There is little information on whether direct-acting antiviral (DAA) treatment can improve liver fibrosis or change glucose and lipid profile in patients with chronic hepatitis C (CHC). We aimed to evaluate the impact of sustained virologic response (SVR) on liver stiffness, glucose and lipid levels.
Methods445 monoinfected CHC patients started treatment with interferon-free DAA therapy from January 2015 to February 2017. Transient elastography (TE), fibrosis scores, glucose and lipid levels were analyzed at baseline and 48 weeks post-treatment (SVR48).
ResultsThe SVR rate was 97.7%. Finally, we evaluated 369 patients who achieved SVR and had reliable TE measurements. Median liver stiffness significantly decreased from 9.3 (IQR 7.3–14.3)kPa at baseline to 6.4 (IQR 4.9–8.9) at SVR48 (p<0.0001). 54.7% of the cohort presented fibrosis regression. Median FIB4 score regressed from 2.0 (IQR 1.1–3.3) to 1.3 (IQR 0.9–2.0) (p<0.0001). Median APRI and Forns values significantly decreased from 0.9 (IQR 0.5–1.7) to 0.3 (IQR 0.2–0.4) and from 6.2 (5.0–7.5) to 4.9 (IQR 3.8–5.9) (p<0.001), respectively. Mean levels of total cholesterol and LDL-C increased from 172mg/dL and 101.5mg/dL to 191mg/dL and 117.5mg/dL (p<0.0001), respectively. In the sub-group of patients with pre-diabetes or diabetes, mean glucose levels decreased from 142.7mg/dL at baseline to 127.2mg/dL at SVR48 (p<0.001).
DiscussionSVR reduces liver stiffness based on TE and fibrosis scores, in patients treated with DAA. Our results show elevated total cholesterol and LDL-C and decreased glucose levels at SVR48.
Se desconoce el efecto a largo plazo de los antivirales de acción directa (AAD) sobre la fibrosis hepática y el perfil metabólico en pacientes con hepatitis crónica C (HCC). Nuestro objetivo fue evaluar el impacto de la respuesta viral sostenida (RVS) sobre la rigidez hepática, la glucosa y el perfil lipídico.
MétodosUn total de 445 pacientes con HCC monoinfectados iniciaron tratamiento con AAD libres de IFN entre enero del 2015 y febrero del 2017. La ET, los marcadores serológicos de fibrosis, los niveles de glucosa y lípidos se analizaron basalmente y 48 semanas tras finalizar el tratamiento (RVS48).
ResultadosLa tasa de RVS fue del 97,7%. Finalmente analizamos 369 pacientes que obtuvieron RVS y tenían medidas fiables en la ET. La mediana de la rigidez hepática descendió de forma significativa de 9,3 (IQR 7,3-14,3) basalmente a 6,4 (IQR 4,9-8,9) kPa en RVS48 (p<0,0001). El 54,7% de la cohorte presentó una regresión de la fibrosis. La mediana del FIB4 disminuyó de 2,0 (IQR 1,1-3,3) a 1,3 (IQR 0,9-2,0) (p<0,0001). Las medianas del APRI y del Forns descendieron significativamente de 0,9 (IQR 0,5-1,7) a 0,3 (IQR 0,2-0,4) y de 6,2 (IQR 5,0-7,5) a 4,9 (3,8-5,9) (p<0,001), respectivamente. La media de los niveles de colesterol total (CT) y LDL-C aumentaron de 172mg/dL y 101,5mg/dL a 191mg/dL y 117,5mg/dL (p<0,0001), respectivamente. En el subgrupo de pacientes con prediabetes o diabetes, los niveles de glucosa descendieron de 142,7mg/dL a 127,2mg/dL en RVS48 (p<0,001).
DiscusiónLa RVS reduce la rigidez hepática determinada mediante ET y marcadores serológicos de fibrosis en pacientes tratados con AAD. Nuestros resultados muestran una elevación en el CT y LDL-C y un descenso en los niveles de glucosa en RVS48.
Hepatitis C virus (HCV) infection represents a major public health issue. Approximately 3% of the world population is infected with HCV according to the World Health Organization (WHO).1 Second generation direct-acting antiviral agents (DAA) are available for the treatment of chronic hepatitis C (CHC) infection, targeting viral proteins (NS3/4A protease, NS5B RNA-dependent RNA polymerase and NS5A serine protease), and allowing to achieve a high success rate with simple regimens2 and few related side effects.3
Liver fibrosis caused by chronic HCV infection determines the risk of morbidity and mortality of the disease, since the amount of fibrosis is correlated with the risk of developing cirrhosis and hepatocellular carcinoma (HCC).4 Therefore, the assessment of the severity of liver fibrosis is essential to establish the management and prognosis of patients. Liver biopsy has traditionally been considered the gold standard for assessing hepatic fibrosis. However, it is limited by its invasive nature, sampling error and poor intra and inter-observer agreement.5 Consequently, liver biopsy has been replaced by noninvasive tests to assess hepatic fibrosis. Fibrosis-4 Score (FIB-4), the Forns test6 and aspartate aminotransferase to platelet ratio index (APRI),7 which combine several biochemical parameters, have been validated for CHC and show acceptable sensitivity and specificity, particularly for advanced fibrosis and cirrhosis.8 The advantages of these scores are that they are simple and easily reproducible. In addition, transient elastography (TE) with FibroScan® device (Echosens, Paris, France) is a non-invasive method that is used for the assessment of hepatic fibrosis in patients with chronic liver diseases.9 Combining TE with serum markers increases the accuracy for staging fibrosis and as a result, liver biopsy could be avoided in most patients with CHC.
Most data on cirrhosis reversibility in the setting of CHC have been generated during studies that analyzed the effect of interferon-based regimens.10 However, there is insufficient clinical investigation studying liver stiffness up to 48 weeks after the completion of DAA in patients with CHC who achieve sustained virological response (SVR). As such, further research is needed, and noninvasive tests and scores could be used.
CHC is associated with insulin resistance (IR), liver steatosis, metabolic disorders and cardiovascular disease.11 Host serum lipids play a role in hepatitis C virion circulation and hepatocyte entry. Moreover, HCV infection is associated with reduced total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C)12 as well as with an increased rate of IR and type 2 diabetes mellitus.13 However, there are scarce data regarding the effect of DAA on glucose and lipid metabolism.
We aimed to assess the impact of SVR on liver fibrosis measured by TE and fibrosis scores within 12 months after the end of DAA treatment in all HCV genotypes and also to evaluate changes in glucose and lipid levels after HCV clearance.
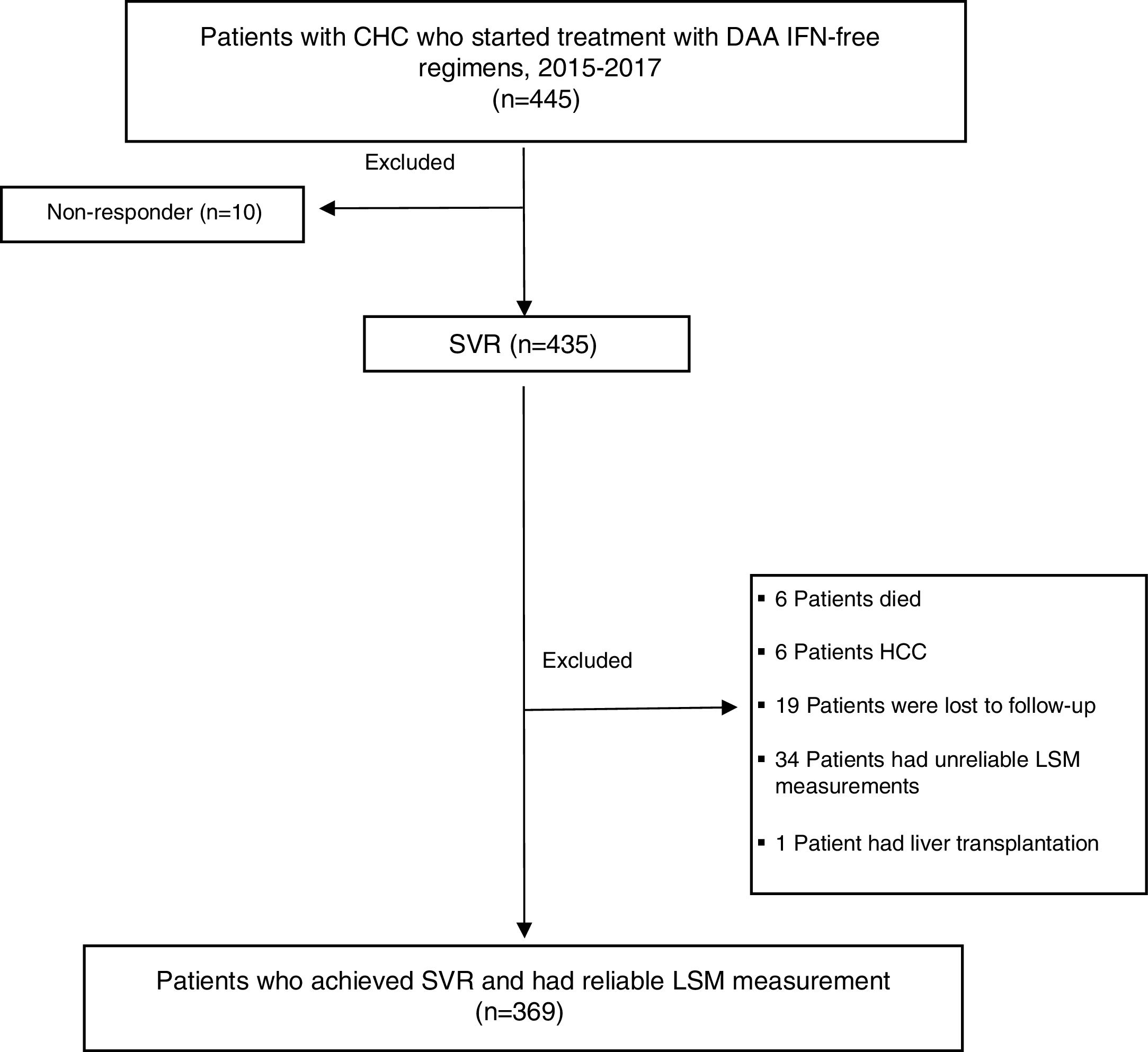
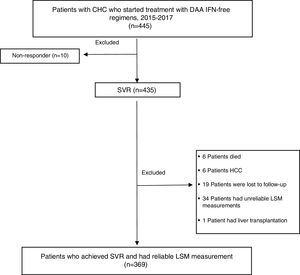
MethodsDesign and study populationThis was a descriptive, observational study. Data were collected prospectively and analyzed retrospectively. 445 CHC monoinfected patients (age ≥18 years) started treatment with DAA IFN-free regimens from January 2015 to February 2017 at the Hospital Complex of Navarra, Spain. Patients co-infected with hepatitis B virus (HBV), human immunodeficiency virus (HIV), diagnosed of HCC or liver transplanted during follow-up were excluded. Only those patients who achieved SVR, with liver stiffness measurement (LSM) and biochemical assessment at the start of treatment and 48 weeks after treatment completion were enrolled. Only 10 patients did not respond to treatment, so SVR was obtained in 97.7% (435/445) of patients. Most excluded patients lacked or had unreliable TE measurements prior to (n=30) or after therapy (n=4). Six patients died of unrelated causes after completing DAA treatment. Six patients had HCC, 19 patients were lost to follow-up and 1 patient received liver transplantation during the one-year period of follow-up (FU). 369 patients fulfilled the inclusion criteria and were included in the statistical evaluation.
The study was approved by the Clinical Research Ethics Committee of Navarra.
Treatment regimenAll patients were treated according to treatment guidelines of the EASL panel14 and approval of the Spanish health authorities. Doses for each DAA were based on the manufacturer's recommendations. The following regimens were used: sofosbuvir (SOF) and simeprevir (SMV)±ribavirin (RBV), SOF and RBV, SOF and daclatasvir (DCV)±RBV, SOF/ledipasvir (SOF/LDV)±RBV, ombitasvir/paritaprevir/ritonavir (OBV/PTV/r)±dasabuvir (DSV)±RBV, elbasvir/grazoprevir (EBR/GZR)±RBV. The SOF+DCV combination was used exclusively for HCV genotype 3 and SOF+RBV for HCV genotype 2. Serum HCV RNA levels were measured before the start of therapy, at the end of treatment (EOT) and 12, 24 and 48 weeks after EOT. SVR was defined as undetectable HCV-RNA 12 weeks after the EOT.
Liver stiffness measurementMeasurement of liver stiffness was performed by TE using Fibroscan® device (Echosens, Paris, France).10 Two officially trained operators were responsible for carrying out the LSM. Ten validated measurements were performed for each patient. Only procedures with a success rate of at least 60% and an inter-quartile range (IQR) of less than 30% of the median value, were considered reliable.10,15 Subjects fasted for 3h prior to each TE to avoid confounding of LSM by stomach distension and postprandial hepatic blood flow.16 M probe was used in most patients, but XL probe was used in obese patients. Subsequent TE time points were before starting treatment and at 48 weeks after EOT (SVR48).
Patients were classified in different categories according to LSM values; <7.1kPa as fibrosis stage F0–F1, 7.2–9.4kPa fibrosis stage F2, 9.5–12.4kPa fibrosis stage F3 and ≥12.5kPa fibrosis stage F4 by Metavir, respectively.17
Serum fibrosis scores and laboratory dataIn the present study we used the APRI7,18 and FIB-419 indexes, as well as the Forns6 test to assess the degree of liver fibrosis. The serum fibrosis scores were calculated from laboratory values at baseline and at SVR48. Cut-off values for significant liver fibrosis and cirrhosis were adopted from the EASL guidelines for non-invasive assessment of liver fibrosis.8 Biochemical tests including aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose level, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were performed at baseline and SVR48. Pre-diabetes and diabetes were defined as fasting plasma glucose 110–125mg/dL or >126mg/dL, respectively.20,21
Statistical analysisCategorical variables were presented as frequencies and percentages. Means with standard deviation or median with IQR for continuous variables. Groups were compared using the t test or the Mann–Whitney test for continuous variables when appropriate, and the Fisher's exact test was used for categorical variables. Multiple logistic regression was performed to evaluate variables associated with fibrosis regression, including variables with a p value ≤0.2 at univariate analysis. Odds ratios (OR) and their 95% confidence intervals (CI) were inferred from the model. A p-value <0.05 and 95% CI were considered as significant for all statistical tests. All statistical analysis were carried out using Statistical Package for the Social Sciences software program (SPSS, V21, IBM, Chicago, Illinois, USA).
ResultsA flow chart of the study is shown in Fig. 1.
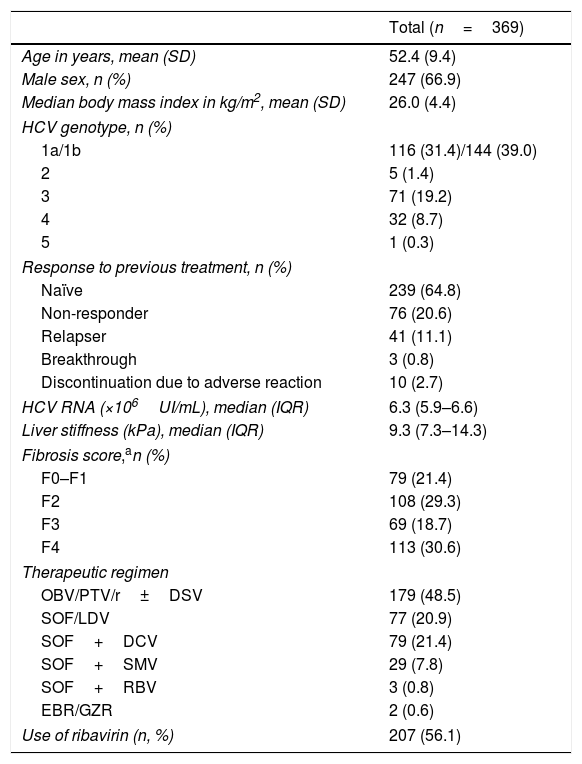
Baseline characteristics445 Patients started a DAA IFN-free regimen during the study period, and 369 fulfilled inclusion criteria and were finally analyzed. The patients’ characteristics included in the study are summarized in Table 1. Most patients were males, their mean age was 52.4 years. Genotype 1a and 1b were the most prevalent. 239 patients (64.8%) were naïve and 130 (35.2%) were treatment-experienced.
Baseline characteristics of the study cohort.
| Total (n=369) | |
|---|---|
| Age in years, mean (SD) | 52.4 (9.4) |
| Male sex, n (%) | 247 (66.9) |
| Median body mass index in kg/m2, mean (SD) | 26.0 (4.4) |
| HCV genotype, n (%) | |
| 1a/1b | 116 (31.4)/144 (39.0) |
| 2 | 5 (1.4) |
| 3 | 71 (19.2) |
| 4 | 32 (8.7) |
| 5 | 1 (0.3) |
| Response to previous treatment, n (%) | |
| Naïve | 239 (64.8) |
| Non-responder | 76 (20.6) |
| Relapser | 41 (11.1) |
| Breakthrough | 3 (0.8) |
| Discontinuation due to adverse reaction | 10 (2.7) |
| HCV RNA (×106UI/mL), median (IQR) | 6.3 (5.9–6.6) |
| Liver stiffness (kPa), median (IQR) | 9.3 (7.3–14.3) |
| Fibrosis score,an (%) | |
| F0–F1 | 79 (21.4) |
| F2 | 108 (29.3) |
| F3 | 69 (18.7) |
| F4 | 113 (30.6) |
| Therapeutic regimen | |
| OBV/PTV/r±DSV | 179 (48.5) |
| SOF/LDV | 77 (20.9) |
| SOF+DCV | 79 (21.4) |
| SOF+SMV | 29 (7.8) |
| SOF+RBV | 3 (0.8) |
| EBR/GZR | 2 (0.6) |
| Use of ribavirin (n, %) | 207 (56.1) |
Abbreviations: OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; DSV, dasabuvir; SOF/LDV, sofosbuvir/ledipasvir; SOF, sofosbuvir; DCV, daclatasvir; SMV, simeprevir; RBV, ribavirin; EBR/GZR, elbasvir/grazoprevir.
Of the initially 445 patients treated, SVR was achieved among a total of 435 (97.7%). All patients who had an undetectable HCV RNA 12 weeks after the EOT, persisted negative at 24 and 48 weeks of FU.
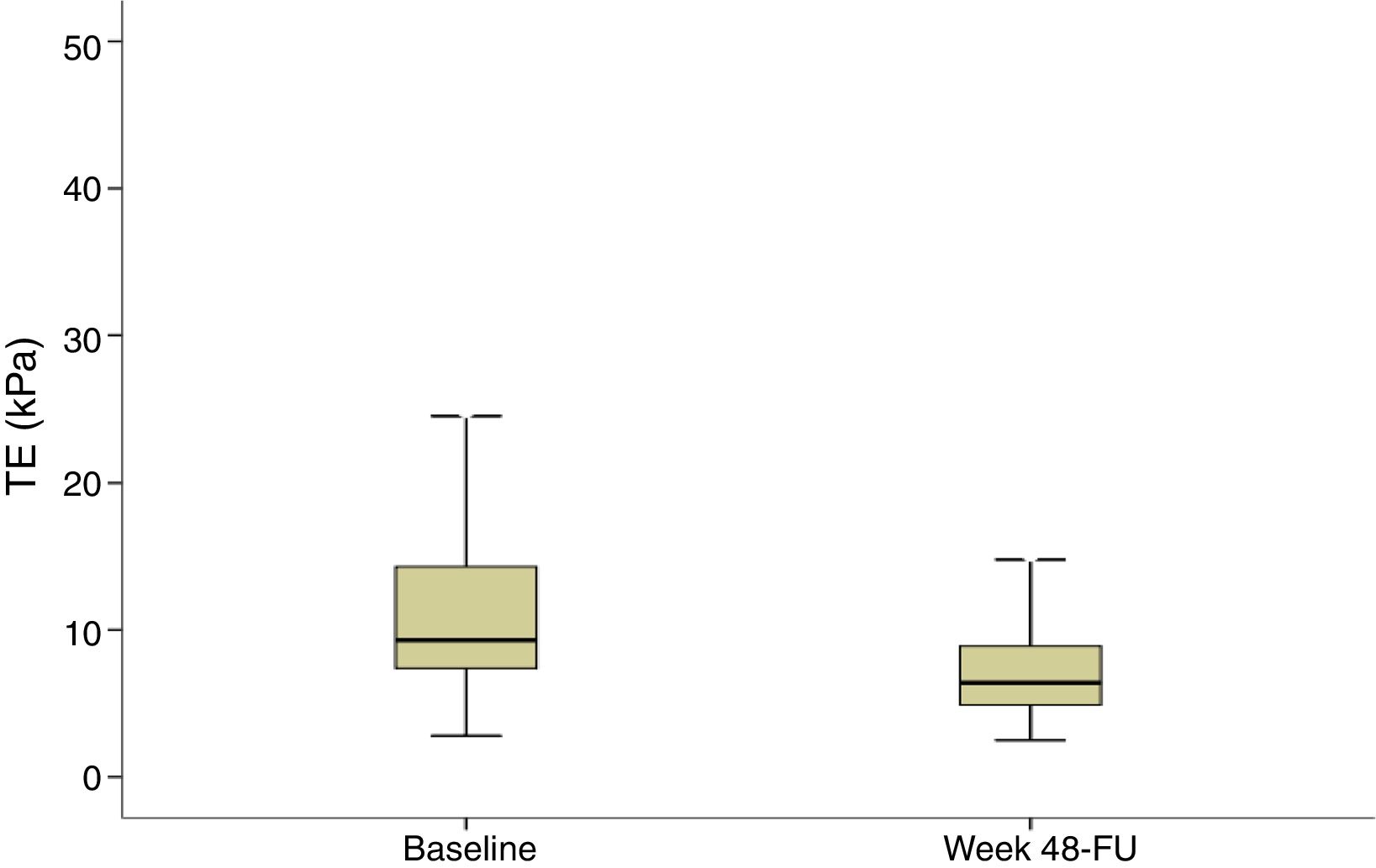
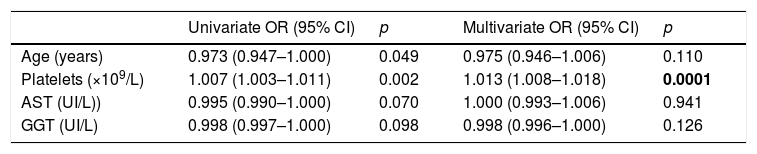
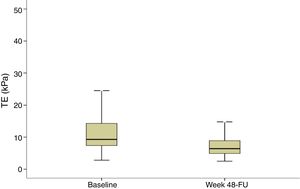
Evolution of liver stiffness and biochemical fibrosis scoresMedian stiffness decreased significantly from 9.3kPa at baseline (IQR 7.3–14.3) to 6.4kPa (IQR 4.9–8.9) (p<0.0001) at SVR48 (Fig. 2). In patients with F4 (cirrhosis) (n=113), LSM decreased from 20.3 (14.8–35.3) to 13.3 (8.35–21.1)kPa (p<0.0001). When LSM was assessed as a categorical variable, 202 (54.7%) patients achieved a regression of at least 1 stage of LS, 165 (44.7%) did not experienced changes and 2 (0.5%) showed progression of fibrosis. These two patients presented obesity and dyslipemia as additional risk factors and neither of them reached the stage of cirrhosis. 49% of cirrhotic patients presented an improvement of at least one fibrosis stage. In order to find if there were any factors influencing a significant improvement in LS, univariate and multiple logistic regression were performed. The baseline variables associated with fibrosis regression at univariate analysis were age, basal platelet count, AST and GGT. However, on multivariate analysis, only basal platelet count emerged as independent predictor of fibrosis regression (Table 2). Fibrosis scores based on laboratory parameters, significantly decreased from baseline to SVR48. The FIB-4 score regressed from 2.0 (IQR 1.1–3.3) to 1.3 (IQR 0.9–2.0). Median APRI and Forns values significantly decreased from 0.9 (IQR 0.5–1.7) and 6.2 (IQR 5.0–7.5) to 0.3 (IQR 0.2–0.4) and 4.9 (3.8–5.9) (p<0.001), respectively.
Transient elastography (TE) at baseline and SVR48. TE values are shown for all patients included (n=369). Liver stiffness measurement at baseline and SVR48 were 9.3kPa (IQR 7.3–14.3) and 6.4kPa (IQR 4.9–8.9) respectively. There was a significant difference between baseline and SVR48 (p<0.001). The box represents the inter-quartile range. The line through the box indicates the median.
Predictors of fibrosis regression.
| Univariate OR (95% CI) | p | Multivariate OR (95% CI) | p | |
|---|---|---|---|---|
| Age (years) | 0.973 (0.947–1.000) | 0.049 | 0.975 (0.946–1.006) | 0.110 |
| Platelets (×109/L) | 1.007 (1.003–1.011) | 0.002 | 1.013 (1.008–1.018) | 0.0001 |
| AST (UI/L)) | 0.995 (0.990–1.000) | 0.070 | 1.000 (0.993–1.006) | 0.941 |
| GGT (UI/L) | 0.998 (0.997–1.000) | 0.098 | 0.998 (0.996–1.000) | 0.126 |
We reported only factors found to be significant at Univariate analysis (p values <0.2). OR: odds ratio. CI: confidence interval.
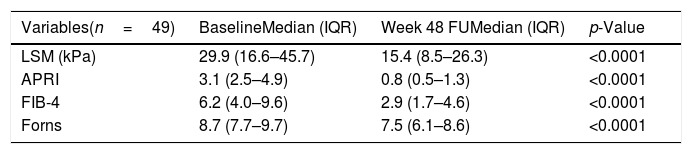
When cirrhotic patients were selected according to 2 diagnostic criteria (TE≥12.5kPa and APRI>2) (n=49), a significant decrease of LSM and fibrosis scores was also observed (Table 3). The group of cirrhotic patients that improve at least one fibrosis stage, had significantly lower basal TE and fibrosis scores levels and higher basal platelet count, regardless of transaminases level. Besides, significant reductions in all non-invasive fibrosis tests were found not only in the patients who improved at least one fibrosis stage, but also in those who did not reach one fibrosis stage improvement (data not shown).
Comparison of transient elastography and fibrosis scores in cirrhotica patients at baseline and 48 weeks after the end of treatment (SVR48).
| Variables(n=49) | BaselineMedian (IQR) | Week 48 FUMedian (IQR) | p-Value |
|---|---|---|---|
| LSM (kPa) | 29.9 (16.6–45.7) | 15.4 (8.5–26.3) | <0.0001 |
| APRI | 3.1 (2.5–4.9) | 0.8 (0.5–1.3) | <0.0001 |
| FIB-4 | 6.2 (4.0–9.6) | 2.9 (1.7–4.6) | <0.0001 |
| Forns | 8.7 (7.7–9.7) | 7.5 (6.1–8.6) | <0.0001 |
LSM: liver stiffness measurement.
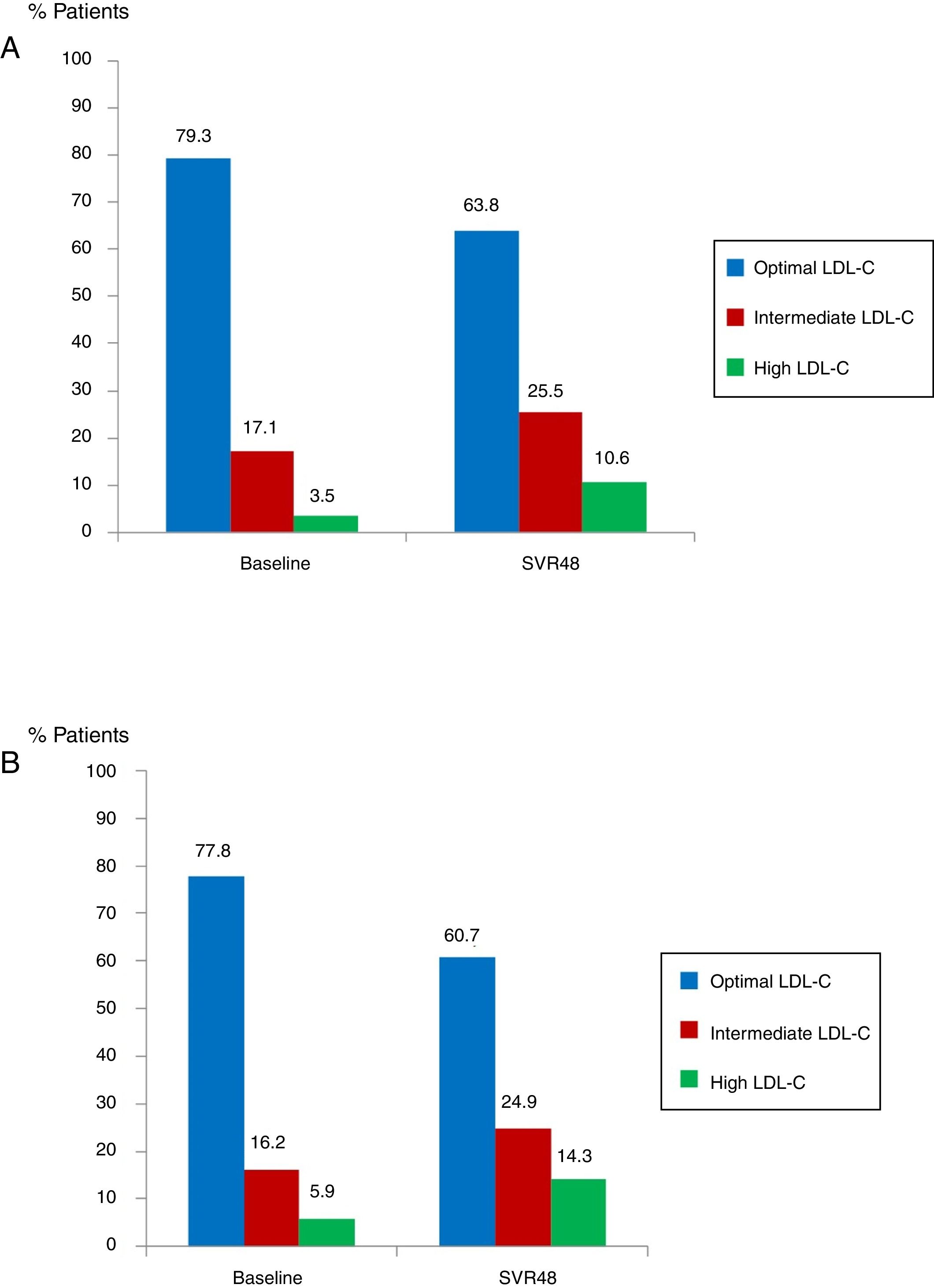
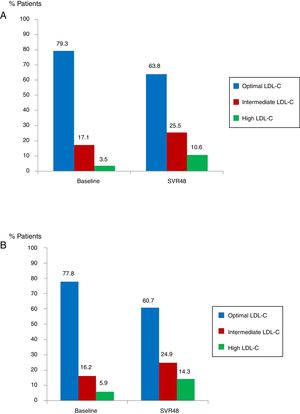
Median glucose serum levels decreased from 95 (88–105) to 94 (86–103) mg/dL (p=0.008). The number of patients with pre-diabetes or diabetes previous treatment was 62 (16.8%). In this group of patients, mean glucose levels decreased from 142.7mg/dL at baseline to 127.2mg/dL at SVR48 (p<0.001). Median levels of TC increased from 172 (151–194) to 191 (164–234)mg/dL (p<0.0001). The number of patients with optimal TC previous treatment was 292 (79.3%) versus 235 (63.8%) at SVR48; intermediate TC was in 63 patients (17.1%) versus 94 (25.5%) SVR48, and high TC was in 13 patients (3.5%) at baseline versus 39 (10.6%) one year after EOT (all p<0.0001) (Fig. 3A). Median LDL-C values significantly increased from 101.5 (81–123) at baseline to 117.5 (96.7–138)mg/dL at SVR48 (p<0.0001). The number of patients with optimal LDL-C was 287 (77.8%) previous treatment versus 224 (60.7%) SVR48, intermediate LDL-C was in 60 patients (16.2%) versus 92 (24.9%) SVR48, and high LDL-C was in 22 patients (5.9%) versus 53 (14.3%) SVR48 (all p<0.0001) (Fig. 3B). However, there were no statistically significant changes in HDL-C.
Proportion of patients with total cholesterol (TC) at baseline and SVR48 (A). Optimal TC (<200mg/dL), intermediate TC (200–239mg/dL) and high TC (≥140mg/dL). The percentage of patients with intermediate and high TC increased one year after treatment with DAA. Proportion of patients with low density lipoprotein cholesterol (LDL-C) at baseline and SVR48 (B). Optimal LDL-C (<130mg/dL), Intermediate LDL-C (130–159mg/dL) and high LDL-C (>160mg/dL). The percentage of patients with intermediate and high LDL-C increased one year after treatment with DAA.
Since data concerning lipid-lowering agents was not collected and taking into account that patients who received the OBV/PTV/r±DSV combination therapy and were taking statins these were interrupted, we evaluated the evolution of the lipid profile in all the patients. The same lipid results were confirmed either in the group of patients taking OBV/PTV/r±DSV or regimens containing SOF (data not shown).
DiscussionLittle is known about the long-term effects of SVR achieved by DAA therapy on liver stiffness in HCV mono-infected patients.22 We proposed a real-life study to evaluate the impact of viral clearance on liver stiffness assessed by TE and biochemical fibrosis scores, over a follow-up period through one-year post-treatment. Because LS is disease specific, only chronic HCV patients were included, while those with other liver diseases such as chronic HBV, HIV or HCC were excluded. We observed a significantly decreased of fibrosis from baseline to one year of FU in a large cohort of patients with CHC who achieved viral eradication with DAA IFN-free regimens. Fibrosis regression was also observed in all stages of fibrosis, including cirrhosis. According to our data, more than half of the patients achieved fibrosis regression and only two patients (0.5%) showed fibrosis progression. In addition, these two patients had other risk factors that probably contributed to the liver damage. A significant reduction in TE values in DAA-treated HCV patients was also reported by Bachofner et al.22 Compared to this study, we offer a longer-term follow-up with TE and biochemical markers measured 12-months after EOT.
Like TE values, validated fibrosis scores FIB-4, APRI and Forns declined significantly within one year of SVR. When all the variables were introduced in a multivariate model, only a higher basal platelet count was associated with fibrosis regression. Therefore, cirrhotic patients that improved at least one fibrosis stage, had significantly lower basal TE and fibrosis scores levels and higher basal platelet count, regardless of transaminases level. So, the improvement shown in the TE may be largely due to a reduction in fibrosis rather to an improvement of necroinflammatory activity, unlike what was previously described in other studies.23 In any case, this observation requires further investigation in a longer period of time, given that fibrosis regression may continue over time.24
On the other hand, a significant decrease in non-invasive fibrosis scores was found in all the patients, including those patients who did not reach a one-stage fibrosis improvement. This finding suggests that fibrosis improvement is universal after SVR with DAA. It is likely that fibrosis improves even more with longer periods of follow-up.
Host lipid metabolism modulates HCV infection and vice versa, indicating that TC can facilitate HCV genome replication.25 Cholesterol increases have recently been reported in CHC patients treated with DAA.26 This negative impact on lipid metabolism is either absent or underestimated in cases of IFN-based therapy, probably due to the frequent presence of anorexia. Few studies have described the impact of DAA on lipid metabolism, and available data are incomplete and contradictory. We demonstrated that patients mono-infected with HCV achieving SVR, showed a relevant increase in total cholesterol, which is maintained after one year of treatment completion. Those increments are mainly related to rise in serum LDL-C. It has been suggested that the rapid suppression of HCV core protein by DAA might result in a decrease of lipid droplet production in HCV-infected liver cells, leading to a massive rebound of circulating LDL.27 Further prospective studies are mandatory to evaluate the mechanisms of these changes and their impact on the cardiovascular risk.
An association of chronic HCV infection with IR and type 2 diabetes mellitus has been also stablished.13 IR was reported to accelerate fibrosis in chronic HCV-infected patients. The high rate of SVR in our HCV mono-infected cohort, may mean that IR is no longer having a predictor role for SVR, as that was reported for de IFN-based regimens. This concept was also supported by other studies.28 In our study, we observed that mean glucose levels in patients with impaired basal glycaemia were significantly reduced one year after EOT.
The main limitation of our study was the lack of histological evidence that asses if LSM improvement could predominantly be related to the regression of fibrosis or resolution of necroinflammatory activity. It has been described that the improvement in fibrosis can be overstated by TE.29 However, given the high specificity of non-invasive tests, future studies with histological correlation will be very difficult to perform. Another limitation of this study was that we were not able to collect detailed data concerning either lipid-lowering agents, anti-diabetic treatment, or behavioral factors that have been associated with the natural history of CHC, including alcohol consumption. However, when we compared lipid changes in patients treated with OBV/PTV/r, in which statins are interrupted during DAA therapy, and those treated with regimens containing SOF, no significant differences were observed. The improvement of the quality of life and food intake after viral eradication could be associated with an increase in body weight, and therefore of TC or LDL-C levels. In our study, body weight or BMI were not collected at SVR48, however, a study by Morales et al.30 demonstrated the same increase in TC and LDL-C without a significant change of BMI pre and post-treatment.
In conclusion, we have shown in a cohort of patients managed in real-world setting, that liver stiffness evaluated using TE and fibrosis scores significantly decreased between baseline and 12-months post-treatment in chronic HCV patients who received DAA therapy and achieved SVR. Viral eradication in patients treated with DAA show an elevation in TC and LDL-C and a decrease of glucose levels.
FundingThis work is part of the EIPT-VHC project, which was supported by the Spanish Ministry of Health and by the Carlos III Health Institute in the context of the “Strategic Plan for Tackling Hepatitis C in the Spanish National Health System”. Regina Juanbeltz and Jesús Castilla received funding from the Carlos III Institute of Health with the European Regional Development Fund (CM17/00095, INT17/00066). These institutions did not have any role in the research and writing of this manuscript.
Conflict of interestThe authors declare no conflict of interest.
The authors thank the staff of the Hepatology and Pharmacy Departments for their cooperation during data collection. All authors have read and approved the final manuscript.