Gastric cancer (GC) has been become the second leading cause for cancer-associated death. This study aimed to investigate Orexin A levels and associated receptors in tumor tissues of GC patients.
Patients and methodsForty-six consecutive gastric cancer patients (GC, n=46) and 13 chronic atrophic gastritis patients (CAG, n=13) were recruited. Meanwhile, 18 health individuals visiting Medical Examination Department were involved as control (N group, n=18). ELISA was used to examine Orexin A concentration. Immunohistochemistry assay was used to examine OX1R and OX2R. HE staining was applied to evaluate inflammation. qRT-PCR was employed to detect OX1R, OX2R, prepro-Orexin mRNAs. Serum Helicobacter pylori (H. pylori) infection was measured.
ResultsOrexin A expression in GC patients was significantly up-regulated compared to N group and CAG group (p<0.05). Orexin A expression was increased in CAG group compared to N group (p<0.05). Gastric cancer tissues exhibited significantly obvious inflammation compared to N group and CAG group (p<0.05). OX1R and OX2R expressions were significantly down-regulated in GC group compared to N group and CAG group (p<0.05). OX1R and OX2R were lower significantly in GC group compared to CAG group (p<0.05). Prepro-Orexin was significantly depleted in tumor tissues of GC group compared to N group and CAG group (p<0.05). Orexin A expression was un-associated with gender, age and differential grades (p>0.05). CAG and GC patients demonstrated higher H. pylori infection rates.
ConclusionOrexin A was associated with inflammation by interacting with OX1R/OX2R receptor and activating prepro-Orexin in tumor tissues of gastric cancer patients.
El cáncer gástrico (CG) se ha convertido en la segunda causa principal de muerte asociada al cáncer. El objetivo de este estudio fue investigar la concentración de orexina-A y de los receptores asociados en tejidos tumorales de pacientes con CG.
Pacientes y métodosSe seleccionó a 46 pacientes consecutivos con CG (n=46) y a 13 pacientes con gastritis atrófica crónica (GAC) (n=13). Al mismo tiempo, se utilizó como control a 18 individuos sanos que visitaron la unidad de reconocimiento médico (grupo N, n=18). Se empleó un ELISA para analizar la concentración de orexina-A. Se usó un ensayo inmunohistoquímico para el análisis de OX1R y OX2R. Se aplicó tinción hematoxilina-eosina para evaluar la inflamación. Se utilizó PCR cuantitativa en tiempo real para detectar el ARNm de OX1R, OX2R y prepo-orexina. Se evaluó la infección por Helicobacter pylori (H. pylori) en suero.
ResultadoLa expresión de orexina-A en pacientes con CG era considerablemente mayor en comparación con el grupo N y el grupo de GAC (p<0,05). La expresión de orexina-A fue mayor en el grupo de GAC en comparación con el grupo N (p<0,05). Los tejidos con cáncer gástrico presentaron una inflamación significativamente visible en comparación con el grupo N y el grupo de GAC (p<0,05). La expresión de OX1R y OX2R fue notablemente menor en el grupo de CG en comparación con el grupo N y el grupo de GAC (p<0,05). OX1R y OX2R fueron significativamente menores en el grupo de CG en comparación con el grupo de GAC (p<0,05). La prepo-orexina se encontraba especialmente disminuida en tejidos tumorales del grupo de CG en comparación con el grupo N y el grupo de GAC (p<0,05). La expresión de la orexina-A no se asoció al sexo, la edad o los grados diferenciales (p>0,05). Los pacientes con GAC y CG registraron tasas de infección por H. pylori más elevadas.
ConclusiónLa orexina-A se asoció con la inflamación al interactuar con los receptores OX1R/OX2R y activar la prepo-orexina en tejidos neoplásicos de pacientes con cáncer gástrico.
Gastric cancer has been become the second leading reason for the cancer-associated death and the fourth most common malignancy in the whole world,1,2 especially in the Eastern Europe, East Asia, South and central American.3 In the recent years, although the diagnostic and therapeutic approaches have been improved, the five years survival rates are also less then 30%.4 Meanwhile, more than 50% gastric cancer patients suffer from the tumor metastasis and tumor recurrence post the tumor resection.5 In clinical, for the late-date gastric cancer (or advanced gastric cancer), more than 40% of patients are resistant to the chemotherapy, which causes the poor survival.6 Therefore, discovering a novel prognostic biomarker to improve the diagnosis, facilitate the metastasis and predict the prognosis is critical and urgent.
Orexin A is the evolutionarily-conserved neuro-peptide that is firstly discovered by the subtractive cDNA cloning and the orphan receptor technology.7 Orexin A derives from a proteolytic cleavage of a common 130 amino acid precursor peptide, which named prepro-Orexin.8 Actually, the Orexin A mainly acts through two G-protein-coupled receptors, including orexin receptor 1 and 2 (OX1R and OX2R).9 The previous study reported that orexin A reduced the cell proliferation of pancreatic tumor cells and stimulated the growth in adrenal gland tumor cells.10,11 Thus, the Orexin A is involved in this study to confirm the relationship between Orexin A and the gastric cancer.
Therefore, the present study investigated the levels of Orexin A and it's associated receptors, OX1R and OX2R, in the tumor tissues of gastric cancer patients. Meanwhile, the correlations between the Orexin A levels and gender/age/differential grade were also evaluated by using the serum of gastric cancer patients.
Materials and methodsSubjectsTotal of 46 consecutive gastric cancer patients (GC group, n=46) and 13 chronic atrophic gastritis patients (CAG group, n=13) received from January 2016 to December 2016 in our hospital were recruited in this study. Meanwhile, 18 health individuals who visit the Medical Examination Department were involved in this study as the Normal control group (N group, n=18). The Normal control group didn’t include the patients with normal gastric histology or patient without atrophic chronic gastritis. The characteristics of the subjects were listed in Table 1.
The usage of the tumor specimens was approved by the Ethics Committee of People's Hospital of Ningxia Hui Autunomous Region, Yinchuan, China. The written informed consents were obtained from all of the patients.
ELISAThe serum samples applied in this study were aliquots (0.5ml) from the original samples (2ml), where the blood from the fasting patients, and were collected into the serum-separator tubes. Then, the blood was centrifuged at 3000r/min for 20min at 4°C. The levels of Orexin A was examined using the Human Orexin (HCRT) ELISA Kit (Cat No. CSB-EL010230HU, CusaBio. Tech., Houston, TA, USA) according to the instruction of manufacturer. The absorbance was detected using the microplate reader (Mode: Multiskan Spectrum, Thermo Electron Corp., Waltham, MA, USA) at 450nm.
Immunohistochemistry assayThe tumor tissues or the normal tissues were fixed with 4% paraformaldehyde (Sangon Biotech, Shanghai, China) for 30min at room temperature and washed by using PBS for 3 times (5min per time). Then, the tissues were sliced into sections and endogenous peroxidase was inactivated with 3% hydrogen peroxide (Sangon Biotech, Shanghai, China) for 10min. The section were blocked with 10% goat serum (Hyclone, Logan, UT, USA) for 20min, and washed with PBS for 3 times. The sections were treated with rabbit anti-human OX2R polyclonal antibody (1:2000, Cat. No. ab224368, Abcam Biotech., Cambridge, Massachusetts, USA) and rabbit anti-human OX2R polyclonal antibody (Cat. No. sc-402343, Santa Cruz Biothch., Santa Cruz, CA, USA) at 4°C overnight. Then, sections were incubated with horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:1000, Cat. No. ab6721, Abcam Biotech., Cambridge, Massachusetts, USA) for 1h at 37°C. Eventually, the sections were immersed in DAB solution (ZSGB Bio. Inc. Co., Beijing, China), and rinsed in distilled water. Images of sections were captured and observed with an inverted microscope (Model: CK40; Olympus, Japan) and analyzed by using an image-scanning system (Model: BH-2, Olympus, Japan).
Hematoxylin–eosin (HE) stainingThe tumor tissues were treated as the above methods. The tumor tissues were cut into section with thickness of 4μm and stained using hematoxylin (Nanjing Jiancheng Bioengineering Inst., Nanjing, China) and eosin (Biyotime Biotech. Shanghai, China), respectively. Finally, the sections were observed with an inverted microscope (Model: CK40; Olympus, Japan). The images were evaluated and analyzed with a professional image analysis software.
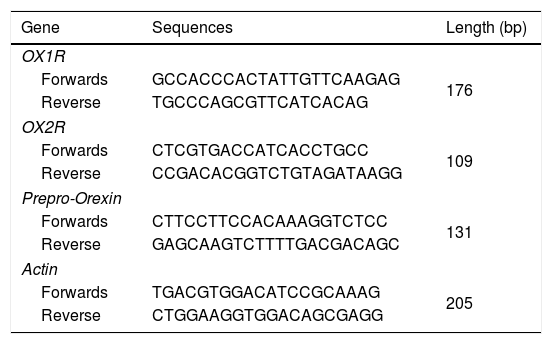
Quantitative real-time PCR (qRT-PCR)Total RNAs in tumor or normal tissues were extracted by using the Trizol regents (Beyotime Biotech., Shanghai, China) due to previous study reported.12 The complementary DNAs (cDNAs) were synthesized with the Reverse Transcription (RT) Regent (Beyotime Biotech., Shanghai, China). mRNAs of OX1R, OX2R and prepro-Orexin were amplified using the Sybr Green I real-time PCR reagents (Western Biotech., Chongqing, China). The primers were synthesized by Western Biotech. (Chongqing, China) (Table 2). qRT-PCR conditions were listed as the followings: pre-denaturation for 4min at 94°C, supplementing with 35 cycles of 94°C for 20s, 60°C for 30s, 72°C for 30s. Finally, the PCR reaction was terminated at 72°C for 10min. The relative mRNAs were analyzed by using a gel scanning system (version: GDS8000, UVP, Sacramento, CA, USA). The relative expression of PCR products were calculated by using the previous 2△△ct method.13
Primers for the RT-PCR assay.
| Gene | Sequences | Length (bp) |
|---|---|---|
| OX1R | ||
| Forwards | GCCACCCACTATTGTTCAAGAG | 176 |
| Reverse | TGCCCAGCGTTCATCACAG | |
| OX2R | ||
| Forwards | CTCGTGACCATCACCTGCC | 109 |
| Reverse | CCGACACGGTCTGTAGATAAGG | |
| Prepro-Orexin | ||
| Forwards | CTTCCTTCCACAAAGGTCTCC | 131 |
| Reverse | GAGCAAGTCTTTTGACGACAGC | |
| Actin | ||
| Forwards | TGACGTGGACATCCGCAAAG | 205 |
| Reverse | CTGGAAGGTGGACAGCGAGG | |
In this study, we measured the serum H. pylori infection by examining serum H. pylori IgG antibody titer using the commercial Helicobacter pylori IgG Detection ELISA Kit (Biohit, Helsinki, Finland) due to protocol of manufacturer. The serum H. pylori was divided into H. pylori positive (H. pylori+) and H. pylori negative (H. pylori−).
Statistical analysisThe Data were represented as mean±standard deviation (SD) and analyzed with a SPSS software 20.0 (SPSS Inc., Chicago, Ull, USA). Tukey's post hoc test validated analysis of variance (ANOVA) was used to compare differences among multiple groups. All tests or experiments at least conducted for 6 repeats. The p values less than 0.05 was assigned as significant difference.
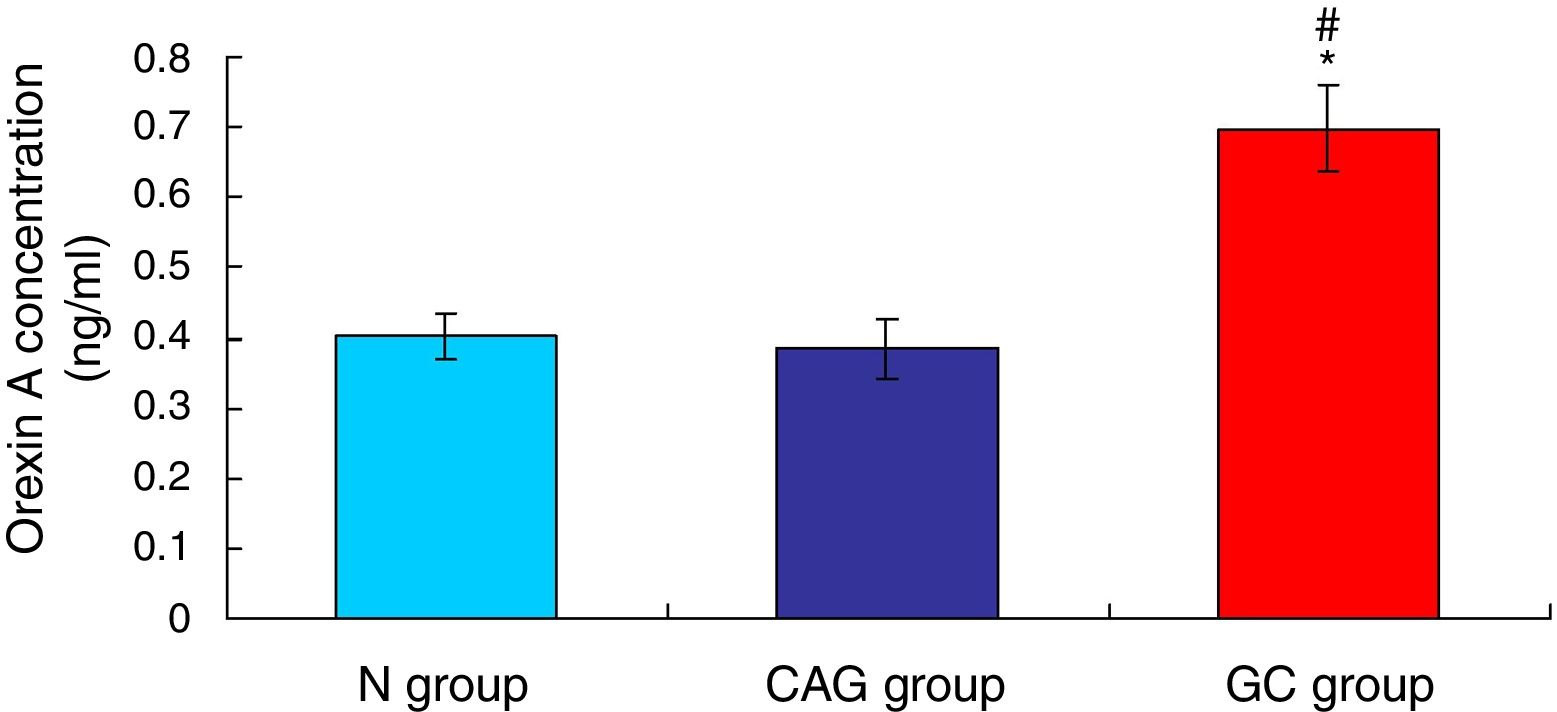
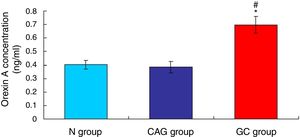
ResultsOrexin A level was up-regulated in gastric cancer patientsThe level of Orexin A in the serum of gastric cancer patients (GC), chronic atrophic gastritis patients (CAG) and normal subjects (N) was examined using ELISA assay. The results indicated that the Orexin A level in GC patients was significantly up-regulated compared to that in N group and CAG group (Fig. 1, p<0.05). Meanwhile, the Orexin A level was also increased in CAG group compared to that in N group (Fig. 1, p<0.05).
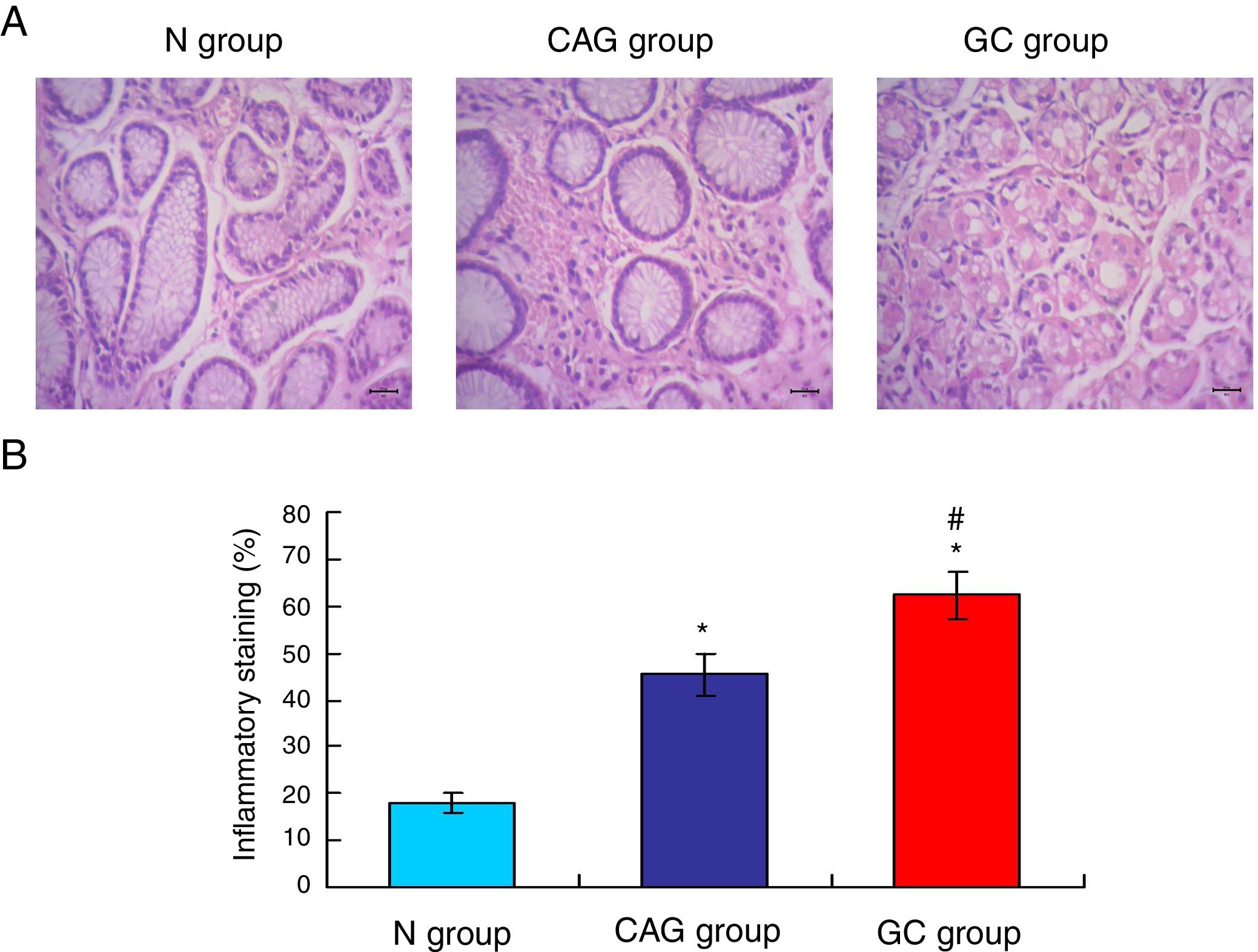
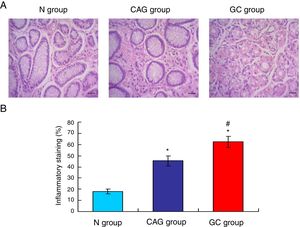
Gastric cancer tissues exhibited obvious inflammationTo compare the inflammation among all of three groups, the HE staining (Fig. 2A) was used in this study. The HE staining results showed that the gastric cancer tissues exhibited the significantly obvious inflammation compared to that of the N group and CAG group (Fig. 2B, p<0.05). Meanwhile, the CAG tissues inhibited significantly obvious inflammation compared to that of the N group (Fig. 2B, p<0.05).
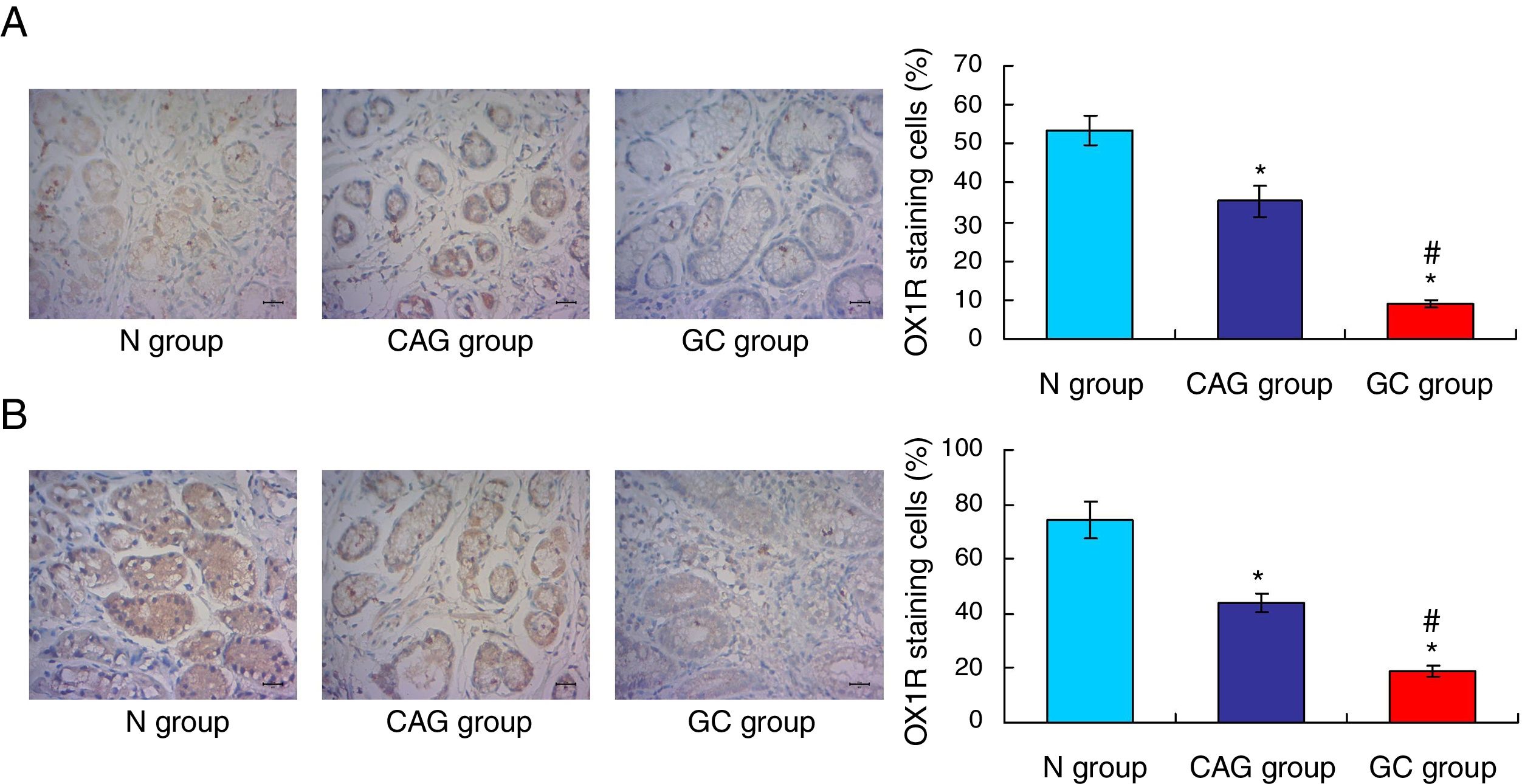
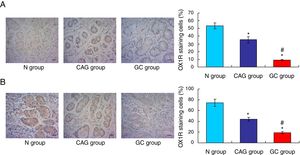
OX1R and OX2R expressions were down-regulated in gastric cancer patientsIn this study, the Orexin A associated receptors, OX1R and OX2R, were examined by using the immunohistochemistry assay. The results showed that the OX1R expression was significantly down-regulated in GC group compared to that in N group and CAG group (Fig. 3A, p<0.05). Meanwhile, OX1R expression in CAG group was also significantly lower compared to that in N group (Fig. 3A, p<0.05). Moreover, OX2R expression was also significantly down-regulated in GC group and CAG group compared to that in N group (Fig. 3B, p<0.05). Meanwhile, OX2R expression was lower significantly in GC group compared to that in CAG group (Fig. 3B, p<0.05).
Immunohistochemistry assay for evaluating OX1R and OX2R expression in the tumor tissues of gastric cancer patients. (A) Evaluation for OX1R expression in tumor tissues. (B) Evaluation for OX2R expression in tumor tissues. *p<0.05 vs. N group, #p<0.05 vs. CAG group. Magnification, 400×.
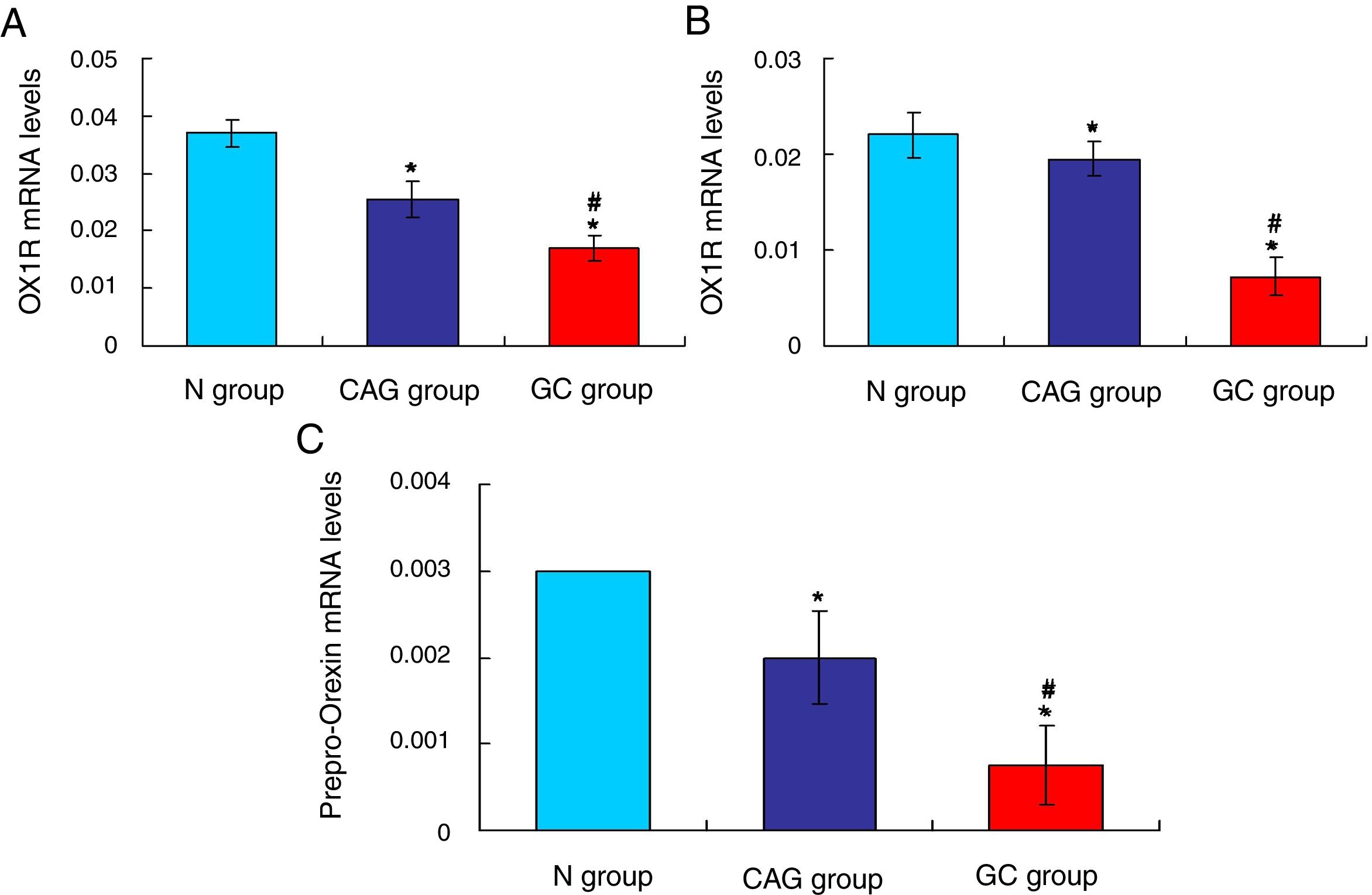
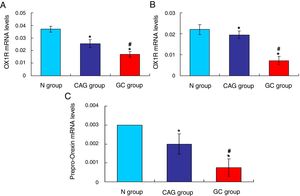
Furthermore, the RT-PCR assay also showed that the OX1R (Fig. 4A) and OX2R (Fig. 4B) mRNA expressions in GC group were lower significantly compared to that in N group and CAG group (p<0.05). Also, OX1R (Fig. 4A) and OX2R (Fig. 4B) mRNA was also significantly decreased in CAG group compared to that in N group (p<0.05).
qRT-PCR assay for evaluating OX1R, OX2R and prepro-Orexin mRNA expression in the tumor tissues of gastric cancer patients. (A) Evaluation for OX1R mRNA expression in tumor tissues. (B) Evaluation for OX2R mRNA expression in tumor tissues. (C) Evaluation for prepro-Orexin mRNA expression in tumor tissues. *p<0.05 vs. N group, #p<0.05 vs. CAG group.
The Orexin A mainly produced or transformed by the prepro-Orexin in the cells, but not by the other channels.14 Therefore, in order to verify the cause that induced the increased levels of Orexin A in tumor tissues of gastric cancer patients, the prepro-Orexin expression was evaluated using RT-PCR assay. The results indicated that prepro-Orexin was significantly depleted in tumor tissues of GC group compared to that in tissues of N group and CAG group (Fig. 4C, p<0.05). Also, the prepro-Orexin mRNA expression was lower significantly in CAG group compared to that in N group (Fig. 4C, p<0.05).
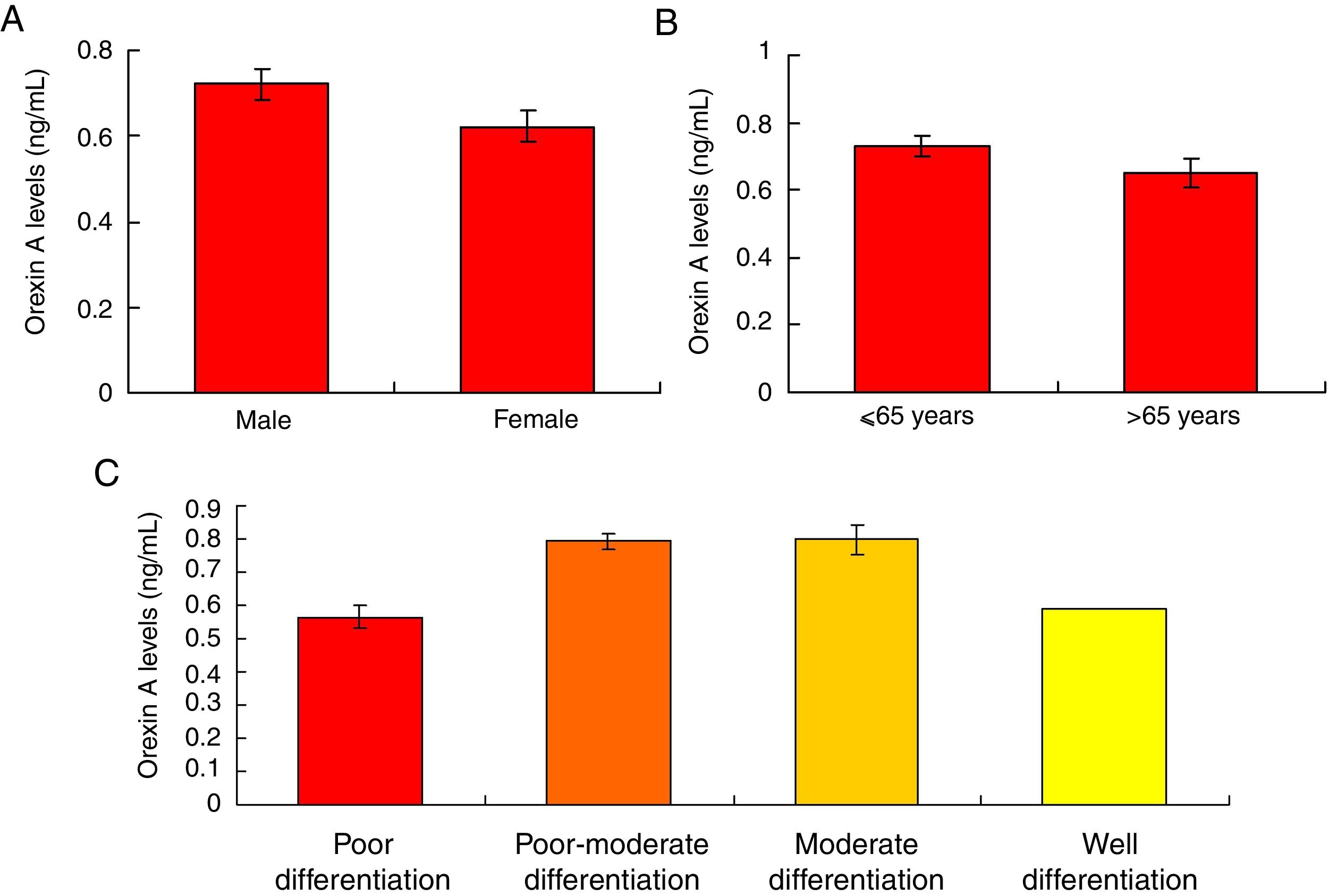
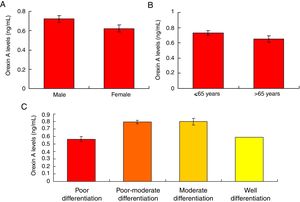
Orexin A expression was un-associated with gender, age and differential gradesOur results showed that there were no significant differences for the Orexin A levels between male gastric cancer patients and female patients (Fig. 5A, p>0.05). There were also no significant differences for the Orexin A levels between gastric patients less than 65 years and more than 65 years (Fig. 5B, p>0.05). Moreover, the Orexin A levels were also un-associated with the differential grades (poor, poor-moderate, moderate and well differentiation) of the gastric cancer (Fig. 5C, p>0.05).
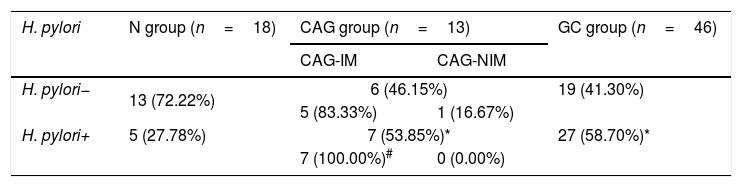
The CAG and GC patients demonstrated higher H. pylori infection ratesAccording to the ELISA results for Helicobacter pylori infection, H. pylori+ rates in both CAG group and GC group were higher significantly compared to that in the N group (Table 3, p<0.05). However, there was no significant difference for H. pylori+ rate between CAG group and GC group (Table 3, p>0.05). Moreover, majority of CAG patients with the atrophic gastritis and intestinal metaplasia in adjacent mucosa (CAG-IM group) demonstrated higher intestinal metaplasia rates (83.33% vs. 16.67 in H. pylori− group and 100% vs. 0.00% in H. pylori+ group), comparing to the CAG patients without intestinal metaplasia (CAG-NIM group) (Table 3, p<0.05).
Measurements for the serum H. pylori in different groups.
| H. pylori | N group (n=18) | CAG group (n=13) | GC group (n=46) | |
|---|---|---|---|---|
| CAG-IM | CAG-NIM | |||
| H. pylori− | 13 (72.22%) | 6 (46.15%) | 19 (41.30%) | |
| 5 (83.33%) | 1 (16.67%) | |||
| H. pylori+ | 5 (27.78%) | 7 (53.85%)* | 27 (58.70%)* | |
| 7 (100.00%)# | 0 (0.00%) | |||
CAG-IM: chronic atrophic gastritis with intestinal metaplasia; CAG-NIM: chronic atrophic gastritis without intestinal metaplasia.
The chronic atrophic gastritis patients were divided into grade 0, I, II, III, IV, according to OLGIM staging protocol. The results indicated that there were no significant differences between H. pylori infection and the OLGIM grading of CAG patients (Table 4, p>0.05).
DiscussionTo our best knowledge, the present study is the first investigation for evaluating Orexin A and it's receptors in tumor tissues of gastric cancer patients.8,15 In order to verify the roles of Orexin A in the tumor tissues of gastric cancer patients and associated mechanisms, the levels of Orexin A and OX1R/OX2R were evaluated in this study.
The previous studies11,16 reported that there Orexin A plays both of proliferative roles and apoptotic roles, according to the different type of tumor cells. The Orexin A triggers the tumor cells proliferation in adrenal gland tumors and inhibited cell growth in the colon cancer.11,16 In our study, the serum Orexin A expression was significantly increased in the tumor tissues of gastric cancer patients compared to that in normal health individuals and CAG patients. This result suggests that the Orexin A was associated with the tumor cell proliferation in tumor tissues, which is consistent with the previous study in vitro.15
The OX1R and OX2R have been discovered to be expressed in plenty of tumor cell lines, and which have been proven to be negatively correlated with Orexin A expression.17,18 Therefore, in order to explore the potential mechanism for the Orexin A associated gastric cancer cells proliferation, the OX1R and OX2R expressions were evaluated in this study. Both of the immunohistochemistry assay and RT-PCR assay results showed that the OX1R and OX2R expressions in tumor tissues of gastric cancer patients were lower significantly compared to that in normal individuals and in tissues of CAG patients. These results suggest that the receptors mediated the tumor growth and were depleted by the increased expression of Orexin A in the tumor tissues of GC patients.
According to the previous study,19 the gender, age and differential grades affect the tumor progression in clinical, therefore, we evaluated the effects of gender, age and differential grades on the serum Orexin levels. Our results illustrated that there were no significant differences for the Orexin A expression between male and female, age less and more than 65 years and among different differential grades, which is consistent with the previous study.20 Therefore, supplementing with the concise clinical trials, the Orexin A might become a promising biomarker for predicting the progression of gastric cancer in clinical.
Moreover, the previous studies21,22 reported that the inflammation, cell proliferation and pro-apoptotic effects are associated with the H. pylori infection. We speculated that the Orexin-associated inflammation might be correlated with the H. pylori infection. Therefore, in this study, we evaluated the H. pylori infection in all normal, CAG and GC patients. The findings showed that CAG and GC patients demonstrated higher H. pylori infection rates. Also, majority of CAG patients illustrated intestinal metaplasia (83.33% in H. pylori− group and 100% in H. pylori+ group). These results suggest that the occurrence of CAG and GC is associated with the H. pylori infection, which are consistent with the previous study.23 Moreover, the chronic atrophic gastritis were divided into grade 0, I, II, III, IV, due to the OLGIM staging protocol. However, we found that there were no significant differences between H. pylori infection (H. pylori− or H. pylori+) and the OLGIM grading of CAG.
ConclusionsThe present study demonstrated that Orexin A was associated with inflammation by interacting with OX1R/OX2R receptor and activating prepro-Orexin in tumor tissues of gastric cancer patients. In summary, the findings in this provided a novel insight to the biological activity of Orexin A on the gastric cancer, which might bring important implication for the health of patients.
Conflict of interestAuthors declare no competing financial or commercial interests in this manuscript.
This work was funded by Ningxia Nature Science Foundation (Grant No. 2019AAC03159), National Natural Science Foundation of China (Grant No. 81460207) and Science and Technology Key R&D Projects in Ningxia (Grant No. 2016KJHM85), and Scientific Research Project of Higher Schools in Ningxia (Grant No. NGY2018-83, NGY2018-73).