Dose “intensification” is a recommended strategy to recover therapeutic benefit in Crohn's disease (CD) patients who have lost initial response to anti-TNF therapy. Once patients have achieved remission, dose “de-intensification” can be used for cost and safety reasons.
ObjectivesThe primary aim of this study was to evaluate the long-term durability of remission after stepping down anti-TNF therapy. The secondary aim was to identify predictive factors associated with loss of response after “de-intensification” and to evaluate the effectiveness of a second “re-intensification” in patients who lost response after the treatment was stepped down.
MethodsWe evaluated CD patients who received at least one standard anti-TNF dosage after achieving remission with “intensified” anti-TNF therapy.
ResultsTwenty-four patients were included. The treatment was “intensified” because of partial response in 11 patients, loss of response in 10, and primary lack of response in 3. Eight of the 24 patients had lost response after a median follow-up of only 7 months after “de-intensification” of the anti-TNF therapy. The anti-TNF drug was “intensified” again in all 8 patients. Three patients did not respond to the new “intensification”, two had partial response and three achieved remission. On univariate analysis, no predictive factors were identified for loss of response after treatment “de-intensification”.
ConclusionsAfter only 7 months of follow-up, one-third of the CD patients who received “de-intensification” therapy lost response; of these, two-thirds did not achieve response after subsequent “re-intensification”.
La “intensificación” del tratamiento anti-TNF podría ser una opción terapéutica en los pacientes con enfermedad de Crohn (EC) pierden la respuesta inicial a la dosis estándar. Una vez que los pacientes alcanzan de nuevo la remission podría plantearse la “desintensification” del tratamiento por motivos de costs y seguridad.
ObjetivosPrimario evaluar la duración de la remisión a largo plazo tras la “desintensificación” del tratamiento anti-TNF. Secundario: Identificar los factores predictivos de recidiva tras la “desintensification” y evaluar la eficacia de una nueva “intensificación” en los pacientes que recidivaron tras la “desintensificación” del tratamiento.
MétodosSe incluyeron pacientes con EC que recibieron al menos una dosis estándar de tratamiento anti-TNF después de alcanzar la remisión con el tratamiento “intensificado”.
ResultadosVeinte y cuatro pacientes fueron incluidos. El tratamiento se “intensificó” por respuesta parcial en 11 pacientes, por pérdida de respuesta en 10 y por falta de respuesta primaria en 3. Ocho de los 24 pacientes perdieron respuesta después de una mediana de 7 meses de seguimiento tras la “desintensification” del anti-TNF. El tratamiento anti-TNF se “intensificó” de nuevo en los 8 pacientes. Tres de ellos no respondieron a la nueva “intensificación”, 2 presentaron una respuesta parcial y 3 alcanzaron de nuevo la remisión. No se identificaron factores predictivos de recidiva después de la “desintensificación” del tratamiento.
ConclusionesDespués de sólo 7 meses de seguimiento, un tercio de los pacientes con EC en los que se “desintensificó” el tratamiento anti-TNF perdió la respuesta; dos tercios de ellos no lograron respuesta tras la “re-intensificación”.
Tumour necrosis factor α (TNF) plays a pivotal role in the pathogenesis of several chronic inflammatory disorders such as Crohn's disease (CD), ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis or psoriasis. Anti-TNF drugs act by controlling inflammation through different mechanisms. Both infliximab and adalimumab are anti-TNF drugs; they bind specifically to TNF, preventing it to attach to specific receptors. Both have been proved to be effective in CD.1–4
Although infliximab and adalimumab are initially effective in a high proportion of patients, approximately 40% of patients treated with a maintenance regimen may lose the therapeutic response over time.5–7 For patients who lose their initial response, consideration can be given to “dose intensification” to regain therapeutic benefit. Dose “intensification” can be achieved by increasing the infliximab dose from 5mg/kg to 10mg/kg every 8 weeks or by decreasing the interval between infusions to every 4 weeks or both; Adalimumab dose is “intensified” administering the drug every week instead of every other week.5–8
Efficacy of anti-TNF drug dose “intensification” to overcome loss of response in CD patients is high: in the ACCENT I study, increasing the infliximab dose to 10mg/kg restored response in 90% of the patients with luminal CD who lose response to 5mg/kg.1 Furthermore, approximately 80% of patients who lost response while on 10mg/kg every 8 weeks regained response after increasing the dose to 15mg/kg.1 Although there is less information available, similar figures have been reported with adalimumab: in the CHARM study, 45% of patients who lost response regained remission after increasing the dosage of adalimumab from 40mg each-other-week to 40mg weekly.9
There are both safety and economic concerns with this intensive treatment regimen in the long-term. Therefore, “desintensification” – the administration of the initial standard dose of anti-TNF – could be considered in patients who achieve remission with the “intensified” regimen. However, predictive factors of relapse after the “desintensification” of the treatment have not been identified. Therefore, it has not been established the group of patients that could benefit from the “desintensification” of the anti-TNF therapy. Furthermore, data on the durability of remission after “desintensification” of therapy are scarce.
The aim of the present study was to evaluate the long-term durability of remission after stepping down the anti-TNF treatment, in patients who achieved clinical remission after anti-TNF treatment “intensification”. Secondary aims were to identify predictive factors associated with loss of response to the “desintensified” treatment, and to evaluate the effectiveness of a subsequent anti-TNF dose “intensification” in patients who lose response after stepping down the treatment.
MethodsPatients who regained clinical remission after “intensification” of the anti-TNF therapy and were thereafter, “desintensificated”, were evaluated in a historical cohort study. Patients were excluded from the study if the anti-TNF drug had been initiated for the treatment of a disease other than CD, if the treatment had been “intensified” for a disease other than CD, if the treatment had been “desintensificated” due to the onset of adverse events with the “intensified” treatment.
Data collectionAs this was a retrospective study, clinical records of each patient were reviewed to obtain all data and multiple variables were recorded: sex, age, smoking status, age at diagnosis, location of disease and disease behaviour (inflammatory, stenosing or fistulizing), perianal disease, use of immunomodulators (start date, dosage, date of discontinuation, reason for discontinuation); indication of anti-TNF therapy, previous use of other anti-TNF drugs, start date, reason for “intensification”, date of “intensification”, “intensification” regimen of the current anti-TNF drug, response to “intensification” and dose and date of the last “intensification”; data regarding the “desintensificated” treatment included the reasons for discontinuation of “intensificated” therapy, clinical activity at the time of the “desintensification”, biochemical markers of inflammation at the time of “desintensification”, disease activity assessed by endoscopic or radiological explorations at the time of “desintensification” and the outcome after the “desintensification” (relapse of the disease, date of the relapse, treatment of the flare, response to a second “intensification” of the treatment).
DefinitionsDose “intensification”: Dose intensification of adalimumab was defined as a decrease in the interval of administration from every-other-week to every-week. In the case of infliximab, dose “intensification” was defined as an increase in infliximab dose, e.g., from 5 to 10mg/kg, a decrease in infliximab infusion interval, e.g., from every 8-week to every 4-week, or a combination of both.
Dose “desintensification”: A reduction in the anti-TNF dosage from the “intensified” regimen to the standard schedule: 5mg every 8 weeks in the case of infliximab and 40mg every-other-week in the case of adalimumab.
Evaluation of response: For luminal disease, response to adalimumab and infliximab was evaluated using the Harvey–Bradshaw index (HBI) at the time of the “desintensification” and, where necessary, four weeks after the second “intensification”. Partial response was defined as a decrease in the HBI of more than 3 points. Remission was defined as a HBI below or equal to 4 without steroids. In perianal CD, complete response was defined as closure of all fistulas and partial response as a 50% or more reduction in the number of draining fistulas.
Loss of efficacy: Loss of efficacy was defined as impairment in patient's symptoms coupled with endoscopic, radiographic, and/or serologic (elevated C-reactive protein) evidence of inflammation that forced the treating physician to intensify treatment schedule or change to another drug.
Disease behaviour and location: Disease behaviour was categorized based on Montreal classification as: (1) inflammatory (B1) or CD without; (2) stricturing (B2) when symptoms of partial or complete obstruction with fixed stenosis with or without proximal dilatation were present; and (3) fistulizing (B3) when internal fistulas, intra-abdominal abscesses or bowel perforation were detected. The location of disease was established by identifying macroscopic evidence of CD in any part of the gastrointestinal tract. Possible categories of disease location included the ileum (L1), colon (L2), ileum and colon (L3), upper gastrointestinal tract (L4), and perianal/perineal area (p).
Smoking history: Smoking was defined as consumption of at least 1 cigarette daily for a period of at least 3 months prior to the study entry.
Statistical methodsThe mean, median, and standard deviations were calculated for continuous variables. Percentages and 95% confidence intervals (95% CI) were provided for categorical variables. The Kaplan–Meier method was used to evaluate the long-term durability of remission after the “de-intensification”, and any differences between survival curves were evaluated with the log-rank test. Univariate analysis was performed in an attempt to investigate factors potentially associated with loss of response after the “de-intensification” of the treatment. In the log-rank test and in the univariate analysis, p<0.05 was considered the level of significance.
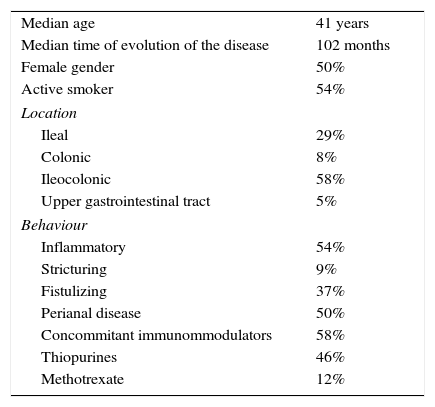
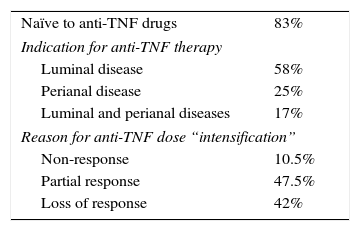
ResultsBaseline characteristicsA total of 24 CD patients from 8 referral centres were included. The main characteristics of the study patients are reported in Table 1. The median age was 41 years (range 26–56 years). Fifty percent of all patients were female and 15 (62.5%) were under infliximab treatment. Fourteen patients (58%) had ileocolic location, 13 (54%) inflammatory behaviour and 12 (50%) perianal disease. The treatment had been “intensified” due to partial response in 11 (47.5%), loss of response in 10 (42%) and primary non-response in 3 (10.5%) patients (Table 2).
Baseline characteristics of the patients.
| Median age | 41 years |
| Median time of evolution of the disease | 102 months |
| Female gender | 50% |
| Active smoker | 54% |
| Location | |
| Ileal | 29% |
| Colonic | 8% |
| Ileocolonic | 58% |
| Upper gastrointestinal tract | 5% |
| Behaviour | |
| Inflammatory | 54% |
| Stricturing | 9% |
| Fistulizing | 37% |
| Perianal disease | 50% |
| Concommitant immunommodulators | 58% |
| Thiopurines | 46% |
| Methotrexate | 12% |
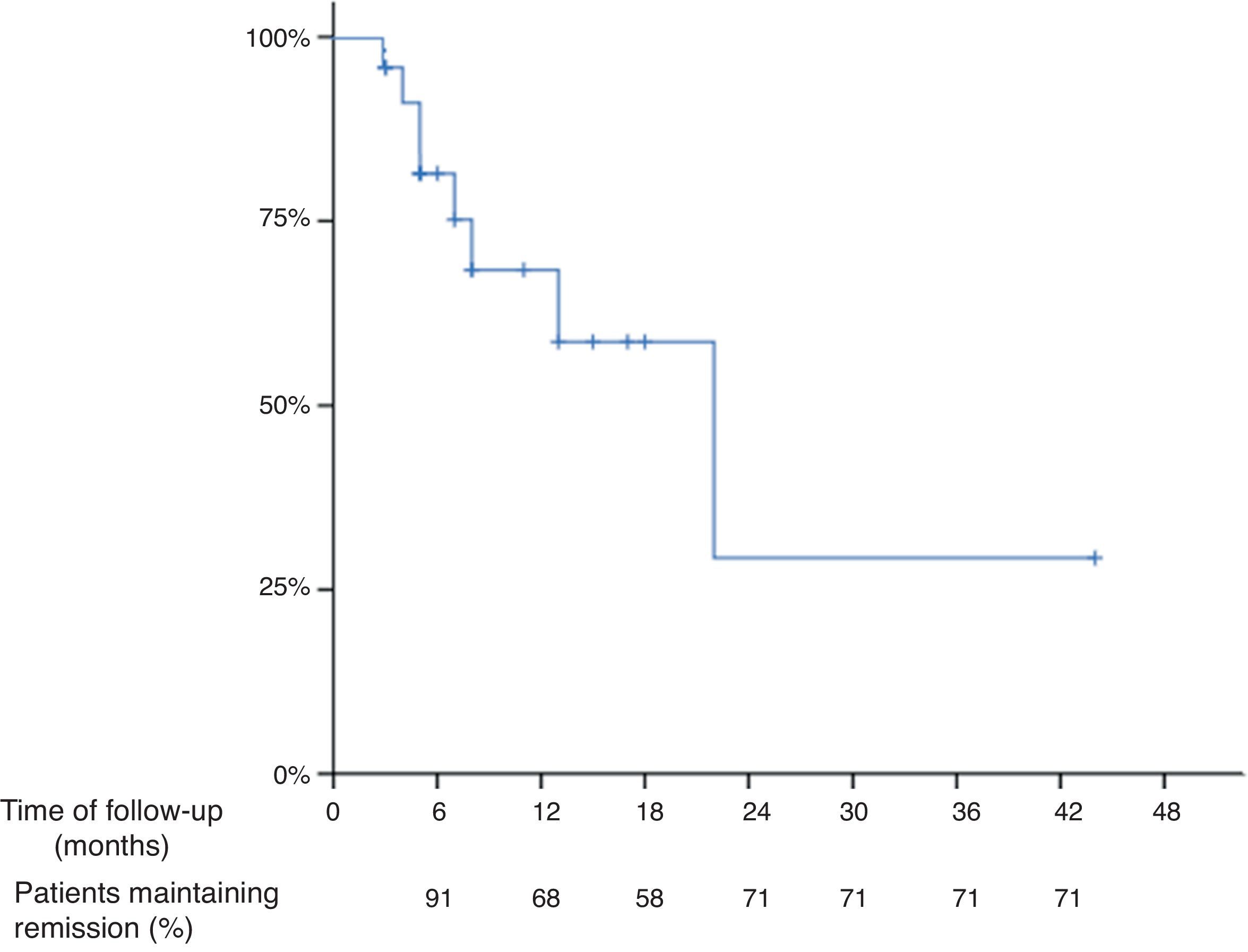
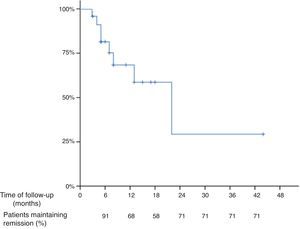
The mean time of follow up after the “de-intensification” of the treatment was 10 months (range 3–44 months) and the median was 7 months. Thirteen patients were followed-up at least for 7 months. Based on Kaplan–Meier survival estimates, 68%, 58% and 29% of all patients who “de-intensified” the treatment after being in remission with the “intensified” dosage maintained response at 12, 18 and 24 months, respectively (Fig. 1), of note that the number of patients at 18 and 24 months were low. The overall incidence of loss of response to the anti-TNF drug was 40% per patient-year of follow-up.
Factors predicting loss of response: univariate analysisNo statistical significance in loss of response was observed depending on the following variables: age at diagnosis, gender, time from CD diagnosis to first anti-TNF administration, disease behaviour, presence of perianal disease, current smoking habit and concomitant treatment with immunosuppressants. The indication for the anti-TNF therapy (luminal CD or perianal disease with or without luminal involvement) was not associated with a higher probability for relapsing after the “desintentification” of the treatment. However, having “intensificated” treatment due to perianal disease tended to be associated with a higher probability of losing response (p=0.06).
On the other hand, the time in remission before the “desintensification”, the clinical activity classified by the HBI score and biological markers of activity at the time of desintensification (such as haemoglobin, platelets, leucocytes, C-reactive protein, ferritin or erythrocyte sedimentation rate) were not associated with a higher probability of relapse after the “desintensification”.
Outcome after a new “intensification”Anti-TNF treatment was “intensificated” in 8 patients, who experienced a new flare after “desintensification”. Five patients were receiving infliximab and 3 adalimumab. Four patients had received “intensified” dose due to partial response, 3 due to loss of response and 1 had primary failure with the standard dose. From them, 4 had concommitant treatment with thiopurines and 1 with methotrexate. The indication for the anti-TNF treatment was luminal disease in 2 of them and both perianal and luminal disease in 2. After the new “intensified” dosage, 3 of them (37.5%) achieved remission again, 2 had only partial response and 3 did not respond.
DiscussionThe results of our study suggest that the risk of relapse is high (40% per-patient year of follow-up) after the “desintensification” of the anti-TNF treatment in CD patients who successfully “intensified” it due to primary failure, partial response or loss of efficacy.
Although anti-TNF drugs have demonstrated a high efficacy in the induction of remission in CD patients,1,3 a relevant proportion of patients need to “intensify” the treatment to maintain remission.5–7 “Desintensification” – the administration of the initial standard dose of anti-TNF – could be considered in patients who achieve remission with the “intensified” regimen. Arguments supporting the “desintensification” of the treatment are the theoretical higher risk of the potential adverse events associated to the anti-TNF therapy –such as infections and tumours – and the higher cost of the “intensified” treatment. On the other hand, there is a risk of relapse after the “desintensification” of the treatment. However, data of the durability of the remission after the “desintensification” of the therapy are scarce.
There are only 2 other studies published assessing the durability of response after the “desintensification”, one of them only in abstract form.10,11 The study by Karmiris et al. included 20 CD patients under “intensification” adalimumab treatment (40mg weekly) due to loss of response to the standard dose.10 The adalimumab dose was “desintensificated” in all of them after achieving remission with the “intensificated” treatment. The treatment had to be “intensificated” again in 35% of patients due to relapse with the standard dose. Authors did not report the effectiveness of the new “intensification”. Predictive factors of relapse after the “desintensification” were not found by authors in this study.10 Baert et al. evaluated the durability of response after anti-TNF “desintensification” in a cohort of 75 patients under adalimumab “intensified” treatment.11 The dose of adalimumab was “desintensificated” after being in remission. Thirty-eight percent of patients relapsed during the follow-up. Those authors found no factors predicting the success of desintensification, may be due to the small subgroups.11
It has been suggested a close relationship between trough levels of anti-TNF drugs and maintenance of response, and there have been described different factors that could decrease the anti-TNF serum levels.12,13 Antidrug antibodies can impede the clinical response by affecting the drug's bioavailability, being partly responsible of a greater clearance of the drug through the formation of immune complexes. On the other hand, some patients might have low serum levels of the drug without the presence of antibodies, suggesting a greater clearance of the drug not related to immunogenicity.12,13
We should acknowledge a limitation of our study that is the small sample size, which is at least in part due to the stringent inclusion criteria used. Furthermore, we believe that this fact represents what happens in clinical practice, where only a low proportion of patients in remission after the dose “intensification” change the treatment back to the standard dose. Nonetheless, our results are in agreement with those previously reported in the same scenario, showing that there is a high risk of loss of response after the “desintensification” of the anti-TNF treatment in CD patients.
Some authors have described that the patients who regain remission after the “intensification” of the anti-TNF treatment presented higher increment of the anti-TNF-α serum levels than patients who did not respond to this therapeutic strategy.14 However there have not been established the anti-TNF-α serum levels that could be considered therapeutic, as there seems to be a high variability among patients. Thus, nowadays the decision of “desintensificating” the dose of the anti-TNF treatment cannot be reliably based on the anti-TNF serum levels and deserves further study.
In addition, there is no clear evidence to support a clear benefit when maintaining anti-TNF therapy at a higher dose long-term. Neither the ACT nor the ACCENT studies detected that high-dose anti-TNF carried higher rates of side effects.
To decrease the risk of developing the adverse events associated with the anti-TNF therapy, such as infections of tumours, might be the main argument to support the “desintensification” strategy. In this respect, in the ACCENT I study, where CD patients were randomized to receive either placebo, infliximab 5 gm/kg or infliximab 10mg/kg, authors did not find differences in terms of adverse events, such as infections, tumours and infusion reactions, between the 3 groups of treatment.1,15 Same figure has been described with adalimumab. Thus, Colombel et al. pooled adalimumab safety data coming from the clinical trials in a recently published metaanalysis, showing that the risk of adverse events was similar between 40mg every-other-week and 40mg weekly groups.16 Therefore, nowadays there is no evidence that support the superiority of the treatment with the standard dose with respect to the “intensified” one in terms of safety.
The second argument supporting the “desintensification” of the dose is the drugs cost associated with the “intensified” regimen. There are not studies that compare the long-term comparative cost-effectiveness of both strategies. It is likely, however, that the savings in drug cost will be compensated by the increased hospital admissions and surgeries associated to the high rate of loss of response.
In conclusion, a relatively high proportion of patients relapse after the “desintensification” of the treatment even in the short-time. Furthermore, only one-third of patients who relapse after the “desintensification” of the treatment regain remission with a new dose “intensification”. As there is no evidence suggesting that “desintensification” may have safety benefits and its cost-effectiveness remains uncertain “desintensification” could not be recommended in CD patients who achieve remission after anti-TNF “intensification”.
Conflict of interestsDra. María Chaparro: conferencias, soporte para investigación y/o actividades formativas: MSD, AbbVie, Hospira, Dr. Falk Pharma.
Dr. Manuel Barreiro-de Acosta: Asesoramiento científico, soporte para investigación y/o actividades formativas: MSD, Abbvie, Hospira, Kern Pharma, Takeda, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceutical.
Dra. Esther García-Planella: Ninguno
Dr. Eugeni Domènech: asesoramiento científico, conferencias, soporte para investigación y/o actividades formativas: MSD, AbbVie, Takeda, Hospira, Kern Pharma, Ferring, Faes Farma, Shire Pharmaceutical, Chiesi, Otsuka Pharmaceuticals, y Gebro Pharma.
Dr. Fernando Bermejo: Conferencias y consultor MSD y AbbVie.
Dra. María Dolores Martín-Arranz: Consultora de Abbvie y MSD. Conferencias para Abbvie, MSD, Janssen, Shire.
Dr. David Monfort: Ninguno
Dr. Gisbert: conferencias, consultor, asesoramiento científico y becas de investigación de MSD, Abbvie, Hospira, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma.
Dr. María Chaparro: conferences, support for research and/or training activities: MSD, AbbVie, Hospira, Dr. Falk Pharma.
Dr. Manuel Barreiro-de Acosta: scientific advice, support for research and/or training activities: MSD, Abbvie, Hospira, Kern Pharma, Takeda, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceutical.
Dra. Esther García-Planella: None.
Dr. Eugeni Domènech: scientific advice, conferences, support for research and/or training activities: MSD, AbbVie, Takeda, Hospira, Kern Pharma, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Otsuka Pharmaceutical and Gebro Pharma.
Dr. Fernando Bermejo: conferences and MSD and AbbVie consultant.
Dra. María Dolores Martín-Arranz: Abbvie and MSD consultant. Conferences for Abbvie, MSD, Janssen, Shire.
Dr. David Monfort: None.
Dr. Gisbert: conferences, consultant, scientific advice and research grants for MSD, Abbvie, Hospira, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma.
CIBEREHD is funded by the Instituto de Salud Carlos III.