Non-alcoholic fatty liver disease (NAFLD) is associated with poorer glycemic control and a higher risk of type-2 diabetes (T2D) complications, extrahepatic and cardiovascular disease (CVD). Our study aim was to evaluate the association between NAFLD, T2D complications, and the development of overall clinical events (OCE) (CV, liver-related, and mortality) in patients with T2D.
MethodsProspective single-center study comprising T2D subjects with no history of CVD and non-T2D matched controls. Patients were selected from the Outpatient Diabetes Clinic of Vall d’Hebron Hospital and related primary care centers.
Results186 diabetics and 57 controls were included. Amongst T2D, 124/186 subjects had NAFLD (66.6%). T2D-NAFLD subjects showed a heavier metabolic burden and higher median liver stiffness (5.6kPa [4.5–7.3] vs 4.8 [4.2–5.8]; p=0.004) compared to non-NAFLD diabetics. During a median follow-up of 5.6 years, 33 (17.7%) T2D patients developed OCE vs 4 (7.0%) controls (p=0.049). No differences were found for OCE between NAFLD and non-NAFLD diabetics (16.9% vs 19.4%; p=0.68). CV was the most reported outcome and only one liver event occurred. NAFLD diabetics showed more often chronic kidney disease (CKD), whereas T2D complications and subclinical CVD rates were similar. A higher liver stiffness, older age, and male gender were independently associated with OCE amongst the entire T2D population and NAFLD diabetics.
ConclusionsNAFLD and liver stiffness were associated with CKD and clinical outcomes in diabetics, respectively. A hepatic evaluation is recommended to identify high-risk T2D patients that would benefit from early referral to specialized care.
La esteatosis hepática metabólica (EHMet) se asocia con un peor control glucémico y un mayor riesgo de complicaciones de la diabetes tipo 2 (DM2), enfermedad extrahepática y cardiovascular (CV). El objetivo fue evaluar la asociación entre EHMet, complicaciones microvasculares y el desarrollo de eventos clínicos globales (ECG) (CV, hepáticos y mortalidad) en diabéticos.
MétodosEstudio unicéntrico prospectivo que incluye diabéticos sin historia de CV y controles sin DM2. Se seleccionaron pacientes de la consulta de Diabetes del Hospital Vall d’Hebron y centros de atención primaria asociados.
ResultadosSe incluyeron 186 diabéticos y 57 controles. Entre los diabéticos, 124/186 presentaron EHMet (66,6%). Los pacientes DM2 con EHMet presentaron mayor carga metabólica y una elasticidad hepática superior (5,6kPa [4,5-7,3] vs. 4,8 [4,2-5,8]; p=0,004) a los diabéticos sin EHMet. Durante una mediana de seguimiento de 5,6 años, 33 (17,7%) diabéticos desarrollaron ECG vs. 4 (7,0%) controles (p=0,049). No hubo diferencias en ECG entre diabéticos con y sin EHMet (16,9% vs. 19,4%; p=0,68). El evento más reportado fue CV y solamente se produjo un evento hepático. La enfermedad renal crónica (ERC) fue más frecuente en diabéticos con EHMet, mientras que los ratios de complicaciones microvasculares y enfermedad CV silente fueron similares. El género masculino, una mayor edad y elasticidad hepática se asociaron de forma independiente a ECG para el total de diabéticos y para aquellos con EHMet.
ConclusionesLa EHMet y la elasticidad hepática se asociaron a ERC y eventos clínicos en diabéticos. Se recomienda una evaluación hepática para identificar pacientes diabéticos de riesgo que se beneficiarían de una derivación precoz al especialista.
Non-alcoholic fatty liver disease (NAFLD) has become a major public health problem, affecting 25–30% of adults in Western countries.1,2 Both the prevalence and incidence of NAFLD have risen sharply in the last decades alongside the main drivers of the current metabolic syndrome epidemics; remarkably type 2 diabetes mellitus (T2D) and obesity.3
NAFLD and T2D frequently coexist and share a close bidirectional relationship.4 NAFLD prevalence rises by over 50% in T2D subjects, and patients with NAFLD are most likely to develop diabetes than non-NAFLD subjects.5 In addition, NAFLD has been shown to worsen the prognosis of T2D, including glycemic control, and microvascular complications, and increase the risk of developing extrahepatic complications, such as cardiovascular or chronic kidney disease (CKD).6–8 Indeed, macrovascular complications constitute the leading cause of death in NAFLD and T2D patients.9,10
The presence and severity of liver fibrosis are major determinants of the development of liver and extrahepatic events in NAFLD, as demonstrated in histological cohorts11,12 and reports on non-invasive estimates of fibrosis.13,14 Furthermore, the independent association between NAFLD and incident CKD has been underlined in a recent meta-analysis,15 being progression to advanced stages of CKD more pronounced in subjects with T2D and high-risk NAFLD fibrosis.16
Until recently,17 guidance on the diagnosis of NAFLD amongst T2D patients offered by international guidelines18 had poor discrimination.19 Diagnostic recommendations are often based on indirect suboptimal methods such as liver ultrasound or serum-based scores, which have been proven less sensitive than vibration-controlled transient elastography (VCTE) controlled attenuation parameter (CAP) for the detection of steatosis.20,21 Moreover, data from cardiovascular disease amongst NAFLD subjects comes from retrospective reports without concomitant liver stiffness measurements (LSM) or with small biopsy series, or from prospective studies with partial cardiovascular evaluation at baseline.22–27
The study aimed to evaluate the association between NAFLD, diabetes complications, and the risk of developing clinical events in a well-characterized prospective cohort of T2D patients with VCTE data.
Material and methodsPatients and settingWe conducted a single-center, prospective study comprising T2D subjects with no history of clinical CVD and non-diabetic controls matched by age.
Study subjects with T2D were recruited from the Outpatient Diabetic Clinic of Vall d’Hebron Hospital and the Primary Healthcare centers within its catchment area (North Barcelona). The non-diabetic controls came from the same Primary Healthcare centers and most were relatives of T2D patients. Participants were enrolled in the PRECISED study (NCT02248311) from September 2014 to June 2017. Follow-up data were collected until October 2021. The PRECISED dataset has served for observational studies assessing subclinical CV risk factors in T2D subjects.28,29
Inclusion criteria were: (1) age from 50 to 79 years; and (2) a history of T2D diagnosed at least one year before the screening according to ADA criteria.30
Exclusion criteria were: (a) medical history of a CV event; (b) type 1 diabetes; (c) any contraindication for the performance of positron-emission tomography/computed tomography (PET/CT) or magnetic resonance imaging (MRI); and (d) any concomitant disease associated with a short life expectancy.
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the local ethics committee: Comité Ético de Investigación Clínica del Hospital Universitario de Vall d’Hebron (Ethical Committee for Clinical Research of the Vall d’Hebron University Hospital) with reference number PR(AG)127/2014.
DefinitionsNAFLD was defined as the presence of steatosis assessed by VCTE as CAP≥275dB/m20,21 in patients with overweight/obesity, T2D, or evidence of metabolic dysregulation, except for patients with liver stiffness measurements (LSM)≥20kPa to avoid exclusion for “burn-out” NASH cirrhosis. Patients with suspicion of other liver etiologies (viral hepatitis, autoimmune diseases) and those with alcohol risk consumption (>30g/day in males and >20g/day in females) were considered non-NAFLD. Overweight and obesity were defined as a body mass index≥25kg/m2 and ≥30kg/m2, respectively. Diabetic nephropathy (DN) was defined as the presence of microalbuminuria>30mg/dL. According to KDIGO guidelines,31 we defined chronic kidney disease (CKD) as an estimated glomerular filtrate rate (eGFR)<60ml/min/1.73m2 and/or the presence of albuminuria>30mg/dL. Subclinical CVD was defined as the composite of coronary arterial calcium score (CACs)≥400AU, carotid plaque≥3mm, or carotid intima-media thickness (CIMT)>1mm.32,33 Cardiovascular events (CVE) encompassed acute coronary syndrome, stroke, or acute peripheral arterial syndrome. Liver-related events (LRE) included the development of ascites, hepatic encephalopathy, upper gastrointestinal bleeding secondary to portal hypertension, or hepatocellular carcinoma (HCC). The composite endpoint of overall clinical events (OCE) embedded CVE, LRE, and all-cause mortality. Organic damage was defined as the presence of diabetic microvascular complications (nephropathy, retinopathy, and/or peripheral neuropathy), chronic kidney diseas,e and/or subclinical CVD. Since controls are unable to develop T2D-related complications, we decided to analyze the presence of organic damage specifically amongst T2D patients.
OutcomesThe main study outcome was to assess the development of OCE during follow-up in the entire study cohort, with a special interest in T2D subjects overall and those diabetics with NAFLD. We also aimed to identify predictors of clinical outcomes in T2D patients.
As secondary outcome, we investigated the association between NAFLD and organic damage in T2D subjects.
ProceduresClinical data were obtained on the first visit by an endocrinologist. Anthropometric data were obtained by standardized protocols at the same visit. LSM and CAP values by VCTE were obtained from all participants at inclusion using Fibroscan 502 Touch devices (Echosens, Paris, France) equipped with M and XL probes. All measurements were performed by a specialized healthcare professional experienced with the procedure (≥ 500 examinations), using the probe recommended by the device for each patient. LSM values were considered reliable if an interquartile range/median (IQR/M) ratio<0.30 was achieved, and only examinations with at least 10 individual measurements were deemed valid.20 Of note, the median time from inclusion to VCTE was 4 days (IQR 2–10 days).
A detailed description of the rest of the procedures is provided in Annex 2 from supplementary material.
Statistical analysisCategorical data are presented as frequencies and percentages. Continuous data are presented as mean±standard deviation or median (interquartile range) when applicable. Differences among groups were assessed using the χ2 test for qualitative variables, while t-Student and non-parametric tests were used for quantitative variables with normal and non-normal distribution, respectively. Missing values were kept as missing, and no specific statistical procedures were used for imputations.
A Cox regression analysis was performed to identify risk factors associated with overall clinical events. The cumulative probability of developing clinical events was calculated using the Kaplan–Meier estimate and adjusted by predictors. For the analysis of risk factors associated with organic damage, a logistic regression model was applied. Only variables with p<0.1 in univariate analysis were included for multivariate modeling.
A p<0.05 was considered statistically significant. Data were collected and edited using Microsoft Excel (version Microsoft Office Pro 2019). Statistical analyses were performed using PAWS Statistics (version 19.0; SPSS Inc., Hong Kong) software.
The description of the sample size calculation for the PRECISED study is provided in the Supplementary material.
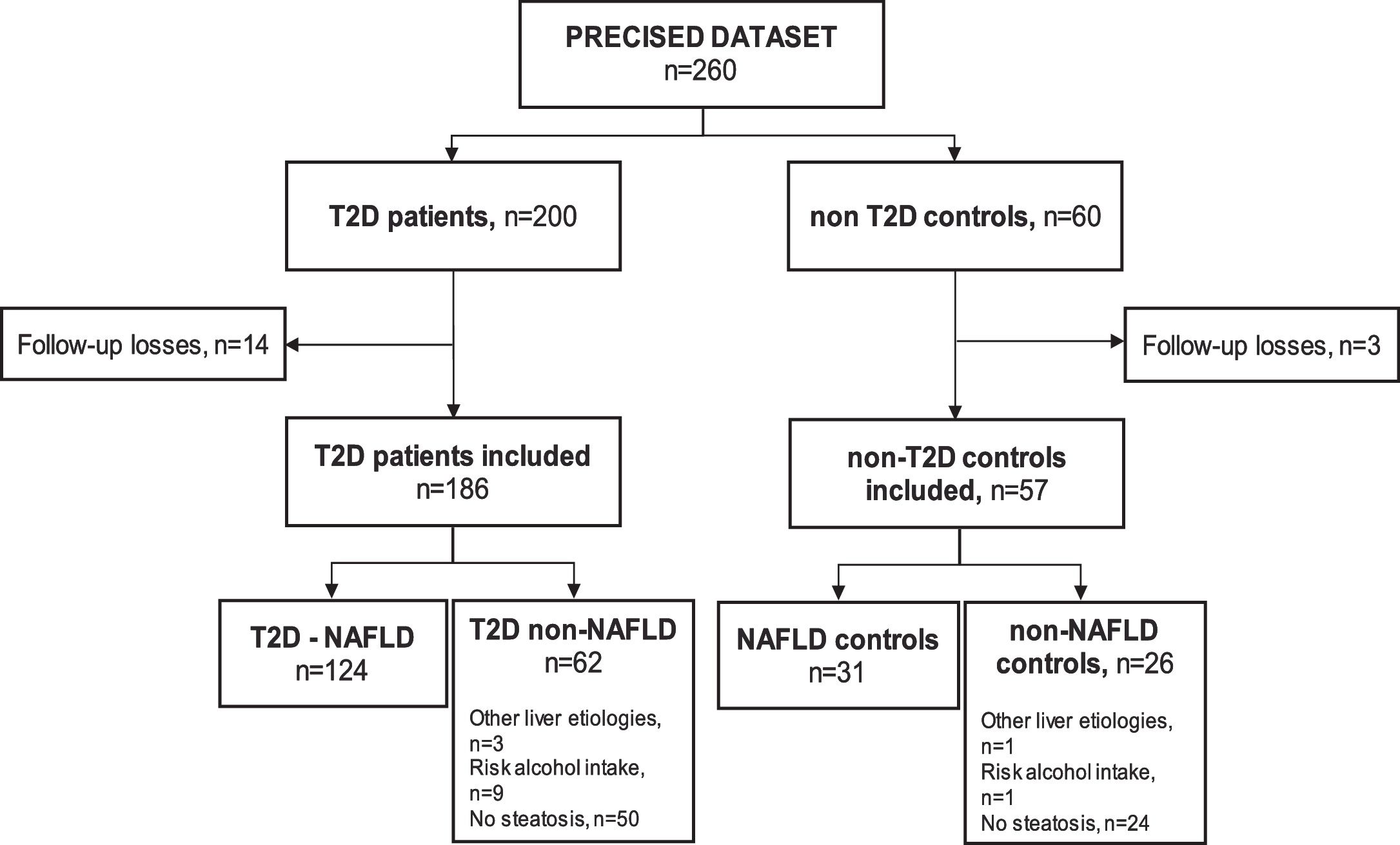
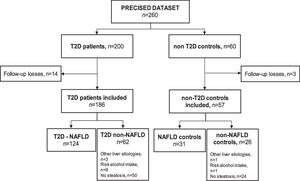
ResultsSampleSeventeen subjects (6.5%) out of the 260 subjects from the PRECISED cohort were excluded due to incomplete information at baseline or losses during follow-up. Out of the 243 remaining subjects included in the study, 186 (76.5%) were diabetic patients and 57 (23.5%) were non-T2D controls (Fig. 1).
Among T2D subjects, 66.6% (124/186) met the diagnostic criteria for NAFLD. Fifty patients out of the 62 T2D patients classified as non-NAFLD did not present liver steatosis, nine showed an alcohol risk intake at baseline, and 3 had a previous chronic liver disease different from NAFLD (HCV, HBV, and primary biliary cholangitis, respectively). Of the non-T2D control group, 54.4% (31/57) had NAFLD, one subject presented an alcohol risk consumption, another had a previous HCV cirrhosis, and 24 controls did not show liver steatosis.
The baseline features of the 186 diabetics and 57 controls included in the analysis are summarized in Supplementary Table 1.
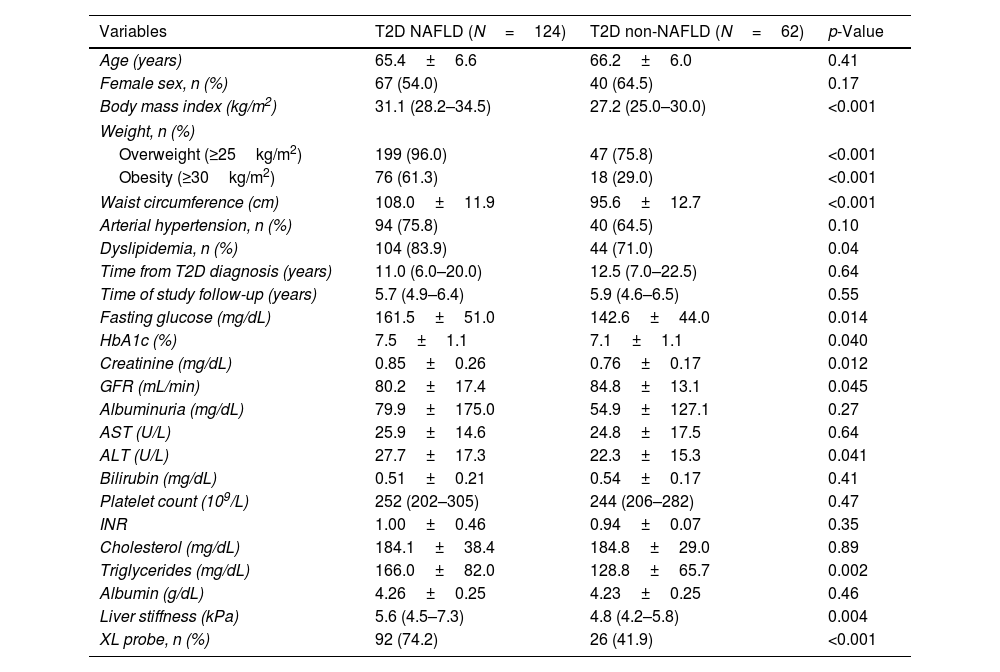
Baseline characteristics of T2D subjects with and without NAFLDThe main characteristics of NAFLD and non-NAFLD T2D patients are shown in Table 1. Female sex, mean age, and rates of arterial hypertension were similar between groups. NAFLD subjects showed higher rates of obesity (61.3% vs 29.0%; p<0.001) and dyslipidemia (83.9% vs 71.0%; p=0.04)
Baseline characteristics of T2D-NAFLD and non-NAFLD patients.
| Variables | T2D NAFLD (N=124) | T2D non-NAFLD (N=62) | p-Value |
|---|---|---|---|
| Age (years) | 65.4±6.6 | 66.2±6.0 | 0.41 |
| Female sex, n (%) | 67 (54.0) | 40 (64.5) | 0.17 |
| Body mass index (kg/m2) | 31.1 (28.2–34.5) | 27.2 (25.0–30.0) | <0.001 |
| Weight, n (%) | |||
| Overweight (≥25kg/m2) | 199 (96.0) | 47 (75.8) | <0.001 |
| Obesity (≥30kg/m2) | 76 (61.3) | 18 (29.0) | <0.001 |
| Waist circumference (cm) | 108.0±11.9 | 95.6±12.7 | <0.001 |
| Arterial hypertension, n (%) | 94 (75.8) | 40 (64.5) | 0.10 |
| Dyslipidemia, n (%) | 104 (83.9) | 44 (71.0) | 0.04 |
| Time from T2D diagnosis (years) | 11.0 (6.0–20.0) | 12.5 (7.0–22.5) | 0.64 |
| Time of study follow-up (years) | 5.7 (4.9–6.4) | 5.9 (4.6–6.5) | 0.55 |
| Fasting glucose (mg/dL) | 161.5±51.0 | 142.6±44.0 | 0.014 |
| HbA1c (%) | 7.5±1.1 | 7.1±1.1 | 0.040 |
| Creatinine (mg/dL) | 0.85±0.26 | 0.76±0.17 | 0.012 |
| GFR (mL/min) | 80.2±17.4 | 84.8±13.1 | 0.045 |
| Albuminuria (mg/dL) | 79.9±175.0 | 54.9±127.1 | 0.27 |
| AST (U/L) | 25.9±14.6 | 24.8±17.5 | 0.64 |
| ALT (U/L) | 27.7±17.3 | 22.3±15.3 | 0.041 |
| Bilirubin (mg/dL) | 0.51±0.21 | 0.54±0.17 | 0.41 |
| Platelet count (109/L) | 252 (202–305) | 244 (206–282) | 0.47 |
| INR | 1.00±0.46 | 0.94±0.07 | 0.35 |
| Cholesterol (mg/dL) | 184.1±38.4 | 184.8±29.0 | 0.89 |
| Triglycerides (mg/dL) | 166.0±82.0 | 128.8±65.7 | 0.002 |
| Albumin (g/dL) | 4.26±0.25 | 4.23±0.25 | 0.46 |
| Liver stiffness (kPa) | 5.6 (4.5–7.3) | 4.8 (4.2–5.8) | 0.004 |
| XL probe, n (%) | 92 (74.2) | 26 (41.9) | <0.001 |
Hypertension: ≥140/90mmHg or requiring treatment; dyslipidemia: serum triglycerides≥150mg/dL and/or total cholesterol>200mg/dL, LDL>130mg/dL, HDL<40mg/dL in men and <50mg/dL in women or requiring treatment.
In NAFLD subjects, mean alanine transaminase values (27.7±17.3 vs 22.3±15.3; p=0.041) and median LSM (5.6kPa vs 4.8kPa; p=0.004) were higher compared to non-NAFLD diabetic group. AST and hepatic function parameters, such as bilirubin or albumin, did not significantly differ between groups.
NAFLD patients showed worse glycemic control (mean glycosylated hemoglobin was 7.5±1.1% vs 7.1±1.1%; p=0.04) and received more often Glucagon-like Peptide-1 receptor analogs (GLP1-RAs) and insulin compared to those diabetics without NAFLD (Supplementary Table 2). Of note, median time from T2D diagnosis was similar between groups.
Development of clinical outcomesDuring a median follow-up time of 5.6 years (IQR 4.8–6.4 years), from the whole PRECISED cohort, 23 diabetic subjects (12.4%) developed a CV event compared to one event (1.8%) in the control group (p=0.019). Only one liver event was observed in a diabetic subject, and 8.1% of patients with T2D died compared to 5.3% in the control group (p=0.48). OCE were significantly higher in T2D subjects compared to controls (17.7% vs 7.0%; p=0.049) (Supplementary Table 3)
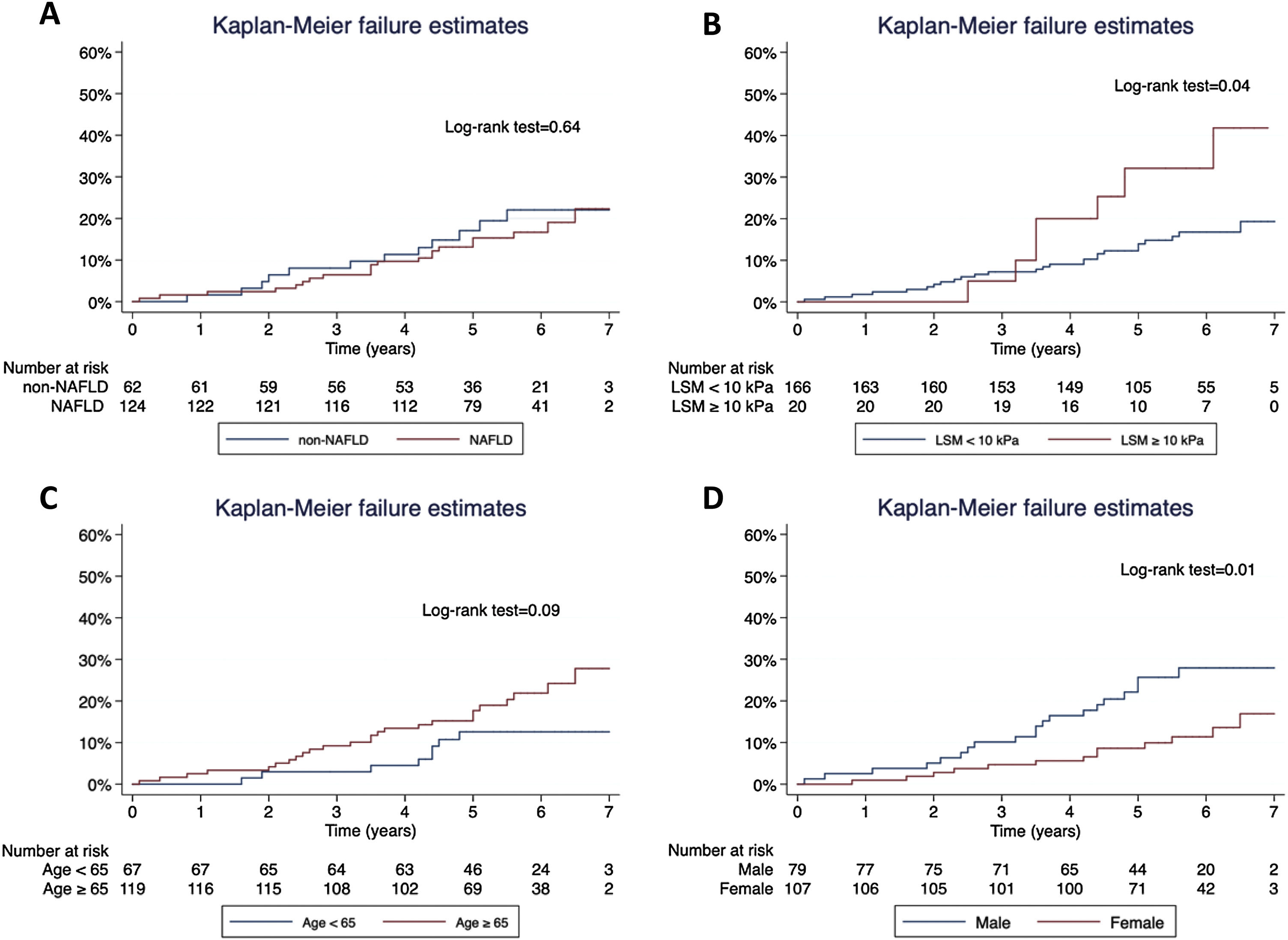
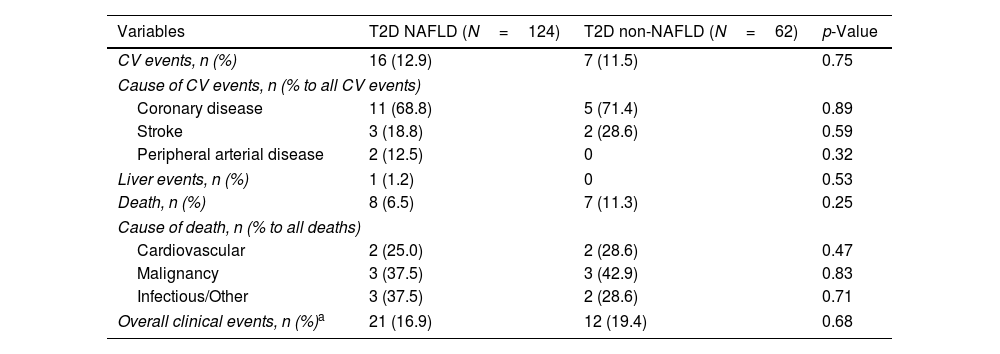
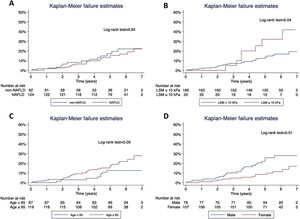
Concerning T2D subjects, we did not find significant differences in the development rate of cardiovascular outcomes between NAFLD and non-NAFLD patients (12.9% vs 11.5%; p=0.75). As shown in Table 2, acute coronary syndrome was the most frequently reported outcome amongst T2D (16/186). One subject with NAFLD presented a LRE (ascites) and 8 NAFLD patients (6.5%) died, 25% of which were due to CVD, with no differences compared to non-NAFLD subjects. Finally, the rate of OCE was similar between T2D patients with and without NAFLD (16.9% vs 19.4%; p=0.68). The occurrence of OCE amongst T2D patients during the study period according to NAFLD, liver stiffness, sex and age is depicted in Fig. 2. As shown, male patients and those with LSM≥10kPa developed significantly more OCE during the study period (long-rank test 0.01 and 0.04, respectively). No association was found for NAFLD or age in survival analysis. The comparison between T2D subjects according to the LSM 10kPa threshold is provided in Supplementary Table 4.
Clinical outcomes in diabetic patients.
| Variables | T2D NAFLD (N=124) | T2D non-NAFLD (N=62) | p-Value |
|---|---|---|---|
| CV events, n (%) | 16 (12.9) | 7 (11.5) | 0.75 |
| Cause of CV events, n (% to all CV events) | |||
| Coronary disease | 11 (68.8) | 5 (71.4) | 0.89 |
| Stroke | 3 (18.8) | 2 (28.6) | 0.59 |
| Peripheral arterial disease | 2 (12.5) | 0 | 0.32 |
| Liver events, n (%) | 1 (1.2) | 0 | 0.53 |
| Death, n (%) | 8 (6.5) | 7 (11.3) | 0.25 |
| Cause of death, n (% to all deaths) | |||
| Cardiovascular | 2 (25.0) | 2 (28.6) | 0.47 |
| Malignancy | 3 (37.5) | 3 (42.9) | 0.83 |
| Infectious/Other | 3 (37.5) | 2 (28.6) | 0.71 |
| Overall clinical events, n (%)a | 21 (16.9) | 12 (19.4) | 0.68 |
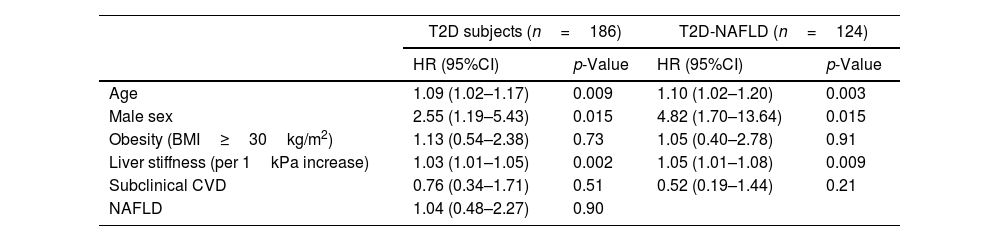
The analysis of predictors of clinical events is shown in Table 3. Identified risk factors for OCE amongst T2D patients globally and T2D subjects with NAFLD were male sex, older age, and increasing values of liver stiffness. However, NAFLD and subclinical CVD were not found to be significantly associated with the occurrence of OCE amongst the T2D population.
Risk factors associated with the development of clinical events.
| T2D subjects (n=186) | T2D-NAFLD (n=124) | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Age | 1.09 (1.02–1.17) | 0.009 | 1.10 (1.02–1.20) | 0.003 |
| Male sex | 2.55 (1.19–5.43) | 0.015 | 4.82 (1.70–13.64) | 0.015 |
| Obesity (BMI≥30kg/m2) | 1.13 (0.54–2.38) | 0.73 | 1.05 (0.40–2.78) | 0.91 |
| Liver stiffness (per 1kPa increase) | 1.03 (1.01–1.05) | 0.002 | 1.05 (1.01–1.08) | 0.009 |
| Subclinical CVD | 0.76 (0.34–1.71) | 0.51 | 0.52 (0.19–1.44) | 0.21 |
| NAFLD | 1.04 (0.48–2.27) | 0.90 | ||
The types and rates of T2D complications are shown in Table 4. No statistical differences were found for diabetic nephropathy, diabetic retinopathy, or peripheral neuropathy between T2D-NAFLD and non-NAFLD subjects.
Comparison of T2D-associated complications and organ damage between NAFLD and non-NAFLD subjects.
| Variables | T2D NAFLD (N=124) | T2D non-NAFLD (N=62) | p-Value |
|---|---|---|---|
| T2D microvascular complications, n (%) | |||
| Nephropathy | 46 (37.7) | 15 (24.6) | 0.07 |
| Retinopathy | 30 (24.2) | 20 (32.3) | 0.24 |
| Neuropathy | 22 (17.7) | 13 (21.0) | 0.59 |
| Any complication | 69 (55.6) | 29 (46.8) | 0.25 |
| Subclinical CV disease, n (%) | |||
| CACs>400AU | 28 (23.5) | 13 (22.4) | 0.86 |
| Carotid plaque≥3mm | 39 (34.8) | 18 (34.0) | 0.91 |
| CIMT>1mm | 14 (12.5) | 5 (9.4) | 0.56 |
| Composite endpoint | 55 (45.8) | 29 (49.2) | 0.67 |
| CKD, n (%) | 55 (45.1) | 15 (24.6) | 0.007 |
CACs, coronary arterial calcium score; CIMT, carotid intima-media thickness; CKD, chronic kidney disease.
Regarding cardiovascular surrogates, we did not find statistical differences between NAFLD and non-NAFLD subjects when analyzing the presence of each subclinical CVD condition separately (CACs>400AU, carotid plaque≥3mm, or CIMT>1mm) or the composite CVD endpoint.
However, patients with NAFLD showed a higher rate of CKD (45.1% vs 24.6%; p=0.007) than non-NAFLD subjects. Accordingly, NAFLD patients showed worse kidney function values assessed by creatinine and GFR than diabetic subjects without NAFLD, as seen in Table 1. In logistic regression multivariate analysis, the presence of NAFLD was associated with the existence of CKD (OR=2.29, 95%CI 1.07–4.87; p=0.031), independently of well-known risk factors, such as arterial hypertension, dyslipidemia or male sex (Supplementary Table 5).
DiscussionMajor findingsIn the present study, conducted in a prospective cohort of T2D patients matched with non-T2D controls without a history of CVD followed for a median time of 5.6 years, we evaluated the incidence of clinical events and the role of NAFLD in the development of outcomes and organic damage. First, we found that 66.6% of T2D had NAFLD compared to 54.4% of controls. Second, T2D subjects had a greater risk of developing clinical events compared to non-T2D subjects, particularly CV events. Third, liver stiffness measured by VCTE was found to be associated with an increased risk over time of developing clinical events in the T2D population overall and NAFLD diabetics as well. Finally, NAFLD was independently associated with chronic kidney disease in diabetics.
Clinical eventsAs primary outcome, we investigated the incidence of clinical events amongst the whole dataset and particularly in patients with T2D, NAFLD, and liver fibrosis, since these conditions have been associated with poorer prognosis and a major incidence of events, especially if they take place simultaneously.11–14
In our study occurrence of CVE and the probability to present any clinical event including both liver and cardiovascular events as well as all-cause mortality, was superior amongst T2D subjects compared to controls, as expected. However, diabetic patients with NAFLD did not present a higher risk over time to develop significantly more events than non-NAFLD T2D patients in our analysis, probably due to the relatively low number of clinical events, which is lower than previously described.10 This could be partially explained by the accurate control of T2D patients, which had a mean HbA1c<7.5% at inclusion and likely benefited from close surveillance for a highly specialized endocrinology team during the study period. Moreover, subclinical CVD prevalence in our cohort was slightly inferior to previously reported,26 decreasing the probability of event occurrence. Lastly, the small proportion of patients with advanced liver disease (2.4% from the whole PRECISED cohort presented a LSM≥20kPa) at inclusion, and the slow progression of NAFLD fibrosis would justify that only one patient presented a liver-related event.
Predictors of clinical eventsMale gender, older age, and liver stiffness were associated as risk factors for clinical outcomes amongst T2D subjects overall and in those diabetics with NAFLD specifically. Our results are consistent with previous data reporting the close association of gender, age, and T2D complications with the development of clinical events.11,12 Remarkably, liver fibrosis was found to be associated with clinical events, suggesting a leading role in the pathophysiology of cardiovascular disease, independently of well-known risk factors,34 whereas NAFLD was not found to be a risk factor to develop clinical events over time amongst diabetics. This does not come as a surprise, since it is well-known that the liver fibrosis stage is the most consistent risk factor for developing complications in liver disease patients, including CV complications and liver events including HCC, with VCTE being a useful tool to estimate advanced fibrosis and for predicting clinical outcomes.11–14,34 Meanwhile, available studies suggest that NAFLD with mild fibrosis is not overall associated with increased complications other than those related to coincidental comorbidities such as T2D since key pathological changes occur in the transition from F2 stage fibrosis to F3, which correlates with specific metabolic signatures.35 The presence of underlying significant fibrosis as a critical prognostic marker underlies current recommendations to identify patients in the community and primary care that should be referred to specialized care.20 Remarkably, Boursier et al. recently found that T2D patients are often not accurately classified by non-invasive tests including FIB-4 score and VCTE36 and therefore they should be closely followed, and non-invasive tests repeated more frequently than in other patients (i.e., yearly instead of each 2–3 years).
Organic damageAs secondary outcome, we aimed to investigate the influence of NAFLD in the existence of organic damage, since it has been consistently shown that NAFLD and T2D share a deleterious bidirectional relationship with systemic implications at cardiovascular and renal levels.4,5 In our study, the rates of subclinical CVD features and T2D microvascular complications were similar between NAFLD and non-NAFLD diabetic subjects. We found that NAFLD patients presented a higher chronic kidney disease ratio compared to those subjects without NAFLD. In addition, NAFLD was independently associated with the existence of CKD in multivariate analysis, along with common predictors such as arterial hypertension or dyslipidemia. The fact that T2D patients with NAFLD showed similar rates of diabetic nephropathy but a higher prevalence of CKD compared to non-NAFLD diabetics reinforces the hypothesis that NAFLD has a major specific impact on renal function.6,8,15,16
LimitationsOur study has several limitations that should be considered. First, it is a single-center study with a relatively low number of clinical events despite a long follow-up, which is determined by the sample size, the low rate of patients with advanced liver disease, and the good metabolic and low cardiovascular burden at baseline. Notwithstanding this, we firmly believe this scenario properly reflects real-world clinical practice, where diabetic patients from high-income countries are progressively most likely to receive new T2D drugs (i.e. SGLT2i and GLP1-RAs) with highly beneficial cardiovascular, renal and metabolic profiles, minimizing the risk of clinical events. Secondly, the diagnosis of NAFLD and fibrosis staging was not histologically back-tested. However, the present study is the first report from Southern Europe with basal CV assessment, prospective follow-up, and concomitant evaluation of NAFLD and liver fibrosis through VCTE, which has been proven to be the most accurate non-invasive tool for fibrosis and steatosis estimates. In addition, the CAP threshold has been chosen according to recent European clinical practice guidelines,20 which reported Sn/PPVs over 90% for the 275dB/m cutoff, which would be even greater in populations with a high prevalence of NAFLD such as diabetics. Lastly, almost all participants were Caucasian and socioeconomic data were not collected, whereas it is well known that the prevalence of NAFLD, metabolic syndrome determinants, and the risk of complications significantly differ across ethnicities and sociocultural and economic backgrounds, reflecting, on the other hand, the need for updated data for each geographical area.37–39
ConclusionIn our prospective controlled study of T2D patients with an accurate CV and metabolic evaluation, diabetic subjects had a higher risk of developing clinical events compared to non-diabetic controls. The presence of NAFLD and related liver fibrosis were strongly associated with the existence of kidney disease and the development of clinical outcomes amongst T2D patients, respectively. A dedicated evaluation of the presence and severity of the liver disease is mandatory in diabetic patients and should be implemented in primary and specialized pathways in order to identify high-risk patients that would benefit from close monitoring and early referral to diabetes units and liver clinics. This approach will be crucial for better T2D phenotyping and to optimize NAFLD trial inclusion or pharmacological treatment when available.
Authors’ contributionsStudy concept and design; JRE, MP, JMP, JG; Patient enrolment and acquisition of data; MP, AP, CH, MJS, AB, OSS, JB; Analysis and interpretation of data, JRE, RMN, AP, JMP, JG; Drafting of the manuscript, JRE, RMN, JMP; Critical revision of the manuscript: JRE, MP, AP, RMN, CH, OSS, MJS, JB, DS, AB, RS, IFG, JMP, JG.
Authorship statementJRE is the guarantor of the article. All the authors approved the final draft which is being submitted.
Informed consent statementInformed consent was obtained from all subjects involved in the study.
Patient consent for publicationNot required.
Data availability statementThe data supporting this study's findings are available from the corresponding author upon reasonable request.
Permission to reproduce material from other sourcesThe material in this paper is original and does not come from other sources.
Financial disclosureThis research was funded by grants from the Spanish Institute of Health (ISCIII) in the setting of Integrative Excellence Projects (PIE 2013/27) and the European Foundation for the Study of Diabetes (EFSD Pilot Research Grant Programme for Innovative Measurement of Diabetes Outcomes 2017). The study funders were not involved in the design of the study. No financial support has been received for data analysis or writing assistance.
JRE is a PhD student at Universitat Autònoma de Barcelona, Spain. JMP reports having received consulting fees from Boehringer Ingelheim and Novo Nordisk. He has received speaking fees from Gilead, and travel expenses from Gilead, Rubió, Pfizer, Astellas, MSD, CUBICIN, and Novo Nordisk. He has received educational and research support from Gilead, Pfizer, Astellas, Accelerate, Novartis, Abbvie, ViiV, and MSD. Funds from European Commission/EFPIA IMI2 853966-2, IMI2 777377, H2020 847989, and ISCIII PI19/01898. IFG reports having received consulting fees from Boehringer, Novo Nordisk, Bayer, Pfizer and Daichii Sanckyo. He has received speaking fees from Novo Nordisk, Pfizer, MSD, Bayer and Boehringer. He has received educational and research support from Novartis. All other authors: none to disclose.
Conflict of interestSA changed affiliations after finalizing the manuscript and its submission to the journal. Boehringer Ingelheim did not fund the study and did not have any role in the study design, analysis or interpretation of the data, writing or revision of the manuscript or the decision to submit the manuscript for publication. The rest of authors declare no conflict of interest.
Share first authorship.
This work was partially presented at the European Association for the Study of the Liver-International Liver Congress (EASL-ILC), 22–26 June 2022, London.