Pyoderma gangrenosum (PG) is the second most common extra-intestinal cutaneous manifestation of inflammatory bowel disease (IBD), accounting for 1–3% of cases. Although more common in ulcerative colitis (UC) (5–12%) than in Crohn disease (CD) (1–2%), 2 recent Italian and Spanish retrospective multicentre studies appear to suggest a change in trend.1,2 The peristomal variant (PGP) is undoubtedly one of the most feared complications affecting a surgical stoma, and while it is believed to occur in this location in 10–15% of all cases, it is probably underdiagnosed. Rapid detection of PG is essential in order to implement prompt therapeutic measures in a multidisciplinary setting. We report a difficult case that required unique management due to its severity and refractoriness to treatment, and because it occurred in the context of a recent neoplasm, which gave rise to a therapeutic dilemma.
The patient was a 43-year-old woman who had been diagnosed in 1993 with colonic (involving the rectum and left colon) and perianal CD according to standardised criteria (A2L2B1p). She commenced immunosuppression with azathioprine in 2000, due to corticosteroid dependence. However, owing to complications in the perianal disease (rectovaginal fistula plus several high transsphincteric fistulae) in 2004, infliximab was added at the beginning of 2005, following the usual induction and maintenance regimen; maximum escalation (shortening of the interval to 4 weeks) was required from the second year of treatment. The medical treatment was combined with multiple surgical interventions (abscess drainage, seton placement in fistula tracts and advancement flaps for the rectovaginal fistula), but the patient did not progress well, with severely limited quality of life, rectal stenosis and repeated perianal abscesses. Definitive terminal colostomy and left hemicolectomy with proctectomy was therefore proposed at the end of 2012 due to refractoriness to maximum medical and surgical treatment (colonoscopy with rectal stenosis biopsies in early 2012 showed no tumours). The patient initially refused to consider this option, preferring to exhaust all other alternatives and requesting inclusion in a clinical trial with intralesional stem cell injections. However, histological study of a lower rectum biopsy taken during a colonoscopy required as part of the inclusion protocol in the trial (June 2013) revealed adenocarcinoma.
At the end of July 2013, she underwent left hemicolectomy and abdominal–perineal resection, with construction of a definitive colostomy using the distal transverse colon, presenting 2 immediate postoperative complications: infection and partial dehiscence of the perineal and vaginal wall sutures. The surgical specimen showed a segment of large intestine severely affected by the CD, with extensive ulceration and transmural inflammation. At rectal level, there was a 2-cm moderately differentiated adenocarcinoma with a mucinous component of more than 50% that infiltrated the muscle layer without reaching the perirectal fat. Neither the surgical margins nor lymph nodes were affected (pT2N0MX). Abdominal-pelvic computed tomography (CT) did not reveal distant metastasis.
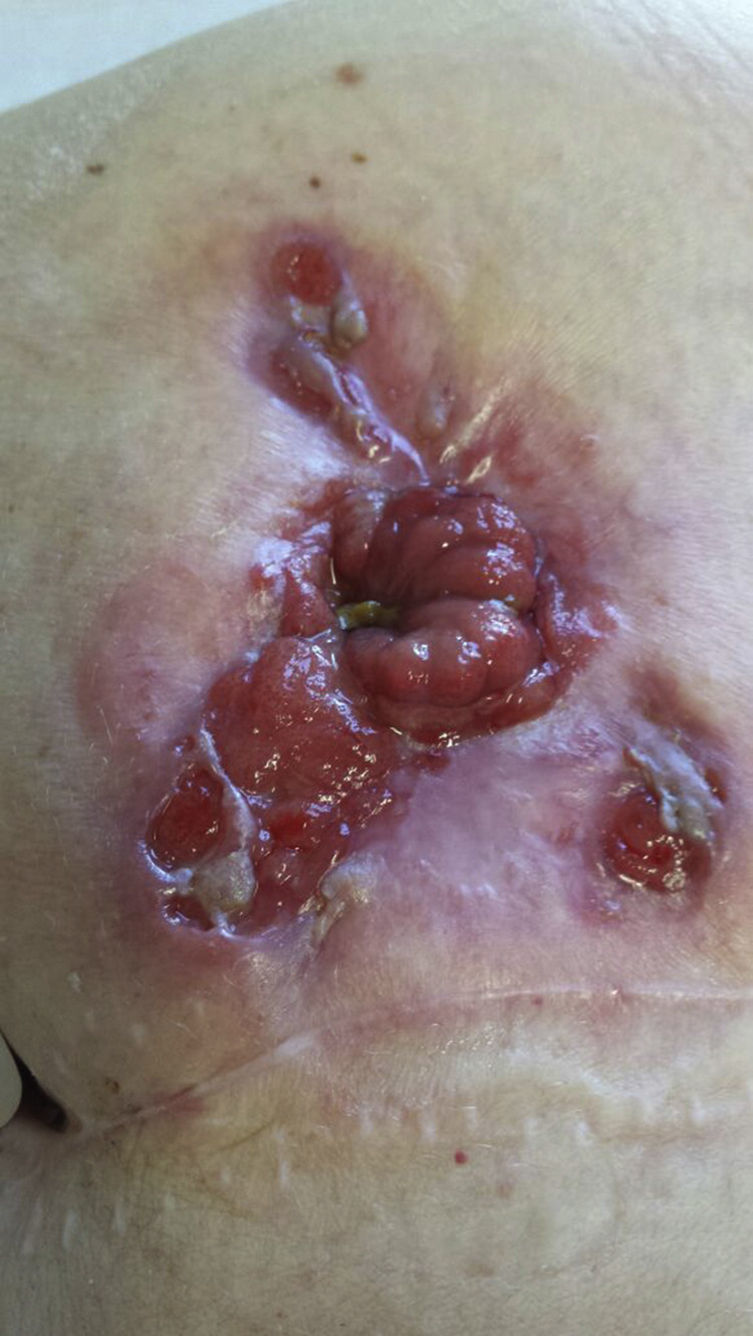
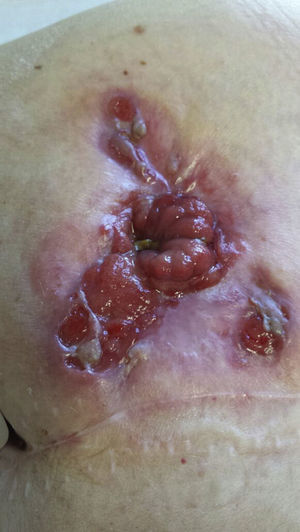
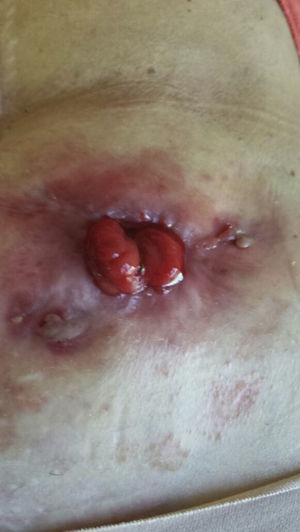
In September 2013, a large, ulcerated peristomal lesion with violaceous, anfractuous borders appeared, completely surrounding the stoma and causing intense local pain, retraction, and compromising its functionality, consistent with PGP (Fig. 1). Biopsies of the lesion were consistent with PGP, while cultures taken from the lesion were negative.
After joint evaluation by the dermatologist, surgeon, stoma care nurse and gastroenterologist, we decided on comprehensive and sequential management.
As a major part of the treatment, the patient's nutritional status was optimised and the pain caused by the pyoderma was controlled according to the World Health Organization pain ladder.
Local management in the stoma care unit consisted of cleaning the ulcers with sterile saline solution under local anaesthesia, application of antibacterial agents (hydrogen peroxide) and hydrocolloid absorbent dressings (carboxymethylcellulose and polyurethane film).
Local medical treatment was started in October 2013 with topical corticosteroids (0.05% clobetasol propionate, 2 applications/day); however, no improvement in the lesions was observed 2 weeks later, so the patient was switched to topical tacrolimus (0.3% ointment, 2 applications/day). After one month with no response, in which the patient was barely able to tolerate treatment, systemic therapy was initiated in December 2013 with oral corticosteroids at a dose of 1mg/kg/day for 1 month. As no clinical improvement was observed, in mid-January 2014, the patient started oral cyclosporine at a dose of 5mg/kg/day together with tapering of the corticosteroid regimen. In February 2014, following a very discrete, transient response of the pyoderma to oral cyclosporine treatment, biological treatment with infliximab was proposed as an option.
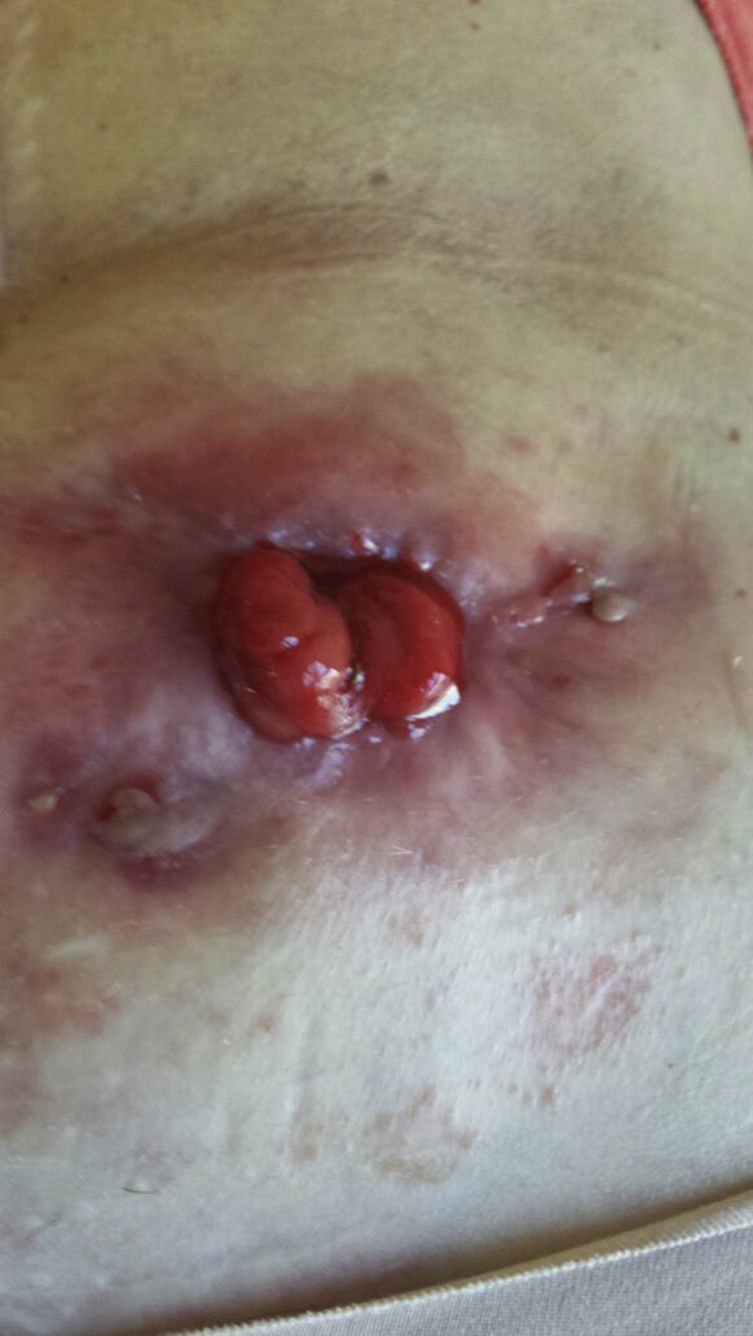
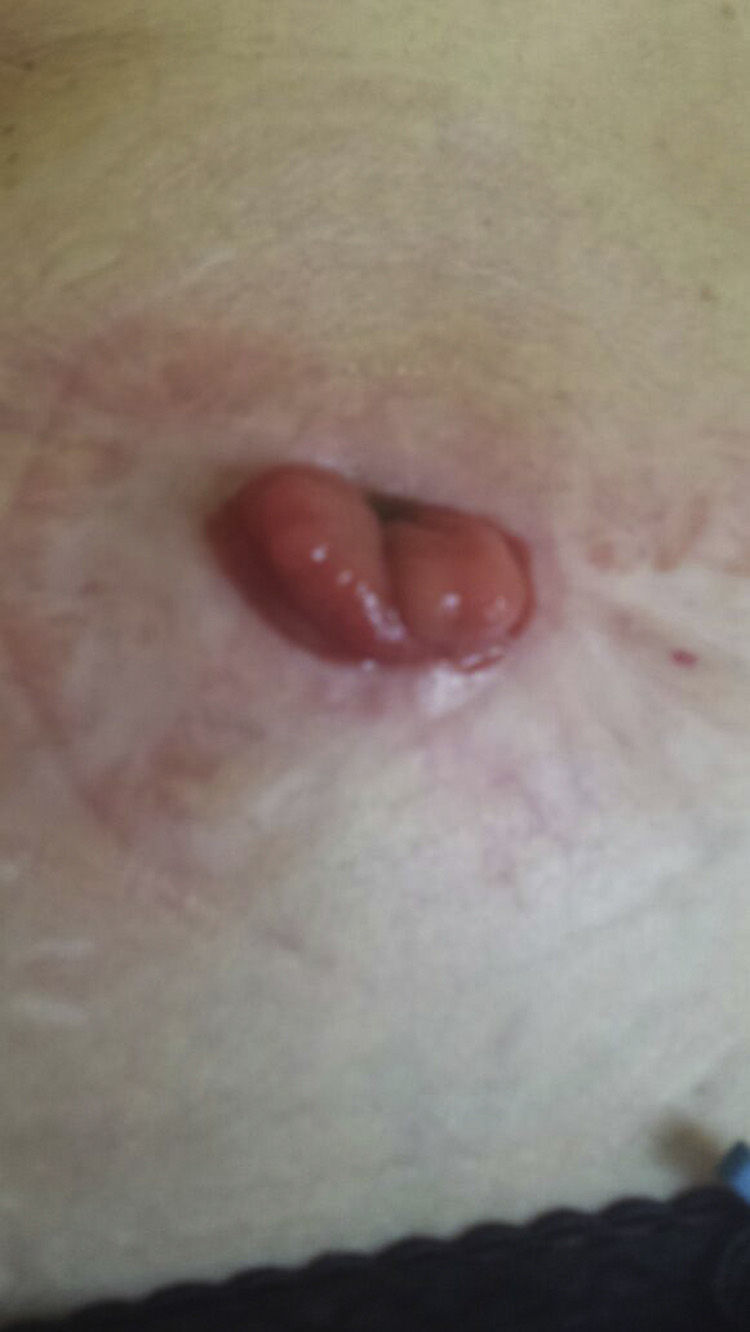
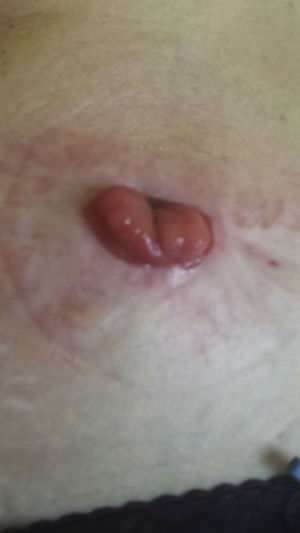
After reaching an agreement with the patient (severe deterioration in quality of life, major psychological impact and failure of the colostomy) and her oncologist (well-differentiated stage I rectal adenocarcinoma), we decided to administer infliximab treatment, following a previous updated anti-TNF-alpha study. The patient was informed of the potential possibility of recurrence of the cancer, but accepted the benefit–risk ratio. Infliximab was introduced at the usual dose of 5mg/kg according to the usual induction regimen (0, 2 and 6 weeks), observing gradual improvement of the lesion until it practically disappeared after the third infusion (Fig. 2). Maintenance treatment was then administered every 8 weeks for a further 3 months; 12 weeks after starting treatment, the biological treatment was discontinued and no lesions were observed (Fig. 3).
As mentioned in the introduction, PGP is a rare, disabling and often underdiagnosed complication that requires a multidisciplinary approach. It is estimated to affect 2–4.3% of patients with IBD in which a surgical stoma is constructed (compared to 0.6% for all-cause stomas). The aetiology of PGP is unclear, but an impaired immune response may be a fundamental factor. It affects women more than men, usually between the ages of 20 and 50 years. It appears more often in ileostomies (70%) compared to colostomies (30%), as in our patient. Its appearance follows 2 basic patterns: immediately after the creation of the stoma (within the first week, more typical of UC), and another more sub-acute form within an average of 2 months (more characteristic of CD).3,4
Although some authors suggest that a peristomal location is more common in CD than in UC, this is still widely debated.3,4
PGP is not unique to surgical stoma in the context of IBD, and cases have been described in neoplasms, diverticulitis or neurological dysfunctions.3,4 A recent case–control study with a small sample of patients with IBD found–within the limitations of the study–that risk factors for the appearance of PGP were high body mass index, female gender and other concurrent autoimmune disorders.5 In our case, the patient had 2 of these factors: CD and female gender.
Diagnosis is essentially clinical (initially pustular peristomal lesions that ulcerate and become painful, with anfractuous erythematous–violaceous borders and with necrotic bases that tend to merge together). Histology is not essential but is recommended to rule out other entities, and is characterised by massive neutrophil infiltration with no microbiological infection.
Treatment should be started promptly after diagnosis, and includes general (control of pain and nutritional status, and correction of any existing anaemia), local, and systemic management, and should be multidisciplinary, involving the gastroenterologist, dermatologist, surgeon and stoma care nurse.
The local aspect of treatment ranges from cleaning and debridement of ulcerated lesions, application of suitable collection devices and creation of an environment favourable to the local treatment chosen.
Topical corticosteroids are the most widely used local treatment, and are administered by intra-lesional injections or occlusive dressings. Topical tacrolimus appears to be superior to corticosteroids in larger, more aggressive PGP. In our case, however, the intense pain experienced by the patient on application hindered its use. Relocating the stoma is not recommended, except in very rare cases, due to the high risk of reappearance.
Systemic treatment should be considered in the case of refractoriness to local and topical therapies, rapidly progressive and aggressive lesions, or activity of the underlying disease. No high-quality, prospective studies have been conducted to recommend guidelines for systemic treatment. Studies have shown systemic steroids to be the most consistently effective treatment in this context. Doses of 1mg of prednisone/kg/day are generally used, with dose reduction every 2 weeks. In the event of unacceptable side effects, corticosteroid dependency or refractoriness to treatment, second-line agents should be used. The most widely used alternative in the case of refractoriness to steroids is cyclosporine at doses of 3–5mg/kg/day for 1 month, having demonstrated its effectiveness in isolated cases and small case series. Other immunosuppressants, such as azathioprine, mycophenolate mofetil, dapsone, minocycline, methotrexate and thalidomide have been used in the treatment of PGP, either as steroid-sparing therapy or as monotherapy, with variable efficacy.6–9
Finally, biological therapies have been added to the therapeutic arsenal of this entity (mostly anti-TNF: infliximab, adalimumab, certolizumab and etanercept), and anecdotally, ustekinumab (anti-IL12 and anti-IL23) has been reported to be effective.6–9
Two factors coincided in our patient: the aggressiveness of the peristomal pyoderma with significant limitations in quality of life and disabling chronic pain, and the recently diagnosed and treated colorectal tumour. For this reason we chose a sequential approach in an attempt to avoid biological treatment until local treatment, systemic corticosteroids and an immunosuppressant (cyclosporine) had proved ineffective. There is scant data on the safety window until re-introduction of a biological treatment in solid tumours. Some societies suggest 10 disease-free years.10 Similarly, and in cases where there are no other therapeutic alternatives, there is no quality evidence on the use of the biologic as monotherapy or combined with an immunosuppressant. In our case, given the short window period from diagnosis of the tumour until introduction of the biologic, and the speed of response, the possibility of combining infliximab with an immunomodulator did not arise.
Please cite this article as: Olmedo Martín RV, Amo Trillo V, López Ortega S, Jiménez Pérez M. Pioderma gangrenoso periestomal tras adenocarcinoma de recto en el contexto de enfermedad de Crohn de localización colónica y perianal compleja. Gastroenterol Hepatol. 2016;39:338–341.