An independent meta-analysis of randomized comparative trials of peginterferons alfa-2a and alfa-2b, both combined with ribavirin, analyzed the probability of achieving a sustained virological response (SVR).
ObjectiveTo estimate the long-term cost-effectiveness of treatment of patients with chronic hepatitis C with peginterferon alfa-2a (180μg/week) plus ribavirin (800–1200mg/day) vs. alfa-2b (1.5μg/kg/week) plus ribavirin (800–1400mg/day), from the perspective of the Spanish National Health System.
MethodsA Markov model was developed with 7 health states to simulate lifetime disease progression. SVR was calculated from the meta-analysis data. Transition probabilities and health state utilities were obtained from published literature. Direct healthcare costs were obtained from the drug catalog, while costs of disease-related complications were obtained from published studies and healthcare cost database. Costs were expressed in 2010€. The annual discount rate applied was 3.5% for both costs and benefits.
ResultsSVR rate for treatment with alfa-2a was higher than with alfa-2b; the differences were 6.0%, 7.6% and 8.7% for all genotypes, genotypes 1/4 and genotypes 2/3, respectively. Each patient would gain 0.469, 0.600 and 0.685 life-years and 0.155, 0.198 and 0.227 quality-adjusted life-years with alfa-2a vs. alfa-2b, for the respective genotypes. The cost saving per patient treated with alfa-2a would be €705, €672 and €1900, for all genotypes and for genotypes 1/4 and 2/3, respectively, alfa-2a being dominant.
ConclusionsAccording to the present model, treatment of patients with chronic hepatitis C with peginterferon alfa-2a is cost-effective compared with peginterferon alfa-2b, both combined with ribavirin.
se analizó las probabilidades de respuesta virológica sostenida (RVS) a través de un metaanálisis independiente que comprendía ensayos comparativos aleatorizados con interferón pegilado alfa-2a y alfa-2b, ambos en combinación con ribavirina.
Objetivoestimar la relación coste-efectividad del tratamiento a largo plazo de pacientes con hepatitis crónica C mediante interferón pegilado alfa-2a (1180μg/semana) más ribavirina (800–1200mg/día) frente al interferón pegilado alfa-2b (1,5μg/kg/kg/semana) más ribavirina (800–1400mg/día), desde la perspectiva del sistema nacional sanitario español.
Métodosse desarrolló un modelo de Markov con 7 estados de salud para simular la evolución de la enfermedad a lo largo de la vida. La SVR se calculó a partir de los datos del metaanálisis. Las probabilidades de transición y las utilidades de los estados de salud se obtuvieron de la literatura publicada. Los costes sanitarios directos se obtuvieron a partir del catálogo de medicamentos, mientras que los costes de las complicaciones relacionadas con la enfermedad se obtuvieron a partir de los estudios publicados y de la base de datos de costes sanitarios. Los costes se expresaron en euros de 2010. La tasa anual de descuento aplicada fue del 3,5% para los costes y los beneficios.
Resultadosla tasa de RVS para el tratamiento con alfa-2a fue superior que con alfa-2b; las diferencias fueron del 6,0%, 7,6% y 8,7% para todos los genotipos, genotipos 1/4 y genotipos 2/3, respectivamente. Cada paciente ganaría 0,469, 0,600 y 0,685 años/vida y 0,155, 0,198 y 0,227 años/vida ajustados por calidad con alfa-2a respecto a alfa-2b, para los respectivos genotipos. El ahorro en costes por paciente tratado con alfa-2a sería de €705, €672 y €1900, para todos los genotipos y genotipos 1/4 y 2/3, respectivamente, siendo alfa-2a dominante.
Conclusionesde acuerdo con el modelo presentado, el tratamiento de pacientes con hepatitis C crónica con interferón pegilado alfa-2a presentan mejor relación coste-efectividad en comparación con el interferón pegilado alfa-2b, ambos combinados con la ribavirina.
Chronic infection with hepatitis C virus (HCV) is a worldwide public health problem affecting approximately 200 million people. The prevalence of antibodies against hepatitis C virus in Spain ranges from 1.6 to 2.6% of the population, so it can be estimated that there must be between 480,000 and 760,000 persons infected with HCV in this country.1 Some 55–85% of patients infected with HCV are unable to clear the virus and develop chronic hepatitis C (CHC) of varying severity, which progresses to cirrhosis and hepatocellular carcinoma in 20% and 1–4% of cases, respectively.2–5 Once the infection becomes chronic, spontaneous eradication is rare.6 Only administration of antiviral therapy is capable of achieving elimination of the infection, with a variable rate of efficacy. The goal of treatment is to achieve a sustained virological response (SVR), defined as absence of detectable VHC ribonucleic acid (RNA) in serum 24 weeks after the end of treatment.7
The last two decades have seen the development of hepatitis C treatment focusing on interferon alfa (IFN), owing to its antiviral, immunomodulatory and antifibrogenic activity. It was initially used in monotherapy and later combined with ribavirin.8
The pegylation process of the interferon molecule, consisting of the attachment of polyethylene glycol chains of variable size and morphology, was developed as a strategy to increase the efficacy by modifying the pharmacokinetic and pharmacodynamic properties of interferon.9 At present, there are two types of pegylated interferons on the market: peginterferon α-2a (Pegasys®, Hoffmann-LaRoche, Basel, Switzerland) and peginterferon α-2b (PegIntron®, Schering-Plough, Kenilworth, NJ, USA), with different characteristics in the pegylation process that confer different properties: mean absorption time (50h and 4.6h, respectively), volume of distribution (8–12L and 0.99L/kg), and clearance (94ml/h and 22ml/h/kg). The significance of these differences is beyond the mere efficacy evaluated as likelihood of achieving SVR, because each treatment regimen was recorded with different costs and dosing, which could mean differences between the costs and efficacy of each treatment regimen.
Recently, the results of a meta-analysis conducted by the Cochrane Hepato-Biliary Group have been published, on clinical trials directly comparing the efficacy of these two peginterferons in CHC.10 The main conclusion was that peginterferon alfa-2a was associated with a higher probability of achieving SVR (47% vs. 41% respectively; odds ratio 1.11, CI 95% 1.04–1.19; P=0.004). These differences in favor of peginterferon alfa-2a remained in subgroup analyses for genotypes 1/4 and 2/3.
Taking into account the differences in efficacy observed in the meta-analysis performed by the Cochrane group, the aim of the present study was to estimate the long-term cost-effectiveness of treating patients with CHC with peginterferon alfa-2a (180μg/week) plus ribavirin (800–1200mg/day) compared to peginterferon alfa-2b (1.5μg/kg/week) plus ribavirin (800–1400mg/day). Due to their clinical relevance, two additional secondary objectives were set: to estimate the cost-effectiveness of long-term antiviral treatment in two subgroups of patients (genotypes 1/4 and genotypes 2/3).
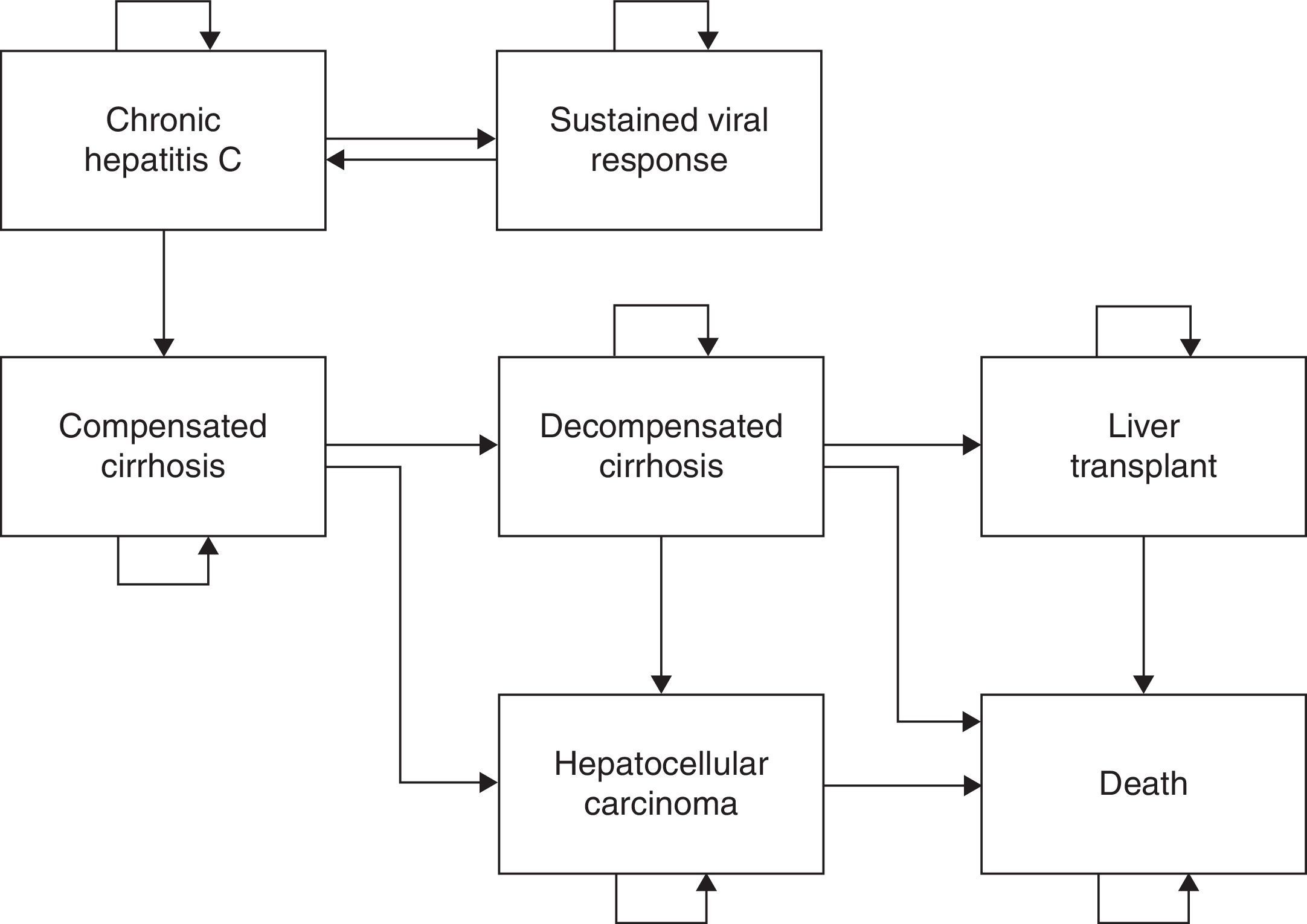
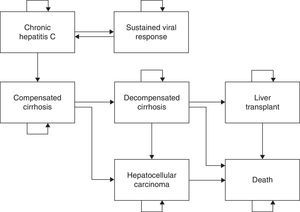
Materials and methodsStructure of the modelWe used a previously published Markov model with 7 states (Fig. 1) of CHC natural history2,11 to assess cost-effectiveness of peginterferon alfa-2a and alfa-2b containing regimen for treatment-naive patients. In brief, it was assumed that transitions between each Markov state would be made in discrete time periods called “cycles”, which had a duration of 1 year in the study.2,4,5 The timeframe of the simulation was up to 100 years of age (patients’ lifetime). The premises and assumptions of the model are summarized in Table 1. Annual progression rates were obtained from previously published studies on the natural history of the disease.2,12–19 The costs and benefits, measured as life year gained (LYG) and quality-adjusted life year (QALY), were calculated in the middle of each cycle.
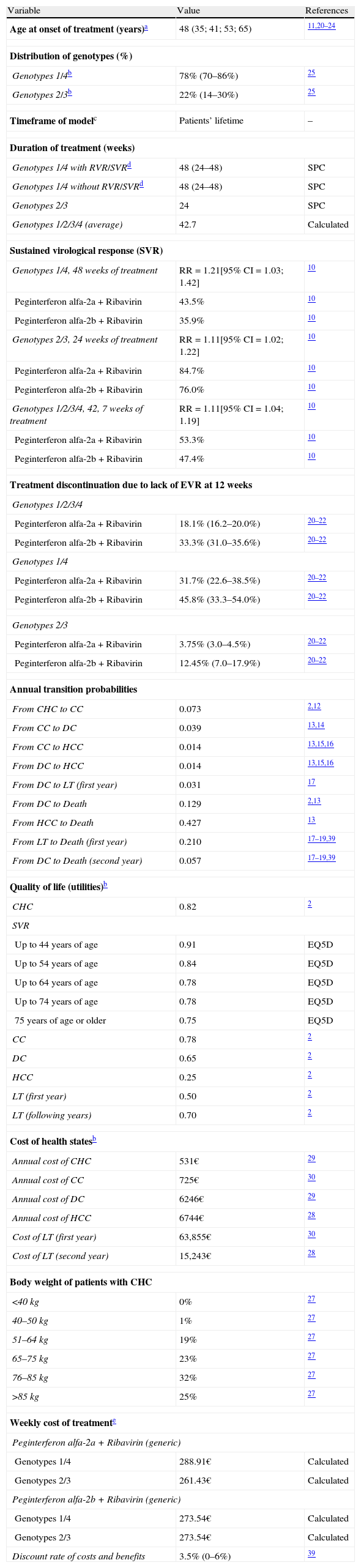
Main premises and estimations considered in the pharmacoeconomic model of treatment of patients with chronic hepatitis C with peginterferon alfa-2a plus ribavirin or peginterferon alfa-2b plus ribavirin.
| Variable | Value | References |
| Age at onset of treatment (years)a | 48 (35; 41; 53; 65) | 11,20–24 |
| Distribution of genotypes (%) | ||
| Genotypes 1/4b | 78% (70–86%) | 25 |
| Genotypes 2/3b | 22% (14–30%) | 25 |
| Timeframe of modelc | Patients’ lifetime | – |
| Duration of treatment (weeks) | ||
| Genotypes 1/4 with RVR/SVRd | 48 (24–48) | SPC |
| Genotypes 1/4 without RVR/SVRd | 48 (24–48) | SPC |
| Genotypes 2/3 | 24 | SPC |
| Genotypes 1/2/3/4 (average) | 42.7 | Calculated |
| Sustained virological response (SVR) | ||
| Genotypes 1/4, 48 weeks of treatment | RR=1.21[95% CI=1.03; 1.42] | 10 |
| Peginterferon alfa-2a+Ribavirin | 43.5% | 10 |
| Peginterferon alfa-2b+Ribavirin | 35.9% | 10 |
| Genotypes 2/3, 24 weeks of treatment | RR=1.11[95% CI=1.02; 1.22] | 10 |
| Peginterferon alfa-2a+Ribavirin | 84.7% | 10 |
| Peginterferon alfa-2b+Ribavirin | 76.0% | 10 |
| Genotypes 1/2/3/4, 42, 7 weeks of treatment | RR=1.11[95% CI=1.04; 1.19] | 10 |
| Peginterferon alfa-2a+Ribavirin | 53.3% | 10 |
| Peginterferon alfa-2b+Ribavirin | 47.4% | 10 |
| Treatment discontinuation due to lack of EVR at 12 weeks | ||
| Genotypes 1/2/3/4 | ||
| Peginterferon alfa-2a+Ribavirin | 18.1% (16.2–20.0%) | 20–22 |
| Peginterferon alfa-2b+Ribavirin | 33.3% (31.0–35.6%) | 20–22 |
| Genotypes 1/4 | ||
| Peginterferon alfa-2a+Ribavirin | 31.7% (22.6–38.5%) | 20–22 |
| Peginterferon alfa-2b+Ribavirin | 45.8% (33.3–54.0%) | 20–22 |
| Genotypes 2/3 | ||
| Peginterferon alfa-2a+Ribavirin | 3.75% (3.0–4.5%) | 20–22 |
| Peginterferon alfa-2b+Ribavirin | 12.45% (7.0–17.9%) | 20–22 |
| Annual transition probabilities | ||
| From CHC to CC | 0.073 | 2,12 |
| From CC to DC | 0.039 | 13,14 |
| From CC to HCC | 0.014 | 13,15,16 |
| From DC to HCC | 0.014 | 13,15,16 |
| From DC to LT (first year) | 0.031 | 17 |
| From DC to Death | 0.129 | 2,13 |
| From HCC to Death | 0.427 | 13 |
| From LT to Death (first year) | 0.210 | 17–19,39 |
| From DC to Death (second year) | 0.057 | 17–19,39 |
| Quality of life (utilities)b | ||
| CHC | 0.82 | 2 |
| SVR | ||
| Up to 44 years of age | 0.91 | EQ5D |
| Up to 54 years of age | 0.84 | EQ5D |
| Up to 64 years of age | 0.78 | EQ5D |
| Up to 74 years of age | 0.78 | EQ5D |
| 75 years of age or older | 0.75 | EQ5D |
| CC | 0.78 | 2 |
| DC | 0.65 | 2 |
| HCC | 0.25 | 2 |
| LT (first year) | 0.50 | 2 |
| LT (following years) | 0.70 | 2 |
| Cost of health statesb | ||
| Annual cost of CHC | 531€ | 29 |
| Annual cost of CC | 725€ | 30 |
| Annual cost of DC | 6246€ | 29 |
| Annual cost of HCC | 6744€ | 28 |
| Cost of LT (first year) | 63,855€ | 30 |
| Cost of LT (second year) | 15,243€ | 28 |
| Body weight of patients with CHC | ||
| <40kg | 0% | 27 |
| 40–50kg | 1% | 27 |
| 51–64kg | 19% | 27 |
| 65–75kg | 23% | 27 |
| 76–85kg | 32% | 27 |
| >85kg | 25% | 27 |
| Weekly cost of treatmente | ||
| Peginterferon alfa-2a+Ribavirin (generic) | ||
| Genotypes 1/4 | 288.91€ | Calculated |
| Genotypes 2/3 | 261.43€ | Calculated |
| Peginterferon alfa-2b+Ribavirin (generic) | ||
| Genotypes 1/4 | 273.54€ | Calculated |
| Genotypes 2/3 | 273.54€ | Calculated |
| Discount rate of costs and benefits | 3.5% (0–6%) | 39 |
Abbreviations: CC: compensated cirrhosis; CD: decompensated cirrhosis; EQ5D: EuroQol 5D; SPC: summary of product characteristics; CHC: chronic hepatitis C; HCC: hepatocellular carcinoma; 95% CI: 95% confidence interval; RR: relative risk; SVR: sustained virological response; RVR: rapid virological response; EVR: early virological response; LT: liver transplant.
Our base-case was a hypothetical cohort of Spanish adult CHC monoinfected patients of both sexes. An age at onset of treatment of 48 years was estimated in accordance with the onset age range of CHC observed in clinical trials comparing the efficacy of two peginterferons.20–24 Patients with HCV genotypes 1, 2, 3 and 4 were considered in the model. The percentage distribution of HCV genotypes in the infected Spanish population shows a very marked predominance of genotype 1.1 In the economic model, it was considered that genotypes 1 and 4 are found in 78% (70–86%) of infected patients and that genotypes 2 and 3 are present in the remaining patients (22%; 14–30%), according to the distribution observed in patients in the study by Sánchez-Tapias et al.25
Estimation of utilitiesUtilities were measured as QALYs, where a QALY was one year of life multiplied by a weighting factor that indicated the quality of life of the person during that year. The utilities of the 7 Markov states were obtained from the study by Kim et al.,2 and those corresponding to the SVR at different ages using the EQ 5D (EuroQol 5D) instrument (Table 1).26
Estimation of costsThe study was conducted from the perspective of the Spanish National Health System (NHS) and therefore only considering direct healthcare costs.
Two types of costs were considered: those related to antiviral therapy and those due to CHC and its complications (CC, DC, HCC and LT). Unit costs of the healthcare resources considered in the analysis are shown in Table 1. Cost of the antiviral drugs was estimated considering the doses of peginterferon alfa-2a (180μg/week) plus ribavirin (800, 1000 or 1200mg/day, for genotypes 2 and 3, or for a body weight <75 and ≥75kg in the case of genotypes 1 and 4, respectively) and the doses of peginterferon alfa-2b (1.5μg/kg/week) plus ribavirin (800–1400mg/day, according to body weight) recommended in the summary of product characteristics of each drug, as well as treatment duration (48 weeks for genotypes 1 and 4 and 24 weeks for genotypes 2 and 3) and the ex-factory price (EFP) of the drugs. The price of ribavirin was estimated for generic drugs. Distribution of body weights was obtained from the Spanish study by Buti et al.,27 in which the body weights of patients with CHC were recorded in the database of Hospital Valle de Hebrón (Barcelona). Unit costs of CHC and its complications were obtained from two Spanish studies28,29 and from a Spanish healthcare cost database (Table 1).30
The costs of the healthcare resources used in the model are presented in 2010 Euro (€)-values. Annual discount rates of 3.5% were applied to costs and benefits according to the recommendation of the UK's National Institute for Clinical Excellence (NICE).31
Cost-effectiveness analysisIn the meta-analysis by Awad et al., which compared the results of clinical trials comparing the two pegylated interferons, higher SVR rates were obtained at the end of treatment with peginterferon alfa-2a than with peginterferon alfa-2b, both combined with ribavirin.10 Consequently, a cost-effectiveness analysis was performed, expressing the results as cost per LYG and cost per QALY gained, assuming the effects of SVR on survival and quality of life of the patient.2,4,5
SVR rates were calculated after adjusting them to the weights of each clinical trial included in the meta-analysis by Awad et al.,10 where the “weight” of the study was equal to the inverse value of the variance obtained in the meta-analysis32; in this way, we obtained SVR rates of 53.3% and 47.4% considering all genotypes; 43.5% and 35.9% considering genotypes 1 and 4; and finally, 84.7% and 76.0% in patients with genotypes 2 and 3, with peginterferon alfa-2a and peginterferon alfa-2b, respectively (Table 1).
Sensitivity analysisAll mean estimations considered in the above sections constituted the base case of the study. To verify the stability of the results and consistency of the estimations made on the base case,33 simple univariate sensitivity analyses were performed in which extreme values of the following variables were taken: (i) patient age at the start of the simulation (35–65 years); (ii) proportion of HCV genotypes 1 and 4 with respect to genotypes 2 and 3 (70–86%); (iii) health state utility values (±10%); (iv) health state unit costs (±20%); (v) prices of non-generic ribavirin (Copegus in peginterferon alfa 2-a treatment and Rebetol in peginterferon alfa-2b treatment); (vi) patients treated with doses of 1.0μg/kg/week of peginterferon alfa-2b were excluded because its overall efficacy could be underestimated; (vii) annual discount rate of costs and benefits (0–6% according to the NICE recommendation)31; (viii) a threshold analysis was performed for treatment discontinuation rates at week 12; (ix) analyses were carried out using extreme values (±20%) of the transition probabilities between Markov states extracted from the literature.
Finally, probabilistic sensitivity analyses were also performed using Monte Carlo simulations.33
ResultsBase case analysisIn agreement with the meta-analysis by Awad et al.,10 sustained virological response (SVR) was higher with peginterferon alfa-2a than with peginterferon alfa-2b, with a relative risk of 1.11 [95% CI 1.04; 1.19], 1.21 [95% CI 1.03; 1.42] and 1.11 [95% CI 1.02; 1.22], for all genotypes, genotypes 1 and 4, and genotypes 2 and 3, respectively.
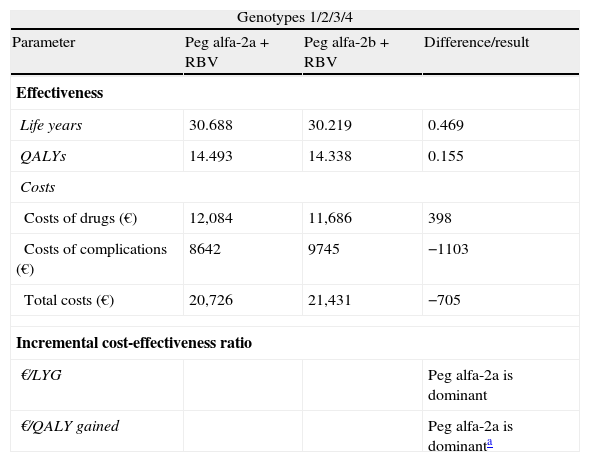
In a hypothetical cohort of patients with CHC (genotypes 1/2/3/4) followed over the patients’ lifetime, each patient treated with peginterferon alfa-2a (plus ribavirin) would gain 0.469 life years (LYG) and 0.155 quality-adjusted life years (QALY) compared to a patient treated with peginterferon alfa-2b (plus ribavirin), obtaining a cost savings of 705€ per patient. Treatment with peginterferon alfa-2a would be “dominant” over treatment with peginterferon alfa-2b, as the former is more effective than the latter, in addition to generating cost savings for the NHS (Table 2).
Cost-effectiveness of treatment of patients with chronic hepatitis C with peginterferon alfa-2a compared to peginterferon alfa-2b, both combined with ribavirin. Base case.
| Genotypes 1/2/3/4 | |||
| Parameter | Peg alfa-2a+RBV | Peg alfa-2b+RBV | Difference/result |
| Effectiveness | |||
| Life years | 30.688 | 30.219 | 0.469 |
| QALYs | 14.493 | 14.338 | 0.155 |
| Costs | |||
| Costs of drugs (€) | 12,084 | 11,686 | 398 |
| Costs of complications (€) | 8642 | 9745 | −1103 |
| Total costs (€) | 20,726 | 21,431 | −705 |
| Incremental cost-effectiveness ratio | |||
| €/LYG | Peg alfa-2a is dominant | ||
| €/QALY gained | Peg alfa-2a is dominanta | ||
| Genotypes 1/4 | |||
| Parameter | Peg alfa-2a+RBV | Peg alfa-2b+RBV | Difference/result |
| Effectiveness | |||
| Life years | 29.915 | 29.315 | 0.600 |
| QALYS | 14.238 | 14.039 | 0.198 |
| Costs | |||
| Costs of drugs (€) | 13,867 | 13,130 | 738 |
| Costs of complications (€) | 10,457 | 11,867 | −1410 |
| Total costs (€) | 24,325 | 24,997 | −672 |
| Incremental cost-effectiveness ratio | |||
| €/LYG | Peg alfa-2a is dominanta | ||
| €/QALY gained | Peg alfa-2a is dominanta | ||
| Genotypes 2/3 | |||
| Parameter | Peg alfa-2a+RBV | Peg alfa-2b+RBV | Difference/result |
| Effectiveness | |||
| Life years | 33.162 | 32.477 | 0.685 |
| QALYS | 15.311 | 15.085 | 0.227 |
| Costs | |||
| Costs of drugs (€) | 6274 | 6565 | −291 |
| Costs of complications (€) | 2830 | 4439 | −1609 |
| Total costs (€) | 9104 | 11,004 | −1900 |
| Incremental cost-effectiveness ratio | |||
| €/LYG | Peg alfa-2a is dominanta | ||
| €/QALY gained | Peg alfa-2a is dominanta | ||
QALY: quality-adjusted life year; LYG: life year gained; Peg: peginterferon; RBV: ribavirin.
Compared to peginterferon alfa-2b, treatment with peginterferon alfa-2a would obtain 0.600 LYG and 0.198 QALY gained per patient, with a cost savings of 672€, treatment with peginterferon alfa-2a also being dominant (Table 2).
Genotypes 2 and 3With respect to HCV genotypes 2/3, peginterferon alfa-2a would also be the dominant treatment, gaining 0.685 life years and 0.227 QALYs, with a cost saving of 1900€ per patient (Table 2).
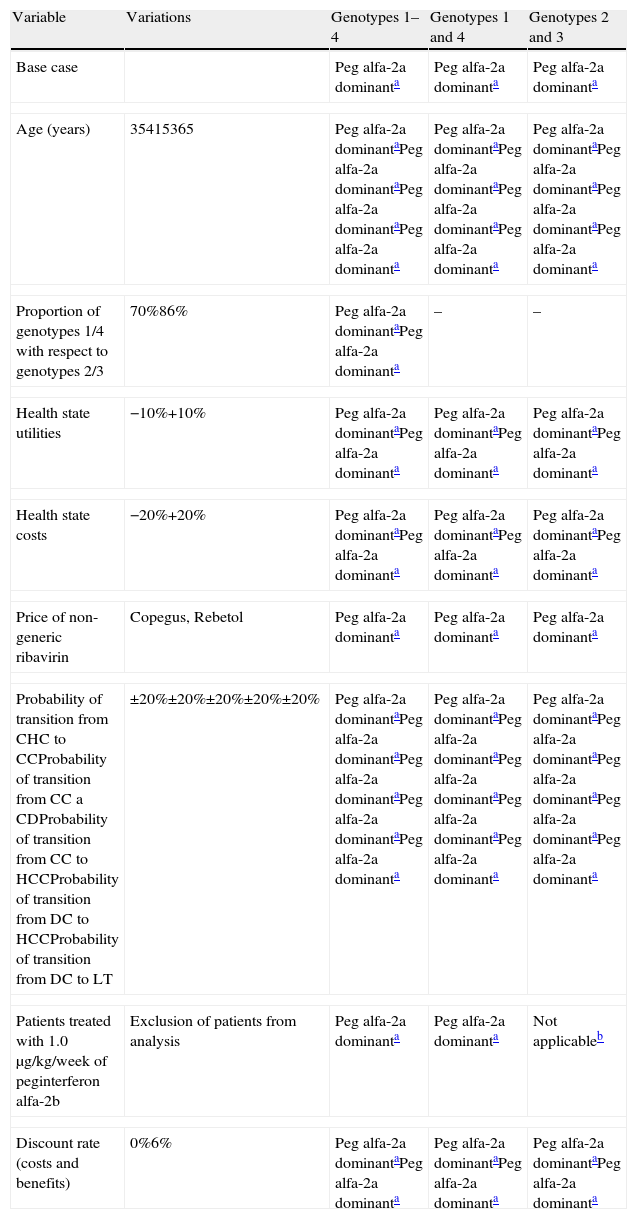
Sensitivity analysisIn all sensitivity analyses, the stability of the base case of the study was confirmed (Table 3), with peginterferon alfa-2a treatment being dominant in all the cases considered, with a 95% confidence interval in the case of the probabilistic analysis.
Simple univariate sensitivity analyses of cost-effectiveness of treatment of patients with chronic hepatitis C with peginterferon alfa-2a compared with peginterferon alfa-2b, both combined with ribavirin. Incremental cost-effectiveness per QALY (€).
| Variable | Variations | Genotypes 1–4 | Genotypes 1 and 4 | Genotypes 2 and 3 |
| Base case | Peg alfa-2a dominanta | Peg alfa-2a dominanta | Peg alfa-2a dominanta | |
| Age (years) | 35415365 | Peg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominanta |
| Proportion of genotypes 1/4 with respect to genotypes 2/3 | 70%86% | Peg alfa-2a dominantaPeg alfa-2a dominanta | – | – |
| Health state utilities | −10%+10% | Peg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominanta |
| Health state costs | −20%+20% | Peg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominanta |
| Price of non-generic ribavirin | Copegus, Rebetol | Peg alfa-2a dominanta | Peg alfa-2a dominanta | Peg alfa-2a dominanta |
| Probability of transition from CHC to CCProbability of transition from CC a CDProbability of transition from CC to HCCProbability of transition from DC to HCCProbability of transition from DC to LT | ±20%±20%±20%±20%±20% | Peg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominantaPeg alfa-2a dominanta |
| Patients treated with 1.0μg/kg/week of peginterferon alfa-2b | Exclusion of patients from analysis | Peg alfa-2a dominanta | Peg alfa-2a dominanta | Not applicableb |
| Discount rate (costs and benefits) | 0%6% | Peg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominanta | Peg alfa-2a dominantaPeg alfa-2a dominanta |
QALY: quality-adjusted life year; CC: compensated cirrhosis; DC: decompensated cirrhosis; CHC: chronic hepatitis C; HCC: hepatocellular carcinoma; Peg: peginterferon; LT: liver transplant.
A treatment is dominant when it is more effective and has fewer costs than the comparator treatment.
Doses of 1.0μg/kg/week of peginterferon alfa-2b were used only in the study by McHutchison6 in genotype-1 patients.
A threshold sensitivity analysis was carried out to determine the percentage of patients who should discontinue treatment in week 12 with peginterferon alfa-2b, so that peginterferon alfa-2a would not be a cost-effective alternative. An extreme theoretical scenario was thus considered in which none of the patients treated with peginterferon alfa-2a discontinued treatment at 12 weeks, and the percentage of patients treated with peginterferon alfa-2b who should discontinue treatment at week 12 was calculated, such that the incremental cost-effectiveness ratio would be greater than 30,000€/QALY gained. The result showed that the percentage of patients who would have to discontinue treatment at 12 weeks would be 61.2% and 67.3% of the patients treated with peginterferon alfa-2b, for all genotypes and for genotypes 1/4, respectively. In the case of genotypes 2/3, peginterferon alfa-2a was always dominant in this analysis.
DiscussionThe overall objective of the economic approach is to identify precisely those interventions or treatments that maximize results, while minimizing the opportunity cost incurred by the use of the limited resources available. In this sense, the treatment of hepatitis C is an area of great interest due to the relatively high prevalence of the disease, the costs associated with management and treatment of adverse effects, and also those derived from disease progression to cirrhosis and its complications. Different treatment strategies developed in recent decades have been analyzed in order to study their greater clinical efficacy (SVR), and also their economic impact.2,11,27,34,35
However, the absence of comparative studies between the two peginterferon alfa molecules has limited the efficacy assessment of these two alternatives for the treatment of hepatitis C, available from the beginning of the 2000s.
According to the results of the present model, treatment with peginterferon alfa-2a is cost-effective in patients with CHC compared to treatment with peginterferon alfa-2b, both combined with ribavirin, since it is more effective and has lower costs than peginterferon alfa-2b.
In the main analysis including all genotypes, it was estimated that, for each patient treated with peginterferon alfa-2a, 0.155 QALYs would be gained, saving 705€ per patient treated when compared to a patient treated with peginterferon alfa-2b. Secondary analyses of the two major treatment regimens based on HCV genotypes (48 weeks for genotypes 1 and 4, and 24 weeks for genotypes 2 and 3) show similar results. In patients with genotypes 1 and 4, it was estimated that 0.198 quality-adjusted life years (QALY) could be gained for each patient treated with peginterferon alfa-2a, achieving cost savings of 672€ per patient treated. Regarding patients with genotypes 2 and 3, the differences again favor the alternative of peginterferon alfa-2a, with a gain of 0.227 QALYs and cost savings of 1900€ per patient treated. It should be clarified that the numerical results of the incremental cost-effectiveness ratio are not expressed in Table 2 (it is only indicated that peginterferon alfa-2a is dominant over peginterferon alfa-2b), because they are negative results that do not provide useful or easily interpretable information, which is why the general recommendation is to report dominance rather than the value of this ratio.33,36
An important aspect when considering the relative cost-effectiveness of the two treatment regimens is the differential impact that the application of stopping rules due to futility of treatment at week 12 could have on this. However, the information from clinical trials comparing the treatment regimens peginterferon alfa-2a vs. alfa-2b is incomplete and its incorporation into the model is not possible. We decided to include a threshold sensitivity analysis, in order to estimate the percentage of therapies with peginterferon alfa-2b that should be discontinued at week 12 for peginterferon alfa-2a not to be cost-effective any longer. In this analysis it was assumed that all patients treated with peginterferon alfa-2a would complete 48 weeks of treatment. In patients with genotypes 1 and 4, 67.3% of patients treated with peginterferon alfa-2b would have to stop treatment at 12 weeks, a figure much higher than that observed in published studies,20,12,21,13,22,14 so it is unlikely that this aspect has any significant impact on our results.
When assessing the results of the study, we should take in account both its possible limitations and consistencies. First, it should be remembered that this is a theoretical model in which nonetheless an attempt has been made to reproduce clinical practice in our setting by incorporating data from Spanish studies. Unlike other previously published models, such as that of Salomon et al.,4 the present model did not consider the Markov states corresponding to progression of portal fibrosis prior to cirrhosis. Despite annual progression rates of fibrosis being different in men and women, this fact has no influence on the cost of the disease, since the healthcare costs are comparable in both genders.
The cost of peginterferon alfa-2b and ribavirin are determined by the weight of the patients, contrary to the practice with peginterferon alfa-2a. In this respect, it should be noted that distribution of body weights was obtained from the Spanish study by Buti et al.,27 in which the body weights of patients with CHC were recorded in the database of Hospital Valle de Hebrón (Barcelona).
The variables included in the model were obtained from the literature, not from an ad hoc study comparing the two peginterferons in Spanish patients. In an attempt to relieve this situation, these variables were subjected to probabilistic and deterministic sensitivity analyses, confirming the conclusions of the base case analysis in all cases.
As strengths of the model, the following aspects should be highlighted: (i) estimation of resource utilization, determined by efficacy of the treatments, was made from an independent meta-analysis of randomized clinical trials directly comparing the efficacy of both peginterferons10; (ii) estimation of transition probabilities in disease progression was made from a systematic review of the medical literature; (iii) all costs of healthcare resources were obtained from Spanish sources28–30; and (iv) in all of the scenarios analyzed, peginterferon alfa-2a was dominant over peginterferon alfa-2b (i.e., it was more effective and had lower costs) with a 95% confidence in the probabilistic analysis.
According to this analysis, the gain in QALYs for a patient would range from 0.155 to 0.227 (depending on the scenario considered); although the increase in QALYs obtained with peginterferon alfa-2a compared with peginterferon alfa-2b seems small, it is important to note that the systematic review by Wright et al.37 considered that a small gain in quality of life in patients with CHC would be 0.01 units per patient. In fact, the health technologies accepted by NICE obtain gains in QALY ranging between 0.03 and 0.05, for example, in the case of antidepressants, and 0.120 in the case of some antirheumatic drugs.38
In conclusion, the results of this pharmacoeconomic analysis indicate that the treatment of chronic hepatitis C monoinfected patients with peginterferon alfa-2a is cost-effective compared with peginterferon alfa-2a, when both are combined with ribavirin.
Conflict of interestJuan Turnes has served as a speaker, a consultant and an advisory board member for Roche, Janssen and MSD.
Manuel Romero-Gómez has served as a speaker, a consultant and an advisory board member for Roche, MSD, BMS, Janssen, Trugene, Abbott, and has received research funding from Gilead, Roche and MSD. Ramón Planas has served as a speaker, a consultant and an advisory board member for Roche, Janssen, Gilead, BMS, MSD, Novartis, and has received research funding from Roche, Gilead, BMS.
Ricard Solà has served as a speaker, a consultant and an advisory board member for Roche, Janssen, Gilead, BMS, MSD, Novartis, and has received research funding from Roche, Gilead, BMS and MSD. Javier García-Samaniego has served as a speaker, a consultant and an advisory board member for Roche, Janssen, Gilead, BMS, MSD, Novartis, and has received research funding from Roche and Gilead. Moises Diago has served as a speaker, a consultant and an advisory board member for Roche, Janssen, BMS, MSD, Abbott and has received research funding from MSD, Roche, Gilead, BMS, and Abbott. Javier Crespo has served as a speaker, a consultant and an advisory board member for Roche, Janssen, Gilead, MSD. Jose Luis Calleja has served as a speaker, a consultant and an advisory board member for MSD; Janssen, Gilead, and BMS, and has received research funding from Gilead and Roche. Carlos Rubio and Pere Ventanyol declare no conflicts of interest.
This study was conducted with a research grant from Roche Farma, SA.