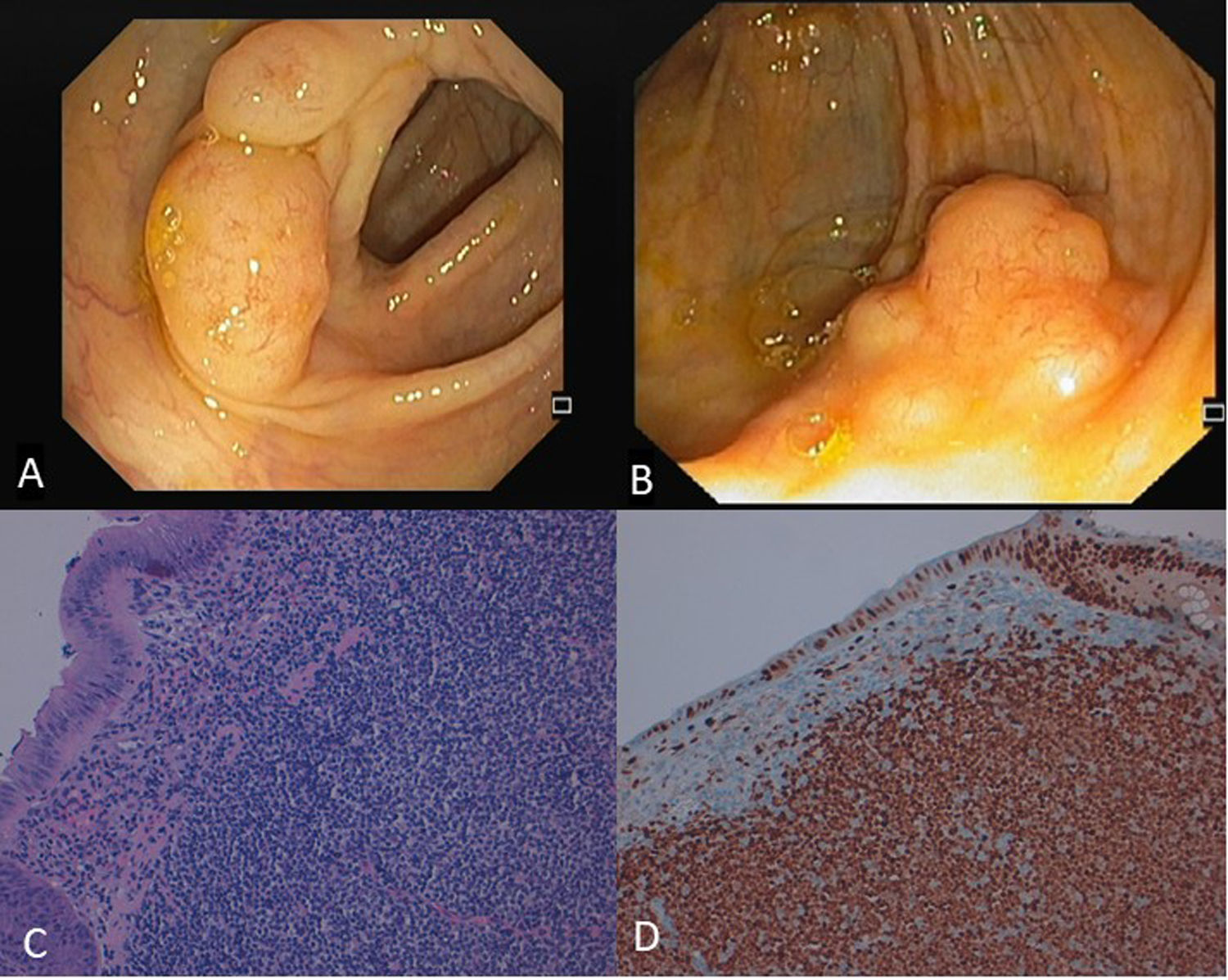
Colon lymphoma is uncommon, representing only 0.2–1.2% of all colonic neoplasms. The most common histological subtype is mantle cell lymphoma, followed by large B-cell diffuse lymphoma, with other types being less common. Clinical manifestations are non-specific and the endoscopic appearance is highly variable, possibly presenting as diffuse infiltration, a single mass or polyps but also with normal mucosa. There is a high rate of morbidity and mortality.1
We report a case of a 67-year-old female with previous medical history of diabetes mellitus and dyslipidemia who performed a screening colonoscopy, where multiple sessile polyps (at least 20) were found along all colon segments, from cecal region to rectum. These polyps were covered by normal mucosa and had diameter between 10–20mm (Fig. 1A). One single polyp removed from ascending colon was histologically hyperplastic. She was asymptomatic and physical examination was normal. Hemogram, albumin, lactate dehydrogenase, urea, creatinine, electrolyte levels and coagulation studies were all normal. Her last colonoscopy performed 8 years earlier was normal. Two sisters had history of colorectal cancer. Because the cause of polyposis was not clear and there was family history of neoplasia, colonoscopy was repeated. At this time, three polyps from transverse colon and a large cecal polypoid lesion (Fig. 1B) were resected. Histopathological examination revealed diffuse nodular infiltration by small-sized atypical lymphoid cells with irregular nuclei and scant cytoplasm involving mucosa and submucosa (Fig. 1C). Immunohistochemical staining was positive for CD20, CD5 and cyclin D1 (Fig. 1D) and negative for CD3 and CD10. Diagnosis of mantle cell lymphoma presenting as multiple lymphomatoid polyposis was established. Computed tomography and positron emission tomography revealed involvement of intra- and extra-abdominal lymph nodes, spleen and Waldeyer's ring, besides gastrointestinal tract, consistent with stage IV of Ann-arbor staging system. She was referred for chemotherapy with rituximab plus bendamustine.
A Multiple small yellowish polyps covered by normal mucosa were found along all colonic segments, from cecal region to rectum. B. A large polypoid lesion with approximately 25mm was identified at the cecum and removed by endoscopic mucosal resection. C. A monomorphic infiltration of mucosa and submucosa by small-sized atypical lymphoid cells may be seen. These cells are characterized by irregular nuclei and scant cytoplasm and demonstrate a nodular infiltrative growth pattern (HE, 200×). D. Immunohistochemistry showing positive stain for cyclin D1.
Mantle cell lymphoma is a rare and aggressive B-cell non-Hodgkin lymphoma, characterized by chromosomal translocation t(11;14) and cyclin D1 overexpression. On immunohistochemistry, tumor cells are characteristically CD5 and pan B-cell antigen positive (CD19, CD20, CD22) and negative for the expression of CD10 and CD23. This type of lymphoma more commonly affects males and usually presents in the fifth or sixth decades of life.2
Gastrointestinal involvement occurs in 5–20% of cases, usually as multiple lymphomatoid polyposis, with multiple polyps involved by lymphoproliferative disease along one or more segments of gastrointestinal tract.3 The most commonly affected segments are colon and rectum, followed by small intestine, stomach and duodenum.4 Less commonly, it may present as a single mass mimicking adenocarcinoma.2 Symptoms of abdominal pain, diarrhea or hematochezia are usually present. Most patients present at advanced stage with extra-intestinal involvement. The main extra digestive sites affected are the bone marrow, peripheral lymph nodes, Waldeyer's ring and liver.5
The current therapeutic approach is based on clinical risk factors, symptoms, patient characteristics and stage of disease. For patients in good condition who are younger than 65 years of age, intensive frontline immunochemotherapy induction regimen combining rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and high dose of cytarabine followed by autologous stem cell transplantation is recommended. For the group of elderly patients or in poor health condition not eligible for autologous stem cell transplantation, conventional immunochemotherapy (e.g. R-CHOP) followed by maintenance with rituximab, appears to be ‘gold standard’.3,4
Unfortunately, despite high response rate to intensive chemotherapy regimens which usually results in regression of macroscopic and sometimes microscopic lesions, remissions are usually short, relapse rate is high and median survival is only 3–4 years.5 Poor prognostic factors include unsatisfactory general clinical condition, involvement of multiple extranodal sites, advanced age (older than 70 years), elevated lactate dehydrogenase levels and bone marrow infiltration.4
In conclusion, although uncommon, lymphoproliferative diseases should be considered in the differential diagnosis of gastrointestinal polyposis, especially at advanced age. This case demonstrates that advanced stage lymphomas may present as multiple lymphomatous polyposis without producing gastrointestinal symptoms and that other types of polyps may occasionally develop in the middle of lymphomatous polyps. Therefore, absence of symptoms and incidental finding of a benign polyp do not preclude this ominous diagnosis.