicity “de novo” in patients treated with another subsequent ICI. The incidence of recurrence of the initial irAE or the appearance of a new one is 40–50% in patients re-treated with ICI. The decision of ICI rechallenges after an irAE should be based on the type and severity of the irAE, the previous response to the immunosuppressive treatment, efficacy expectations and therapeutic alternatives. It seems conceivable to rechallenge patients with ICI after recovery from non-severe ICI-induced colitis, and this is an aspect of treatment for which some data are available (two meta-analysis and 11 retrospective studies).61,67–69,238,294–301 A total of 789 ICI rechallenge cases after toxicity were analyzed and the incidence of irAEs of any grade and grade 3/4 after rechallenge was 34.2% and 11.7%, respectively. Rechallenge compared to initial ICI treatment showed a higher incidence of irAEs, but a similar rate of severe irAEs. Previous GI irAEs and the interval between initial irAEs and ICI reissue were associated with a higher recurrence of high grade irAEs.296
For patients initially treated with anti-PD-1/PD-L1, anti-CTLA-4 rechallenge had a significantly higher incidence of irAEs than anti-PD-1/PD-L1 rechallenge, whereas for those initially treated with anti-CTLA-4 or combination, no significant difference existed in the incidence of irAEs in different rechallenged ICIs.
A meta-analysis including 437 patients from 10 studies who were retreated with ICI after irAEs, confirmed an incidence of recurrence of any grade and grade 3/4 irAEs of 47%, and 13.2%, respectively.297 This incidence is comparable to the historical rate of irAEs in patients with initial ICI while serious events were lower on re-exposure than on previous treatment. The risk of serious irAEs at rechallenge was higher in the subgroup that received combination therapy as the initial regimen.
The study with the largest series is based on cases from the VigiBase, an international pharmacovigilance database.61 Out 452 informative rechallenges, the recurrence rate was 29% after anti-PD-1/PD-L1, 47% after anti-CTLA-4, and 43% after combination therapy resumption. One-quarter to one-third of patients had the same irAE, being colitis in 37%. In the multivariate analysis, anti-CTLA-4 regimen, colitis, hepatitis, and pneumonitis initial irAEs were associated with a higher irAE recurrence rate.61
Pollack MH et al. evaluated the safety of anti-PD-1 treatment in patients who had prior adverse events (grades 2–4) under combination therapy (ipilimumab plus nivolumab).67 Out of eighty patients included, diarrhea/colitis was the most previous prevalent event (41%) and required CS and/or immunosuppressive treatment in all cases. The data of recurrence and appearance of colitis “de novo” were similar to those previously reported (6% and 19% respectively). This risk was associated with a short time interval between the adverse event and the start of the second treatment (56 vs. 62 days, p=0.03) and the continuation of CS treatment at the time of second line therapy; neither severity nor the need of immunosuppressants were associated with the risk of recurrence. Data from a retrospective study described the evolution of 80 patients who developed GI toxicity due to ICIs and received a second line of treatment (27% grades 3–4).298 After one year of follow-up, 80% of the patients were free of the appearance of diarrhea/colitis. Although there was an increased risk of developing colitis in patients who were exposed to anti-CTLA-4 as a second-line treatment, these differences were not significant.
Given that the evidence is scarce and the risk of colitis “de novo” is not negligible, it seems reasonable that the decision to start new lines of immunotherapy treatment is strictly related with the characteristics of the oncological disease and the oncologist's criteria. There should be close monitoring of potential toxicity.
Recommendation 26
We recommend permanently discontinuing ICI in the following cases: diarrhea or grade 4 colitis, recurrent grade 3 diarrhea or colitis or grade 2 diarrhea that does not resolve after 3 months of treatment.
Quality of evidence: low.
Recommendation: strong in favor.
Recommendation 27
We recommend considering the resumption of immunotherapy if the following requirements are met: disappearance of gastrointestinal symptoms, the dose of prednisone could be reduced to ≤10mg/d without the need to add any other maintenance immunosuppressive therapy, the benefit of immunotherapy exceeds the potential risks.
Quality of evidence: low.
Recommendation: weak in favor.
Recommendation 28
The introduction of other ICI in patients with prior digestive toxicity secondary to ICI should be balanced by a multidisciplinary team, due to the possible occurrence or diarrhea/colitis.
Quality of evidence: low.
Recommendation: strong in favor.
Prophylactic therapy to decrease the risk of ICI-induced colitisOnly one RCT has evaluated budesonide prophylaxis as a prevention of ICI-induced colitis,285 however, prophylactic budesonide (9mg/d orally) did not prevent ipilimumab-induced diarrhea or colitis.302
Recommendation 29
Prophylactic therapy with budesonide is not recommended to decrease the risk of ICI-induced colitis.
Quality of evidence: high.
Recommendation: strong against.
Prognostic factors: association between ICI-induced colitis risk and anti-PD-1 efficacyAlthough immunotherapy increases survival in diverse advanced cancers, it only benefited a subset of patients with no clear evidence of predictive factors beyond PD-L1 expression or microsatellite instability.160 The increase in OS in patients developing grades 3–4 ICI-induced colitis is probably a projection of the magnitude of the immune response, reflects the anti-tumor efficacy of ICIs and could be a predictor of survival.303 Since irAE is not a baseline but a time-dependent event, appropriate statistical methods should be used to compare outcomes in patients with ICI to avoid bias such as time exposition and spurious conclusions.304
Two retrospective studies of advanced melanoma patients treated with ICI showed that colitis of any grade was associated with increased OS and improved performance status (PS) scale.302,304 In 576 nivolumab-treated patients with melanoma (phase I and phase III studies) a significantly higher response rate with no impact on PS was found in patients with irAEs of any grade (n=255) compared to those without.285 Another phase III trial compared pembrolizumab vs placebo in patients with melanoma, the development of irAEs was associated with a longer recurrence free survival (Hazard Ratio [HR] 0.61, 95% CI: 0.39–0.95, p=0.03).305
Between the different irAEs, endocrine, cutaneous and GI were associated with an improve OS, while hepatic and pulmonary irAEs conditioned a poor OS outcome (p=0.001).306 A pooled analysis of seven trials in 1747 patients with metastatic or locally advanced urothelial cancer that led to approval of an anti-PD-1/PD-L1, found an improvement in OS among patients who developed irAE (HR, 0.53; 95% CI, 0.43–0.66) when adjusted for baseline covariates and for duration of exposure.307
The prospective non-interventional ST-ICI trial investigated the prognostic role of irAEs (n=29) in patients with non-melanoma solid cancer treated with anti-PD-L1 alone and in combination with radiotherapy (n=104). OS was higher in patients with vs. without irAEs (22.8 vs 9.0 months, p=0.001). On multivariate analysis, only PD-L1 on tumor cells (p=0.049) and irAEs (p=0.001) remained independent predictors of OS.308 In a French series of patients with advanced solid tumors treated with ICI (n=410), when defining irAEs as a time-varying covariate and adjusting on potential confounding factors, grade≥2 irAEs (n=126) increased OS in the overall population (HR 0.57, 95% CI: 0.43–0.74, p<0.0001) and in patients treated with anti-PD-L1 (HR 0.50, 95% CI: 0.36–0.70, p<0.0001). Regarding types of irAEs, thyroiditis, colitis and rash were statistically significantly associated with better OS.309
In a meta-analysis including 30 studies (n=4971), cancer patients who developed irAEs experienced an OS and PS benefit from ICI compared to patients without irAEs (OS: HR 0.54, 95% CI: 0.45–0.65, p<0.001). In sensitivity analysis, the pooled results for OS and PS remained significant indicating that the association between irAE and ICI efficacy was robust. The pooled HR for OS in large studies (n>100) was comparable to the overall effect (0.58 vs 0.54). Notably, neither the Beggfunnel plot nor Egger's test revealed publication bias for OS, further confirmed the stability of the OS results.310
In conclusion, irAEs appear to represent a clinical biomarker for response to ICI, although the true nature of this association is unknown and does not appear to be due to exposure time bias.
Statement 12
Immuno-related adverse events have a prognostic role for the response to ICI, although the true nature of this association is unknown.
Quality of evidence: low.
Immunotherapy toxicity in the upper digestive tractAlthough the literature is scarce, and the scientific evidence is mostly poor quality (many case reports and two retrospective series), it shows that immunotherapy can affect the upper gastrointestinal tract, mainly at the gastric level. One of the first reports of upper digestive involvement was published by Beck KE et al. describing the cases of enterocolitis caused by ipilimumab in 137 patients with melanoma. Cases were identified by suggestive symptoms and compatible endoscopic/histological involvement. Sixteen of the 41 patients diagnosed with enterocolitis had also undergone gastroscopy, of which 10/16 presented macroscopic alterations and 14/16 histological alterations.115 Cheung V et al. publish data on the incidence of gastritis associated with the use of anti-PD-1/PD-L1 or in combination with anti-CLTA-4 in a hospital setting. Of the 205 patients included, 11 (5.4%) developed gastritis.311 Finally, Tang T et al. published data on upper gastrointestinal involvement in a retrospective, single-center series of 4716 patients treated with different lines of immunotherapy. One percent of the patients (n=60) were diagnosed with upper gastrointestinal involvement through endoscopic or histological confirmation, within these, 48% had received treatment with anti-PD-1, 33% anti-CTLA-4 and 18% on combination therapy.312
Despite limited published data, it is likely that upper digestive involvement is underdiagnosed because the symptoms it produces are nonspecific, they may scarcely be reported by patients and little suspected by clinicians. Symptoms associated with digestive toxicity include nausea/vomiting (78%), abdominal pain (28%), hyporexia and weight loss or upper gastrointestinal bleeding (hematemesis/melena) in up to 18% of cases. It is important to note that many patients may present symptoms of associated diarrhea/colitis (68%) with endoscopic and histological involvement in the upper and lower digestive tracts (35%).312,313 The time to onset can also be variable, with a median time of 3 months (range 1–8).312 There are also published cases of late onset.92,314
Although there is grading of the severity of gastritis within the CTCAE classification, this classification has not been used in any study, nor are there any recommendations about its use in clinical practice.137
There are no recommendations for establishing the diagnosis of ICI-induced gastritis. Considering that upper gastrointestinal involvement presents nonspecific symptoms, it is important to perform a diagnostic study that includes an esophagogastroduodenoscopy with taking biopsies in all segments. In published series, endoscopic involvement can occur in 68–77% of cases with the presence of erythema, erosions, ulcerations, exudates or mucosal atrophy. The most frequent form of presentation could be gastritis without ulcerations (56.7%). In the study by Tang T et al. the presence of ulcerations was more frequent in patients who received anti-PD-1 when compared with anti-CTLA-4 (21% vs 5%). The predominant involvement was gastric (57%), followed by duodenal (45%) or gastroduodenal (14%).312 There are two case reports published with esophageal involvement.315,316
Histological findings are important for differential diagnosis with other disorders and when there are no endoscopic changes. Histological alterations can appear in 83–100% of cases, even with no endoscopic involvement and duodenal changes can be less prevalent than gastric ones (38%).312 Johncilla M et al. analyzed specifically histological patterns. The most common presentation was the presence of active and diffuse chronic gastritis with lymphoplasmacytic infiltrate in the lamina propria together with the presence of apoptosis and the second one was gastritis with focal enhancement and neutrophilic infiltration of the glandular epithelium.313–316 There are published cases of CMV superinfection which makes it important to perform biopsies in these patients.317,318
Therapeutic management of upper GI toxicity is not well established and there are no recommendations in different clinical guidelines. In the largest series (n=60 patients), 89% received treatment with PPIs and anti-H2; although there are no data regarding clinical response to this therapy, 42% required CS treatment, 22% IFX, and 15% vedolizumab. Either data on response to CS or biological therapy were reported, although a relapse rate of 15% was documented. As an interesting data, the percentage of patients who required biological therapy was higher when they had upper and lower digestive involvement in comparison with those with isolated lower tract involvement (62% vs 6%).312 In the series by Johncilla M et al. 10 of the 12 patients were treated with CS and two with IFX (one of them due to CS failure), in the follow-up (range 1–37 months) 9 of the 10 patients responded to the initial treatment, but there were 3 relapses. However, in most, the immunotherapy was able to be reintroduced.313 Obviously, larger prospective studies are needed to investigate upper GI tract irAEs to include this toxicity in clinical guidelines and provide help to physicians for treatment management.
Likewise, there are few cases of pancreatic injury due to immunotherapy, ranging from asymptomatic hyperamylasemia to symptomatic acute pancreatitis.319,320 PD-1 inhibitors have been associated with a significant higher risk of pancreatic irAEs compared with anti-PD-L1 agents and those undergoing combined ICI therapy have a significant higher risk compared to subjects undergoing single ICI therapy.320
Statement 13
GI toxicity secondary to ICI can involve upper tract, mainly gastric area. Main clinical manifestations include nausea/vomiting and/or abdominal pain. Upper endoscopy with biopsies is required for diagnosis. Therapeutic management is not well established and includes PPIs, CS and biological therapy for refractory cases.
Quality of evidence: low.
Funding statementThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestMRB has received research educational and/or travel grants from Gilead and has served as a speaker for Gilead and GSK; ADG has received travel grants from Intercept/Advanzpharma. Speaker for Intercept/Advanzpharma. Consultant or advisory role for GlaxoSmithKline and IPSEN; MM has served as a speaker, advisory member or has received research funding from Takeda, Abbvie, Janssen, Pfizer, TillottsPharma and Faes; JGP has acted as consultant or advisory role for AAA, Amgen, Bayer, BMS, Eisai, Ipsen, Lilly, Merck, MSD, Pierre-Fabre, Roche, Servier, Veracyte. Research funding: Astellas, AstraZeneca, BMS, Daiichi-Sankyo, Lilly, Servier. Speaker role: AAA, Amgen, Bayer, BMS, Ipsen, Lilly, Merck, Novartis, Servier. Educational activities: Amgen, Ipsen, Merck, Novartis, Pierre-Fabre, Roche; MV: lecture fees, consulting work, travel support and congress registration: Astra-Zeneca, Eisai-MSD, Roche; LM received unrestricted grants from MSD and Abbvie, and served as advisory board member and/or speaker for MSD, Abbvie, Pfizer, Janssen, Takeda, Biogen, Tillotts, Dr. Falk Pharma, Ferring, Medtronic and General Electric; BS reports consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, Eisai, Eli Lilly, Incyte, Ipsen, Novartis, MSD, Roche, Sanofi, Sirtex Medical, Terumo; speaker fees from Astra Zeneca, Bayer, BMS, Eisai, Eli Lilly, Incyte, Ipsen, Novartis, Roche, Sirtex Medical, Terumo; research grants (to Institution) from BMS and Sirtex Medical; AFM has received grants as invited speaker for BMS, MSD and Roche; MARG has received research educational and/or travel grants from GORE and has served as a speaker for GORE; FR: Consultant or Advisory Role: Roche, Merck-Serono, Amgen, MSD, BMS, Lilly, Celgene, Sanofi-Aventis, Servier, Astra-Zeneca, Bayer, Atellas; Research Funding: Roche, Merck-Serono, Amgen, MSD, Lilly, Celgene, Sanofi-Aventis, Servier, Bayer. Speaking: Roche, Merck-Serono, Amgen, MSD, BMS, Lilly, Celgene, Sanofi-Aventis, Servier, Bayer; Grant support: Amgen; MCL has received travel grants from Intercept/Advanz pharma. Speaker for Intercept/Advanz pharma. Consultant or advisory role for Intercept/Advanz pharma, GlaxoSmithKline and IPSEN.
The rest of authors have no conflict of interest to declare.
English writing support was provided by Fidelma Greaves.
Este artículo se publica conjuntamente, en el marco del correspondiente acuerdo de publicación y derechos de autor, en Revista Española de Enfermedades Digestivas.