The dose of thiopurine drugs in combined treatments with anti-TNF in inflammatory bowel disease (IBD) has not been clearly established. The purpose of this study is to assess whether the dose of azathioprine influences clinical and biochemical response/remission rates, and anti-TNF drug levels/antibody formation.
Material and methodsPatients with IBD on combined maintenance treatment with azathioprine and infliximab or adalimumab were selected. Based on the dose of azathioprine, two groups were defined (standard: 2–2.5mg/kg/day; and decreased: less than 2mg/kg/day).
ResultsIn the IFX group, there were no statistically significant differences (p=0.204) in the rates of remission (39% vs 41.3%), response (10% vs 21.7%) or failure (51.5% vs 37%) depending on the dose of thiopurine drugs. No differences were found between AZA-dose dependent IFX levels (2.46 vs 3.21μg/mL; p=0.211). In the adalimumab group, there were no statistically significant differences (p=0.83) in the rates of remission (66% vs 56%), response without remission (15.38% vs 25%) or failure (18% vs 18%) depending on the dose of thiopurines. With respect to ADA-levels, no differences were found in both groups (7.69 vs 8.23μg/mL; p=0.37).
ConclusionIn our experience, no statistically significant differences were found in either anti-TNF levels or clinical-biological response/remission rates based on doses of azathioprine.
La dosis adecuada de los fármacos tiopurínicos en tratamientos combinados con anti-TNF en la enfermedad inflamatoria intestinal (EII) no ha sido establecida con claridad. El propósito de este estudio es evaluar si la dosis de azatioprina influye en las tasas de respuesta/remisión clínica y bioquímica y en los niveles de fármaco anti-TNF/formación de anticuerpos.
Material y métodosSe seleccionaron pacientes con EII en tratamiento combinado de mantenimiento con azatioprina (AZA) e infliximab (IFX) o adalimumab (ADA). En función de la dosis de AZA, se definieron dos grupos (estándar: 2-2,5 mg/kg/día o disminuida: menos de 2 mg/kg/día).
ResultadosEn el grupo IFX no hubo diferencias estadísticamente significativas (p = 0,204) en las tasas de remisión (39 vs. 41,3%), respuesta (10 vs. 21,7%) o fracaso (51,5 vs. 37%), dependiendo de la dosis de fármacos tiopurínicos. No se encontraron diferencias entre los niveles de IFX dependientes de la dosis de AZA (2,46 vs. 3,21 μg/mL; p = 0,211). En el grupo de ADA no hubo diferencias estadísticamente significativas (p = 0,83) en las tasas de remisión (66 vs. 56%), respuesta sin remisión (15,38 vs. 25%) o fallo (18 vs. 18%), dependiendo de la dosis de tiopurinas. Con respecto a los niveles de ADA, no se encontraron diferencias en ambos grupos (7,69 vs. 8,23 μg/mL; p = 0,37).
ConclusiónEn nuestra experiencia, no se encontraron diferencias estadísticamente significativas ni en los niveles de anti-TNF ni en las tasas de respuesta/remisión clínico-biológica basadas en las dosis de azatioprina.
The introduction of biological drug treatments has led to a breakthrough in the management of patients with inflammatory bowel disease (IBD). The traditional management of IBD during outbreaks and periods of remission was based on drugs such as corticosteroids, aminosalicylates, antibiotics, methotrexate and immunosuppressants such as thiopurines (azathioprine (AZA) and its 6-mercaptopurine metabolite). The approval, in the late 1990s, of infliximab (IFX), a monoclonal antibody directed against TNF (tumour necrosis factor), for the treatment of IBD ushered in a new era, as it demonstrated efficacy both in the induction and maintenance of disease remission. This group of biological drugs includes Anti-TNFs, which are the first line of treatment in IBD, the most widely used being IFX and Adalimumab (ADA), both in Crohn's Disease (CD) and Ulcerative Colitis (UC).1
However, despite their efficacy, a factor that hampers the prospects of these Anti-TNF drugs in the treatment of IBD is the loss of response (primary and secondary), with annual rates ranging from 10 to 50%.2 This lack or loss of response is attributed to various factors, with immunogenicity, involving the development of antibodies blocking the action of the drug, being one of the most relevant.
In recent years, it has been shown that Anti-TNF drug levels correlate with clinical and endoscopic response and remission, so that if they are adequate, a higher remission rate is achieved, both in CD and in UC4,5 with both IFX and ADA.
One of the scenarios to consider when using Anti-TNF drugs is their use in monotherapy or combined therapy with azathioprine. Different studies show that adding a thiopurine immunosuppressive drug to the biological drug means less formation of antibodies against biological drugs (ATT), adequate levels of the biological drug in a greater proportion of patients3 and that combined therapy is more effective in terms of remission without corticosteroid dependence and mucosal healing than monotherapy with IFX or AZA.
The improved response rates with combined therapy appear not to be due exclusively to the combined effect of the two drugs (antiTNF/thiopurine) separately, but to the decrease in immunogenicity of antiTNF with the thiopurine drug. It should be noted that the SONIC6 study (Study of Biologic and Immunomodulator Naive Patients in CD), in which 56.8% of the patients in combination therapy were in clinical remission and free of steroids, compared with 44.4% receiving infliximab in monotherapy (p=0.02) and 30% receiving azathioprine in monotherapy (p<0.001 for comparison with combination therapy). Antiinfliximab antibodies (ATI) were also detected in 14.6% of patients treated in monotherapy with this biological drug, but they were only detected in 0.09% of patients treated with the combination of IFX and azathioprine (AZA), which probably contributed to their greater clinical efficacy.
It can therefore be deduced from the results of the different studies that in both induction of remission and maintenance of response, the addition of thiopurines results in superior responses but with a likely increase in the rate of adverse events in patients.
However, the dose of the thiopurine drug AZA used in combination treatments with antiTNF has not been clearly established and the recommended dose for the treatment of IBD with AZA in monotherapy (2–2.5mg/kg/day) has been adopted.
However, in clinical practice some authors consider that this dose could be lower. Regarding the doses to be used to avoid immunogenicity,6 two recent studies show that lower doses of thiopurines are effective in maintaining remission in the medium term, with higher antiTNF concentrations and a lower rate of antibody formation than patients not treated with thiopurines.7,8
In this regard, we reviewed the 2017 prospective study by Roblin et al.,7 in which patients were randomised in 3 groups: stable azathioprine (2.5mg/kg), decrease of azathioprine (half dose 1.25mg/kg) and suspension of azathioprine. The conclusion of this study is that under combination therapy, a reduction (but not suspension) of the dose of azathioprine appears to be as effective as continuing AZA in full dose, both clinically and pharmacokinetically.
With respect to adalimumab, the Matsumoto et al.8 study, which uses low doses of AZA (25–100mg) in combination therapy, demonstrates trends towards higher ADA levels and a lower rate of antibody formation in the combined treatment group compared to those in the monotherapy group.
The adverse effects of thiopurines can be divided into idiosyncratic (dose-independent) effects such as pancreatitis, fever, arthralgia, myalgia, rash and dose-dependent adverse effects such as myelotoxicity and some cases of digestive intolerance and hepatotoxicity.
IBD patients treated with thiopurines have an increased risk of skin tumours other than melanoma, lymphoproliferative syndrome, urinary tract tumours, and probably cervical intraepithelial neoplasm,9 as well as an increased risk of infections,10 so a lower dose may be beneficial in reducing these complications.
A lower dose of immunosuppressant (AZA) can provide better drug tolerability, an improved safety profile, particularly in terms of dose-dependent effects, and potential better adherence to treatment as the cost is lower and fewer tablets are required; this also has an impact on the patient's quality of life, economic and administrative aspects and possible adverse effects.
Particular beneficiaries of a lower dose of AZA may be high-risk populations, such as the immunosuppressed, young people with Epstein-Bar negative virus, pregnant women and those aged over 65, due to the risk of haem phagocytic activation syndrome, lymphoproliferative syndromes and urinary tract tumours.
Given that the AZA doses needed to curb the phenomenon of antibody formation against the biological drug are not clearly established and achieving comparable effects with a lower dose would be a potential advantage.
This study aims to evaluate whether patients treated with lower doses of AZA in combined treatment with Anti-TNF have biological drug titres, biological antipharmaceutical antibody formation phenomena and clinical response comparable to those of patients who received the standard dose of 2–2.5mg/kg/day.
Material and methodsStudy design and objectivesA retrospective, observational study was conducted, selecting all patients with established diagnosis of IBD under the supervision of the IBD Unit at the Hospital La Paz University Hospital, from April 2006 to December 2018, under combined maintenance treatment (minimum 6 months), with AZA and IFX or ADA.
For analysis purposes, two groups of patients were established depending on the dose of AZA they were receiving. “Standard dose” was defined as AZA 2–2.5mg/kg and AZA in doses lower than 2mg/kg was defined as the “decreased dose”.
The reason why, these patients were being treated with AZA at a lower dose than usual was both due to medical criteria and the appearance of adverse effects that required a dose reduction.
The baseline characteristics of the patients were collected, together with data related to their IBD such as age at diagnosis, sex, type of disease (Crohn's disease/ulcerative colitis), disease pattern, smoking, location, time of disease progression, time up to the start of combined treatment, time of combined treatment and prior treatment with another biological drug.
During the study period, the results of the most recent analyses were collected, including the biological drug levels and antibody titres; and also serological inflammation markers (CRP) and faecal inflammation markers (faecal calprotectin), both secondary variables of the study.
The clinical remission of the patients and possible clinical activity during the follow-up were evaluated according to habitual clinical practice, using validated indices, the Harvey–Bradshow score (HBI) for Crohn's disease and the Walmsley's total score for ulcerative colitis.
The primary objective is to evaluate both the clinical efficacy of the combined treatment (antiTNF+AZA) with different doses of thiopurine, as well as the pharmacokinetic response, through the measurement of biological drug levels (mean of the last two determinations) and the formation of antiTNF antibodies (ATT). Loss of response or clinical failure is defined as the occurrence of significant clinical deterioration(HBI>10 with an increase of HBI>5 of the previous evaluation for Crohn's disease and Walmsley's total score>6 points for ulcerative colitis, and/or need to change the original therapeutic regimen due to adverse events, or clinical commitment, which requires either intensification of the biological medication (on schedule or in doses) or change of biological treatment.
The percentage of patients with an unfavourable pharmacokinetic progression during follow-up was also evaluated – this was defined in IFX as serum levels lower than 1μg/mL or undetectable levels and/or anti-drug antibodies, and in the case of ADA serum levels lower than 2μg/mL or undetectable levels and/or anti-drug antibodies.
As inclusion criteria, only those patients under stable combined treatment (>6 months) will be selected retrospectively. They will have to follow antiTNF treatment at standard doses: every 8 weeks in the case of IFX (5mg/kg intravenous) and 40mg subcutaneous every 2 weeks in the case of ADA. Patients with intensified anti-TNF treatment (IFX and/or ADA), either in dose or on schedule, were excluded.
Measurement of biological drug levels and anti-TNF antibodiesFor the measurement of IFX, ADA and ATT concentrations, the PREMONITOR immunoabsorbent assay kit (ELISA) Lisa-Tracker11 is used, and trough levels of the drug are determined. IFX and ADA were considered undetectable for a concentration of <40ng/mL. The ATT detection level reported by the manufacturer was >10ng/mL. Due to interference with circulating IFX and ADA, antibody concentration was measured only in samples with undetectable biological drug levels (<40ng/mL). This is due to the formation of immunocomplexes, which interfere with the detection of antibodies by a competitive ELISA test.
Statistical analysis and ethical considerationsThe continuous variables measured in the study are expressed as means±standard deviation and the categorical data are presented as absolute numbers and percentages with respect to the absolute frequency. Differences in continuous quantitative variables were evaluated using the Student's t-test. The chi-square test and the exact Fisher test were used to analyse the differences of the categorical variables. A bilateral value of p<0.05 was considered statistically significant. The number of patients included of this study is suitable to find statistically significant differences. All statistical analyses were performed using Stata for Mac.
The study was approved by the Medical Research Ethics Committee of the La Paz University Hospital (Madrid, Spain), which confirms that the data collection procedure maintains the confidentiality of personal data and meets the ethical and legal requirements for this type of study.
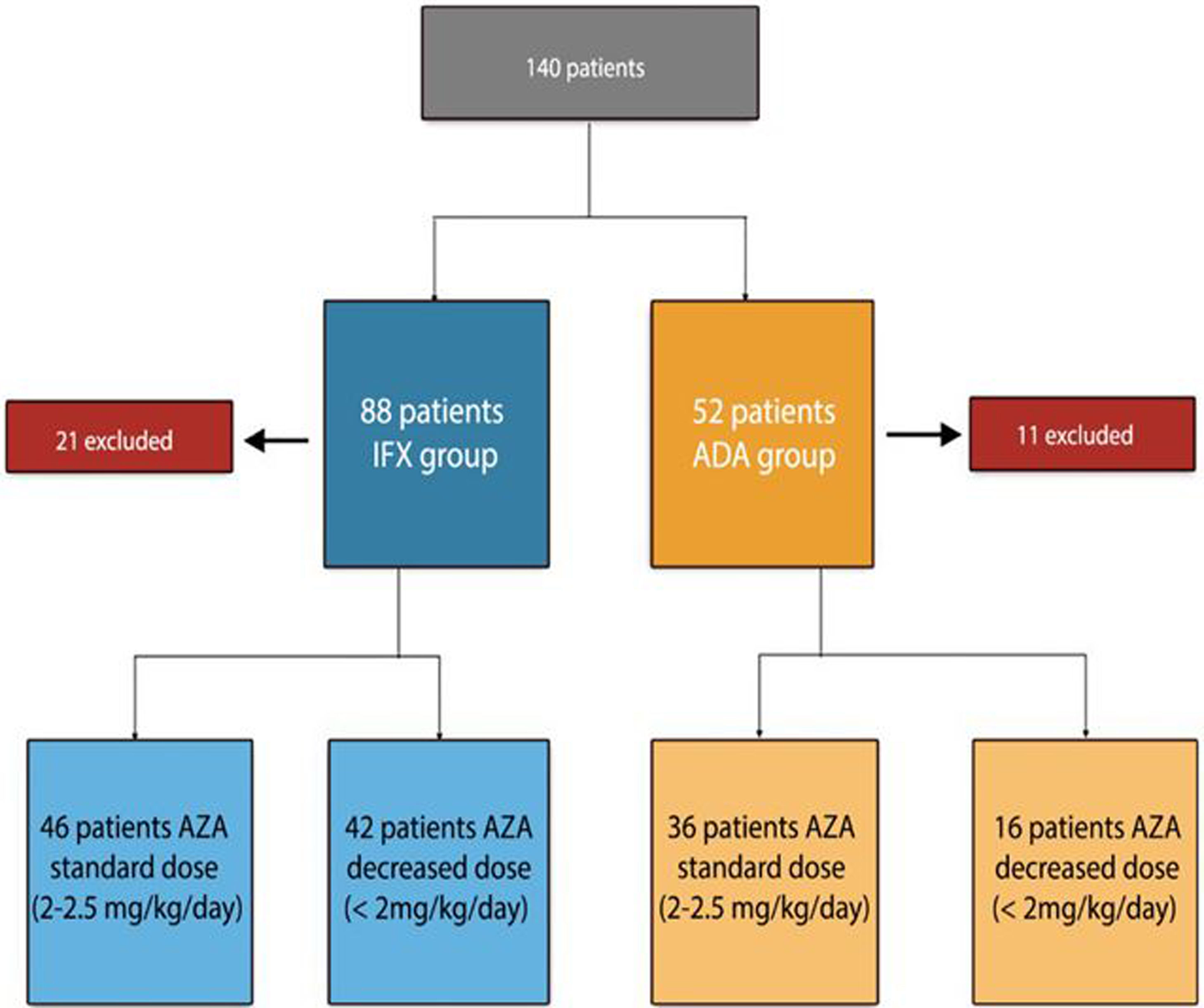
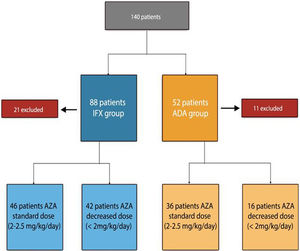
ResultsPatient baseline dataA total of 140 patients were recruited, 88 patients on IFX treatment and 52 on ADA treatment (excluding 21 patients initially selected on IFX treatment and 11 patients with ADA, for not meeting inclusion criteria). The flowchart of the study is described in Fig. 1.
The reason why, the patients (n=58) were being treated with AZA at a lower dose than usual: 31 under medical indication (53%), 4 due to digestive intolerance at full dose (7%), 6 due to elevation of transaminases (10%), 12 due to myelosuppression (20%), 2 due to asthenia (3%), general malaise and 3 patients (5%) due to the patient refusing to receive higher doses.
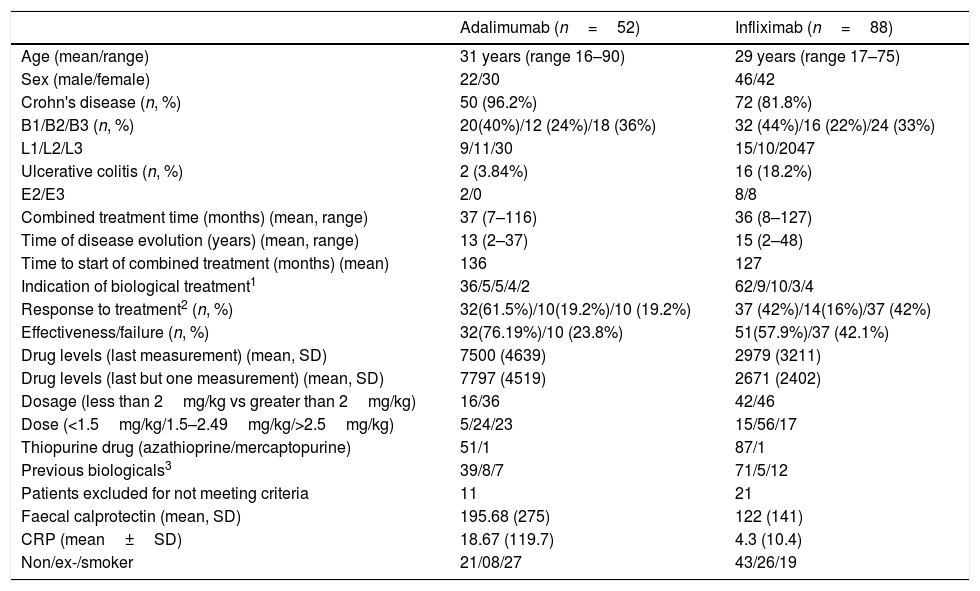
The baseline data of the patients are presented in Table 1.
Baseline data of patients according to treatment with infliximab or adalimumab.
| Adalimumab (n=52) | Infliximab (n=88) | |
|---|---|---|
| Age (mean/range) | 31 years (range 16–90) | 29 years (range 17–75) |
| Sex (male/female) | 22/30 | 46/42 |
| Crohn's disease (n, %) | 50 (96.2%) | 72 (81.8%) |
| B1/B2/B3 (n, %) | 20(40%)/12 (24%)/18 (36%) | 32 (44%)/16 (22%)/24 (33%) |
| L1/L2/L3 | 9/11/30 | 15/10/2047 |
| Ulcerative colitis (n, %) | 2 (3.84%) | 16 (18.2%) |
| E2/E3 | 2/0 | 8/8 |
| Combined treatment time (months) (mean, range) | 37 (7–116) | 36 (8–127) |
| Time of disease evolution (years) (mean, range) | 13 (2–37) | 15 (2–48) |
| Time to start of combined treatment (months) (mean) | 136 | 127 |
| Indication of biological treatment1 | 36/5/5/4/2 | 62/9/10/3/4 |
| Response to treatment2 (n, %) | 32(61.5%)/10(19.2%)/10 (19.2%) | 37 (42%)/14(16%)/37 (42%) |
| Effectiveness/failure (n, %) | 32(76.19%)/10 (23.8%) | 51(57.9%)/37 (42.1%) |
| Drug levels (last measurement) (mean, SD) | 7500 (4639) | 2979 (3211) |
| Drug levels (last but one measurement) (mean, SD) | 7797 (4519) | 2671 (2402) |
| Dosage (less than 2mg/kg vs greater than 2mg/kg) | 16/36 | 42/46 |
| Dose (<1.5mg/kg/1.5–2.49mg/kg/>2.5mg/kg) | 5/24/23 | 15/56/17 |
| Thiopurine drug (azathioprine/mercaptopurine) | 51/1 | 87/1 |
| Previous biologicals3 | 39/8/7 | 71/5/12 |
| Patients excluded for not meeting criteria | 11 | 21 |
| Faecal calprotectin (mean, SD) | 195.68 (275) | 122 (141) |
| CRP (mean±SD) | 18.67 (119.7) | 4.3 (10.4) |
| Non/ex-/smoker | 21/08/27 | 43/26/19 |
Treatment indication: induction of remission/prophylaxis recurrence/perianal disease/maintenance/fistulas.
Response to treatment: remission/response/loss of response.
No previous biological treatment/infusion reaction/loss of response.
In patients under IFX treatment as a function of AZA dose, 46 patients (52%) belonged to the standard dose group and 42 patients (48%) to the decreased dose group. The reason for the decreased azathioprine dose was: 24 patients due to medical indications (57%), and 18 due to adverse effects (42%). The duration of combination therapy was similar in both groups: 37 months (range 7–116) in the standard dose group and 36 months (range 8–127) in the decreased dose group (p=0.105).
Among patients receiving combined treatment with ADA: 36 patients (69%) belonged to the standard dose group while 16 patients (31%) belonged to the decreased dose group. The cause for the decreased azathioprine dose was: 7 patients due to medical indications (43%), and 9 due to adverse effects (57%). Treatment duration was 40 months (range 7–67) in the standard dose group and 33 months (range 7–116) in the decreased dose group, with no statistically significant differences (p=0.231).
Therapeutic response during the study period and biomarkersIn the IFX group, there is no statistically significant difference (p=0.204) in remission (39% vs 41.3%), response (10% vs 21.7%) or failure (51.5% vs 37%) rates depending on the dose of thiopurine drugs (standard or decreased).
If we group response and remission together (efficacy) against clinical failure (defined as the need to modify the treatment regime), in the AZA standard dose group a total of 23 patients (50%) presented response loss, while in the AZA decreased dose group 28 patients (66.6%) maintained clinical efficacy, without objectifying statistically significant differences depending on the dose of thiopurines (p=0.114).
Patients on IFX treatment who had a clinical failure mostly needed to intensify doses of antiTNF (29 patients (33%), requiring a change of biology in 8 cases (9%). There were no statistically significant differences in the management of clinical failure by AZA dose.
Relative to AZA decreased dose group, there are no statistically significant differences (p=0.653) in effectiveness (62.5% vs 68.2%) or failure (37.5% vs 30.8%) rates depending on the indication of decreased dose (adverse effects or medical indication)
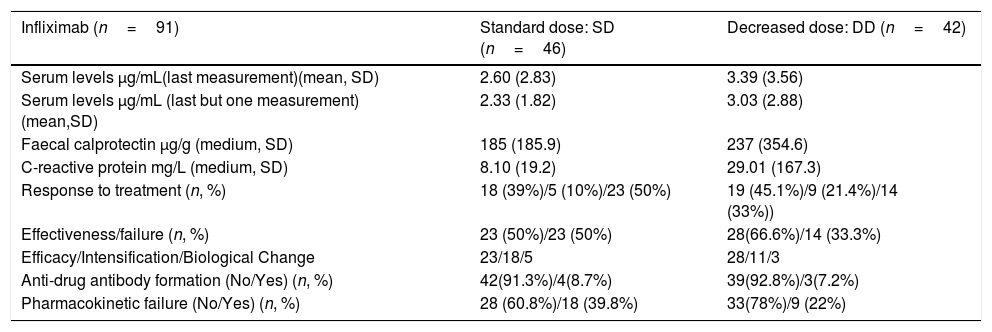
Mean CRP and faecal calprotectin levels did not reach statistically significant differences (p=0.408 and p=0.52 respectively) between the AZA group standard dose and the AZA group decreased dose Their main characteristics are summarised in Table 2.
Descriptive Analysis Infliximab.
| Infliximab (n=91) | Standard dose: SD (n=46) | Decreased dose: DD (n=42) |
|---|---|---|
| Serum levels μg/mL(last measurement)(mean, SD) | 2.60 (2.83) | 3.39 (3.56) |
| Serum levels μg/mL (last but one measurement) (mean,SD) | 2.33 (1.82) | 3.03 (2.88) |
| Faecal calprotectin μg/g (medium, SD) | 185 (185.9) | 237 (354.6) |
| C-reactive protein mg/L (medium, SD) | 8.10 (19.2) | 29.01 (167.3) |
| Response to treatment (n, %) | 18 (39%)/5 (10%)/23 (50%) | 19 (45.1%)/9 (21.4%)/14 (33%)) |
| Effectiveness/failure (n, %) | 23 (50%)/23 (50%) | 28(66.6%)/14 (33.3%) |
| Efficacy/Intensification/Biological Change | 23/18/5 | 28/11/3 |
| Anti-drug antibody formation (No/Yes) (n, %) | 42(91.3%)/4(8.7%) | 39(92.8%)/3(7.2%) |
| Pharmacokinetic failure (No/Yes) (n, %) | 28 (60.8%)/18 (39.8%) | 33(78%)/9 (22%) |
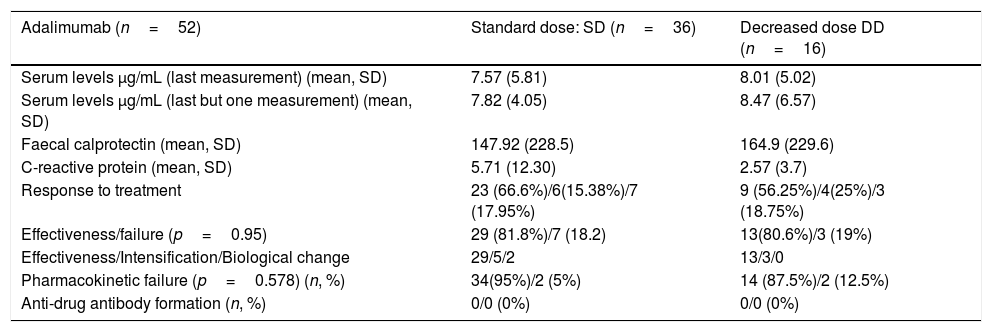
With respect to the group of patients treated with ADA, there are no statistically significant differences (p=0.83) in the rates of remission (66% vs 56.25%), response (15.3% vs 14%) or failure (17.9% vs 18.25%) depending on the dose of thiopurine drugs (standard or decreased).
If we group response and remission together (efficacy) against clinical failure, in the AZA standard dose group a total of 6 patients (19.4,%) presented response loss, while in the AZA decreased dose group 3 patients (18.2%) had clinical failure, without objectifying statistically significant differences depending on the dose of thiopurines (p=0.95). There were no statistically significant differences in the management of clinical failure according to the AZA dose.
With respect to AZA decreased dose group, a total of 2 patients (22.2%) in the AZA decreased dose group for adverse events, presented response loss, while in the AZA decreased dose group for medical indication 1 patient (14.9%) had clinical failure, without objectifying statistically significant differences depending on the indication of decreased dose of thiopurines (p=0.687).
Mean CRP and calprotectin levels in patients on combined ADA treatment were similar between the two groups of patients (standard dose vs decreased dose respectively),without statistically significant differences (p=0.188 and p=0.41). Its main characteristics are summarised in Table 3.
Descriptive analysis adalimumab.
| Adalimumab (n=52) | Standard dose: SD (n=36) | Decreased dose DD (n=16) |
|---|---|---|
| Serum levels μg/mL (last measurement) (mean, SD) | 7.57 (5.81) | 8.01 (5.02) |
| Serum levels μg/mL (last but one measurement) (mean, SD) | 7.82 (4.05) | 8.47 (6.57) |
| Faecal calprotectin (mean, SD) | 147.92 (228.5) | 164.9 (229.6) |
| C-reactive protein (mean, SD) | 5.71 (12.30) | 2.57 (3.7) |
| Response to treatment | 23 (66.6%)/6(15.38%)/7 (17.95%) | 9 (56.25%)/4(25%)/3 (18.75%) |
| Effectiveness/failure (p=0.95) | 29 (81.8%)/7 (18.2) | 13(80.6%)/3 (19%) |
| Effectiveness/Intensification/Biological change | 29/5/2 | 13/3/0 |
| Pharmacokinetic failure (p=0.578) (n, %) | 34(95%)/2 (5%) | 14 (87.5%)/2 (12.5%) |
| Anti-drug antibody formation (n, %) | 0/0 (0%) | 0/0 (0%) |
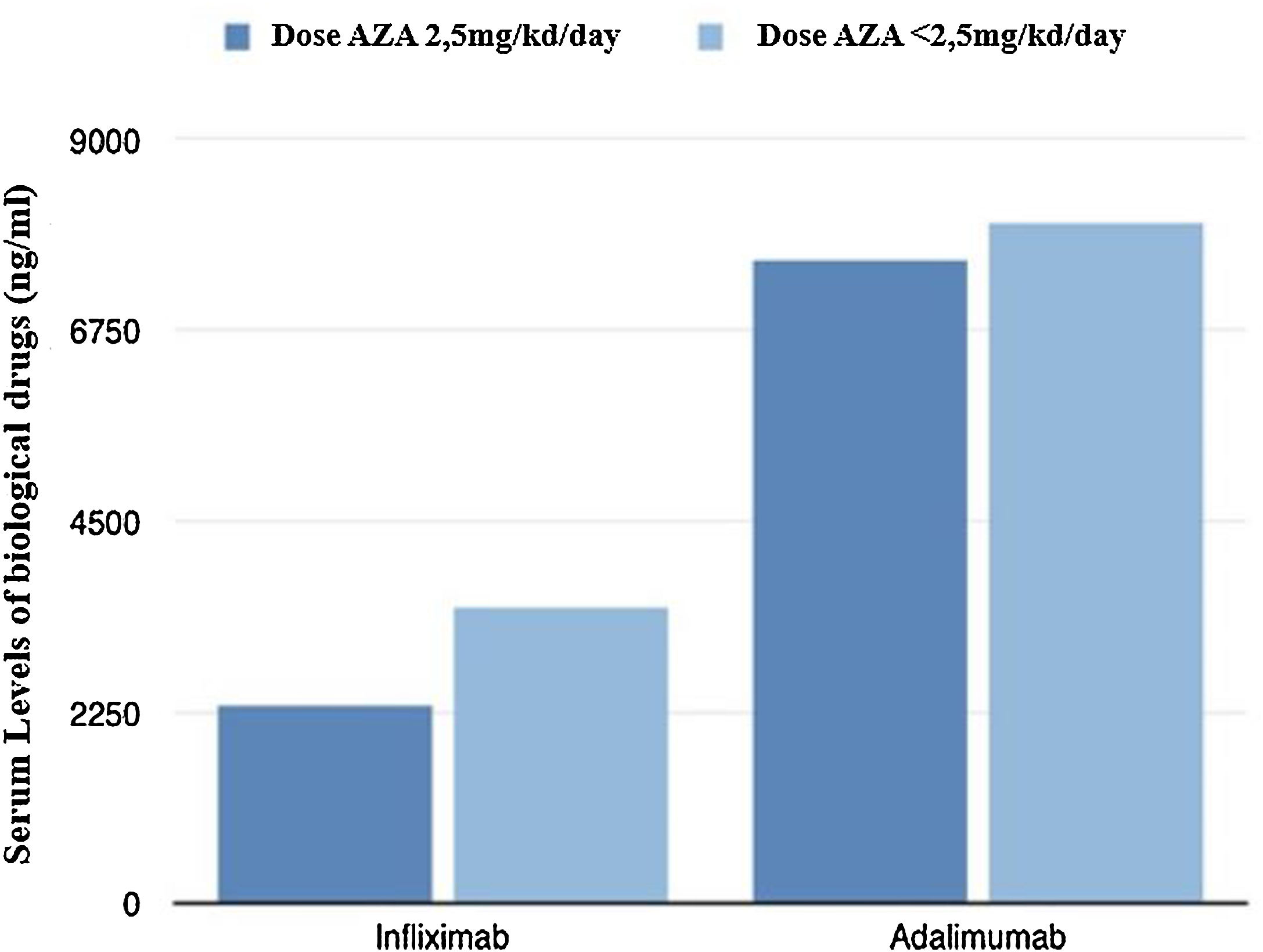
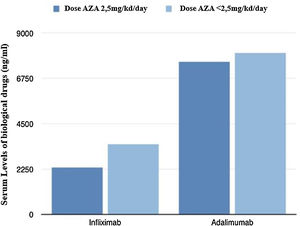
From a pharmacokinetic point of view, there were no differences between the IFX levels (mean between the last two determinations) depending on the AZA dose (2.46 vs 3.21μg/mL; p=0.211).
If we consider pharmacokinetic failure (levels of IFX<1μg/mL or undetectable levels with/without antibody formation). In the standard dose group, a total of 18 patients (39.2%) (of which 4 patients (8.6%) presented development of ATT), compared to 9 patients in the group AZA decreased dose (22%) (of which 3 (7.2%) developed ATT), had an unfavourable pharmacokinetic evolution. No statistically significant differences were found between the two groups in either pharmacokinetic failure or antibody formation (p=0.072 and 0.788).
In the AZA decreased dose group, there were no statistically significant differences between IFX levels (3.29 vs 3.34μg/mL; p=0.887) nor ATT developed (5.6% vs 8.3%; p=0.729) depending on the indication of decreased dose (adverse effects or medical indication).
With respect to ADA levels (mean of the last two determinations) no differences were found in both groups 7.69 vs 8.23μg/mL (p=0.37).
If we consider pharmacokinetic failure (levels of ADA<2μg/mL or undetectable levels with/without antibody formation). In the standard dose group, a total of 2 patients (5%) versus 2 patients in the reduced dose AZA group (12.5%) had pharmacokinetic failure. There were also no statistically significant differences between the two groups (p=0.578). No antibody formation was detected in either group.
In the AZA decreased dose group, there were no statistically significant differences between ADA levels (7.33 vs 7.16μg/mL; p=0.976), depending on the indication of decreased dose (adverse effects or medical indication). No antibody formation was detected in either group.
Fig. 2 shows graphically the last serum levels of the biological drug.
DiscussionLoss of response to Anti-TNF treatment ranges from 10 to 50%, with secondary failure occurring in 20–40% of patients with an initial response to anti-TNF.12 For the correct management of the loss of secondary response to antiTNF treatment, drug levels and the presence of antibodies are a fundamental tool that has been incorporated into the daily clinical practice of most centres.13–15 There is a positive relationship between anti-TNF levels and the likelihood of clinical response, clinical remission and mucosal healing. The presence of antibodies is closely associated with the loss of secondary response16. On the other hand, the presence of subtherapeutic levels of the drug increases the risk of the appearance of antibodies and the activity of the disease17-23.
Intensification of treatment and/or addition of immunosuppressive therapy may reduce the appearance of antibodies and thus the loss of efficacy, especially during the first year of treatment of patients.24,25 Different studies6–8 show that the addiction of an immunosuppressor to biological treatment allows obtaining higher clinical response rates, higher drug levels and less antibody formation. However, the appropriate dose of immunosuppressive drug to use is unknown, and the indication of the dose used in monotherapy has been moved.
In our study, patients receiving combined therapy with IFX and AZA, there was no significant difference between the group of standard dose and decreased dose of immunosuppressant, with respect to the clinical outcome. In fact 50% of patients at standard dose and 66.6% of patients with decreased dose, maintained clinical efficacy, without statistically significant differences depending on the dose of AZA (p=0.114). From a pharmacokinetic point of view, no significant differences were found in either antibody formation or mean levels of the last two IFX determinations between the two groups. There were also no differences found in the mean values of CRP and faecal calprotectin in both groups.
The reason for the indication for dose reduction, either by medical decision or due to adverse effects, does not show influence on the clinical or pharmacokinetic response. The results of our study do not seem to depend on the reason for reducing the dose.
This data matches the clinical trial performed by Roblin et al.,7 in which patients were randomised into 3 groups: stable azathioprine (2.5mg/kg), decreased azathioprine (half dose 1.25mg/kg) and suspension of azathioprine. In this study, it was concluded that there were no significant differences between the two doses of AZA and that the combined treatment was superior to monotherapy. In that study, the percentage of treatment failure after stopping AZA was 31% during follow-up and 42% of patients developed levels of infliximab<1μg/mL one year later. In contrast, halving the AZA dose was not associated with an increased risk of failure at 1 year, and was not associated with an unfavourable evolution of drug levels, without finding pharmacokinetic differences with respect to patients in the AZA stable dose group.
It is worth mentioning the work of Van Assche et al.,26 which assessed the influence of the suspension of the immunosuppressor in patients in remission without steroids under combined therapy (IFX and AZA) for at least 6 months. It was observed that interrupting the immunosuppressant drug resulted in a gradual decrease in biological drug levels over time and more patients had undetectable levels and had developed ATT antibodies (12.5% vs 5.0%).
With regards to the group of patients on ADA treatment, there was no difference between patients with standard dose of AZA versus low dose in terms of clinical outcome. Patients had similar response/remission rates in both groups (81.8% vs 80.6%) without reaching statistical significance (p=0.953).
From the pharmacokinetic viewpoint for ADA, no significant differences were found either in antibody formation or in the mean levels of the last two ADA determinations between the two groups. nor were there differences in the mean values of CRP and faecal calprotectin between the standard dose or the decreased dose of thiopurines.
Our data is consistent with the study by Matsumoto et al.,8 where patients were randomised to receive ADA or combined treatment of ADA and AZA at low doses. From a pharmacokinetic point of view, higher levels of antiTNF were obtained in the low-dose thiopurine group (7.6 vs 6.5μg/mL, p=0.084). In addition, anti-Adalimumab antibody formation was positive in 13.2% of patients in the monotherapy group and 4% in the combination group (p=0.078).
The use of AZA involves a series of side effects that can become serious and in many cases are dose-dependent, so a reduction in dose in which the same efficacy of treatment is achieved would be a possible benefit, due to the decrease in these dose-dependent adverse effects. The most frequent adverse effect is digestive intolerance, which appears in up to 8% of cases, improving in some cases with dose reduction. The most serious dose-dependent adverse effects are myelotoxicity and hepatotoxicity.
The benefit of a low dose of thiopurines may also be a reduction in the theoretical risk of opportunistic infections and cancer, since combined therapy with thiopurines and anti-TNF involves an increase in the risk. Evidence suggests that combined treatment with azathioprine and anti-TNF drugs poses an increased risk of hepatosplenic T cell lymphoma,27 sepsis and opportunistic infections10 such as herpes simplex, shingles, thrush, cervical dysplasia from Human Papilloma Virus, and tuberculosis.28
With regard to the increased risk of neoplasm in patients on immunosuppressive therapy, it has been observed in studies conducted in other diseases and appears to have a dose-dependent relationship. For example, there was an increased risk of skin cancers in kidney transplant recipients treated with azathioprine, which was associated with increased concentrations of 6-TGN (metabolite of azathioprine).29 In a study conducted in Australia30 in liver, heart and lung transplant recipients, higher average daily doses of azathioprine were associated with an increased risk of both early and late non-Hodgkin's lymphoma. These results suggest that high doses of azathioprine may lead to DNA damage and may contribute to promoting carcinogenesis.
On the other hand, the use of fragmented doses of AZA due to the appearance of adverse effects has been described as a limiting factor for adherence to treatments, particularly in the case of chronic medication, such as thiopurine therapy, so that the use of a lower dose of AZA could facilitate better treatment compliance.
Our study has several limitations; it is mainly a retrospective study, and the sample size is small, which hinders the analysis of subgroups and multivariables. Additionally, as this is a retrospective study, confusion factors related to the patient cannot be ruled out. Another limitation is the absence of endoscopic indices, and, other objective data such as endoscopic activity were not taken into account in our study.
However, this study is a real clinical practice study valid for certifying pharmacokinetic evolution in patients undergoing combined treatment depending on the dose of thiopurines. In order to determine the maintenance dose of AZA in combination with antiTNF, further studies of methodological quality are needed to support our results.
In conclusion, our experience suggests that there are no differences between antiTNF levels, nor in clinical-biological response/remission rates based on doses of AZA in patients under combination treatment with antiTNF. The use of a lower dose of an immunosuppressant (AZA) may provide better drug tolerability with a better safety profile and lower expenditure, although prospective and long-term studies are required to confirm these results.
Authors’ contributionsAll authors have made a significant contribution to the research described in this manuscript.
Guarantor of the article: Javier Lucas Ramos.
JLR, CSF, MMD performed the concept and design study, data acquisition (DA), data analysis and interpretation, manuscript and writing.
JPZ, MSA, EMA, JLRD: data analysis and interpretation.
JYC, IAG: DA (data acquisition).
All authors approve the final manuscript as well as the list of authorship.
Conflict of interestNo funding has been received for this work. The authors confirm that there is no financial or personal relationship with other persons or organisations that could give rise to a conflict of interest in relation to the study submitted for publication.