Acute-on-chronic liver failure (ACLF) is a common syndrome that occurs in patients with advanced chronic liver disease. It consists of the rapid failure of various organs and is associated with high short-term mortality. We aim to describe the main features and outcomes of inpatients who developed ACLF and to identify the factors associated with in-hospital and 28-day mortality.
Patients and methodsAll patients meeting ACLF criteria with advanced chronic liver disease admitted for decompensation from January 2014 to December 2016 were identified. Clinical and biological data were collected at the time of ACLF diagnosis and at 3–7 days thereafter, as well as in-hospital and 28-day mortality.
ResultsEighty nine out of 354 admission episodes (28%) developed ACLF, which was present at the time of admission in 72% of cases. A precipitating factor was identified in 83% of cases, the most frequent being infection (53%) and gastrointestinal bleeding (19%). In the multivariate regression analysis, the ACLF grade at 3–7 days after diagnosis was predictive of in-hospital mortality and 28-day mortality, and lower creatinine and bilirubin levels at the time of ACLF diagnosis and a precipitating factor other than bacterial infection were associated with ACLF reversion at 3–7 days.
ConclusionsACLF is a frequent complication among patients with chronic liver disease admitted for acute decompensations and is associated with a high mortality rate and is related to the number of organs involved. Bacterial infection is the most frequent precipitating factor of ACLF and probably entails a worse prognosis.
La insuficiencia hepática crónica agudizada (IHCA) es una complicación frecuente en pacientes con enfermedad hepática crónica avanzada. Consiste en el fracaso de varios órganos y se asocia a una elevada mortalidad a corto plazo. El objetivo fue describir las características y evolución de los pacientes ingresados que desarrollan IHCA e identificar los factores asociados con mortalidad intrahospitalaria y a 28 días.
Pacientes y métodosSe identificaron los pacientes que cumplían criterios de IHCA con enfermedad hepática avanzada ingresados por descompensación de Enero 2014 a Diciembre 2016. Se recogieron datos clínicos y analíticos en el momento de presentar IHCA y a los 3-7 días así como mortalidad intrahospitalaria y a los 28 días.
ResultadosOchenta y nueve de 354 ingresos (28%) desarrollaron IHCA, el 72% de los casos IHCA era presente al ingreso. Se identificó un factor precipitante en el 83% de los casos, el más frecuentes fue la infección (53%). En el análisis multivariante, el grado de IHCA a los 3-7 días del diagnóstico se asoció a mortalidad intrahospitalaria y a los 28 días. Niveles de creatinina y bilirrubina en el momento del diagnóstico de IHCA y un factor precipitante distinto de infección bacteriana, se asoció con mejoría del grado de IHCA a los 3-7 días.
ConclusionesIHCA es una complicación frecuente en los pacientes con enfermedad hepática crónica avanzada ingresados por descompensación aguda y se asocia a una elevada mortalidad. Las infecciones bacterianas es el factor precipitante mas frecuente de IHCA y probablemente conlleva un peor pronóstico.
Initially, acute-on-chronic liver failure (ACLF) was defined as a syndrome that develops in patients with acute decompensation of cirrhosis and it was characterized by organ failure and a high short-term mortality rate.1 Its reported incidence rate is roughly equivalent to one third of the cirrhotic patients admitted for major decompensation (ascites, encephalopathy, gastrointestinal bleeding or bacterial infections).1 Different diagnostic criteria have been applied over the years, based mainly on a theoretical rather than an empirical approach.2,3 The CANONIC study,1 a European, multicenter study, in which more than 1300 cirrhotic patients admitted for acute decompensation were included, established the current diagnostic criteria for ACLF. In addition, it was recently proposed that ACLF should include all patients with chronic liver disease (with or without cirrhosis) with acute hepatic decompensation.4 Taking this latter definition into account, ACLF may be divided into three categories depending on the presence of underlying cirrhosis and, in patients with cirrhosis, the existence of a history of previous hepatic decompensation. Likewise, it can also be graded in three degrees depending on the number of failing organs.5
The pathogenic mechanisms of ACLF are not well established. It has been suggested that an excessive inflammatory response and a decreased tolerance of different organs to the inflammatory response of the host may induce tissue damage and organ failure. The systemic circulatory dysfunction related to cirrhosis may also contribute to the failure of different organs as a consequence, partly due to a hypoperfusion of the tissues and, partly to direct damage to the organs.6 A precipitating event for ACLF (such as bacterial infections, active alcoholism or the reactivation of the hepatitis B virus) is often identified.7,8 However, no potential precipitating event is identified in up to 40% of patients with ACLF1,9; in these patients, it has been posed that bacterial products derived from bacterial translocation or damage-associated molecular patterns resulting from injured liver tissue cells may act as triggering factors.
Despite a growing interest in ACLF, no clear predictors of mortality have been identified beyond the number and type of failing organs.5,10 Shi et al.11 reported that clinical presentation and prognosis may depend on whether the precipitating factor was directly related to liver disease or not. On the other hand, subsequent studies observed a worse evolution and a higher mortality rate in episodes of ACLF triggered by a bacterial infection.12–14
The aims of the present study were to describe the prevalence, and the clinical and epidemiological characteristics of ACLF episodes in patients admitted at a liver unit, and to evaluate the factors associated with mortality.
Patients and methodsStudy designThis is an observational, retrospective study in which all adult patients with chronic liver disease admitted to the liver unit at Germans Trias i Pujol University Hospital (Badalona, Catalonia, Spain) from January 2014 to December 2016 for acute decompensation were identified. Only those meeting the ACLF diagnostic criteria established by the EASL-CLIF Consortium1 also adding those patients with advanced chronic liver disease, were included. The diagnosis of advanced chronic liver disease was based on liver biopsy or non-invasive test for hepatic fibrosis or a composite of clinical signs (clinical decompensation of cirrhosis like ascites, hepatic encephalopy, spontaneous bacterial peritonitis, portal hypertension-related acute gastrointestinal bleeding or bacterial infection) and findings provided by laboratory test results, endoscopy and radiologic imaging. Patients with hepatocellular carcinoma (HCC) outside Milan criteria were excluded to avoid a bias on mortality. The study was approved by the local Ethics Committee.
Data collectionElectronic medical records were accurately reviewed in order to collect epidemiological and demographic characteristics, relevant comorbidities, characteristics of liver disease (etiology, date of diagnosis, previous decompensation, presence of esophageal varices, treatment within 4 weeks before admission with norfloxacin, rifaximin and/or beta-blockers), as well as clinical features at admission (reason for admission, presence and severity of acute alcoholic hepatitis, presence of acute renal injury according to the stablished diagnosis criteria of EASL guideline, MELD and Child–Pugh scores, and ACLF criteria). When more than one decompensation was present at admission, the criteria used to define the hierarchic causality of admission were portal hypertensive bleeding, infection and hepatic encephalopathy and/or ascites. This arbitrary criterion was based in decompensation prognosis and has been used before in other studies.15 All decompensation were managed according the currents EASL guidelines.
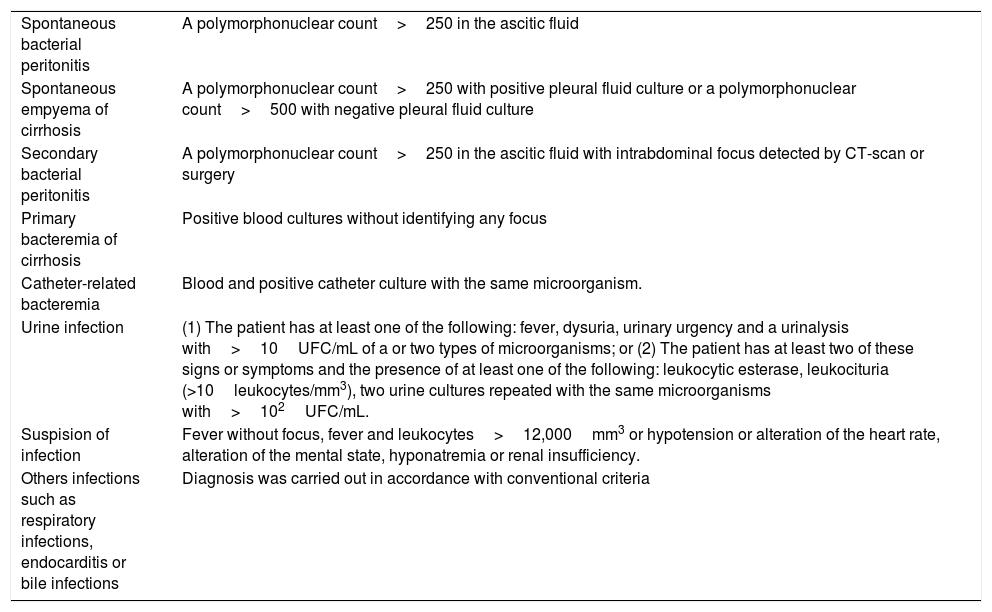
Regarding ACLF features, the type, grade, and detection of a precipitating factor were recorded. The main precipitating factors collected included bacterial infection, gastrointestinal bleeding, acute alcoholic hepatitis, surgery and acute pancreatitis.1,11 The diagnostic criteria for different types of bacterial infection are described in Table 1. Likewise, biological parameters (leukocytes, C-reactive protein (CRP), bilirubin, prothrombin time, creatinine, sodium) were collected at admission, at the time of ACLF diagnosis and 3 and 7 days after ACLF diagnosis. Finally, in-hospital mortality, 28-day mortality and cause of death were recorded.
Diagnostic criteria of bacterial infection.
| Spontaneous bacterial peritonitis | A polymorphonuclear count>250 in the ascitic fluid |
| Spontaneous empyema of cirrhosis | A polymorphonuclear count>250 with positive pleural fluid culture or a polymorphonuclear count>500 with negative pleural fluid culture |
| Secondary bacterial peritonitis | A polymorphonuclear count>250 in the ascitic fluid with intrabdominal focus detected by CT-scan or surgery |
| Primary bacteremia of cirrhosis | Positive blood cultures without identifying any focus |
| Catheter-related bacteremia | Blood and positive catheter culture with the same microorganism. |
| Urine infection | (1) The patient has at least one of the following: fever, dysuria, urinary urgency and a urinalysis with>10UFC/mL of a or two types of microorganisms; or (2) The patient has at least two of these signs or symptoms and the presence of at least one of the following: leukocytic esterase, leukocituria (>10leukocytes/mm3), two urine cultures repeated with the same microorganisms with>102UFC/mL. |
| Suspision of infection | Fever without focus, fever and leukocytes>12,000mm3 or hypotension or alteration of the heart rate, alteration of the mental state, hyponatremia or renal insufficiency. |
| Others infections such as respiratory infections, endocarditis or bile infections | Diagnosis was carried out in accordance with conventional criteria |
Categorical variables are expressed as absolute values and frequencies, and continuous variables are expressed as the median and interquartile range.
To analyze the factors associated with in-hospital and 28-day mortality, the Mann–Whitney's U test was used for the comparison of continuous variables with a non-parametric distribution, and the Chi-square test was used for qualitative variables. In all analyses, the significance level was set at p<0.05. To analyze the risk factors associated with in-hospital and 28-day mortality, a binary logistic regression test was used. All statistical analyses were carried out with the SPSS version 23 statistical package.
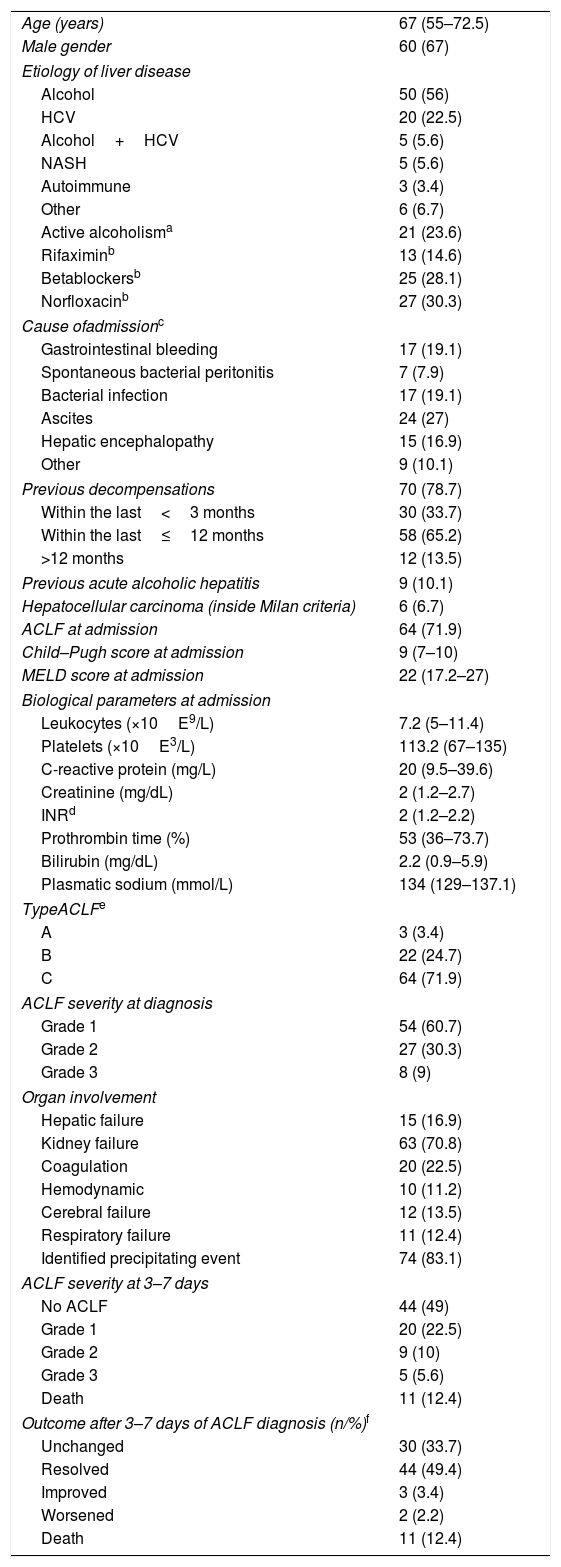
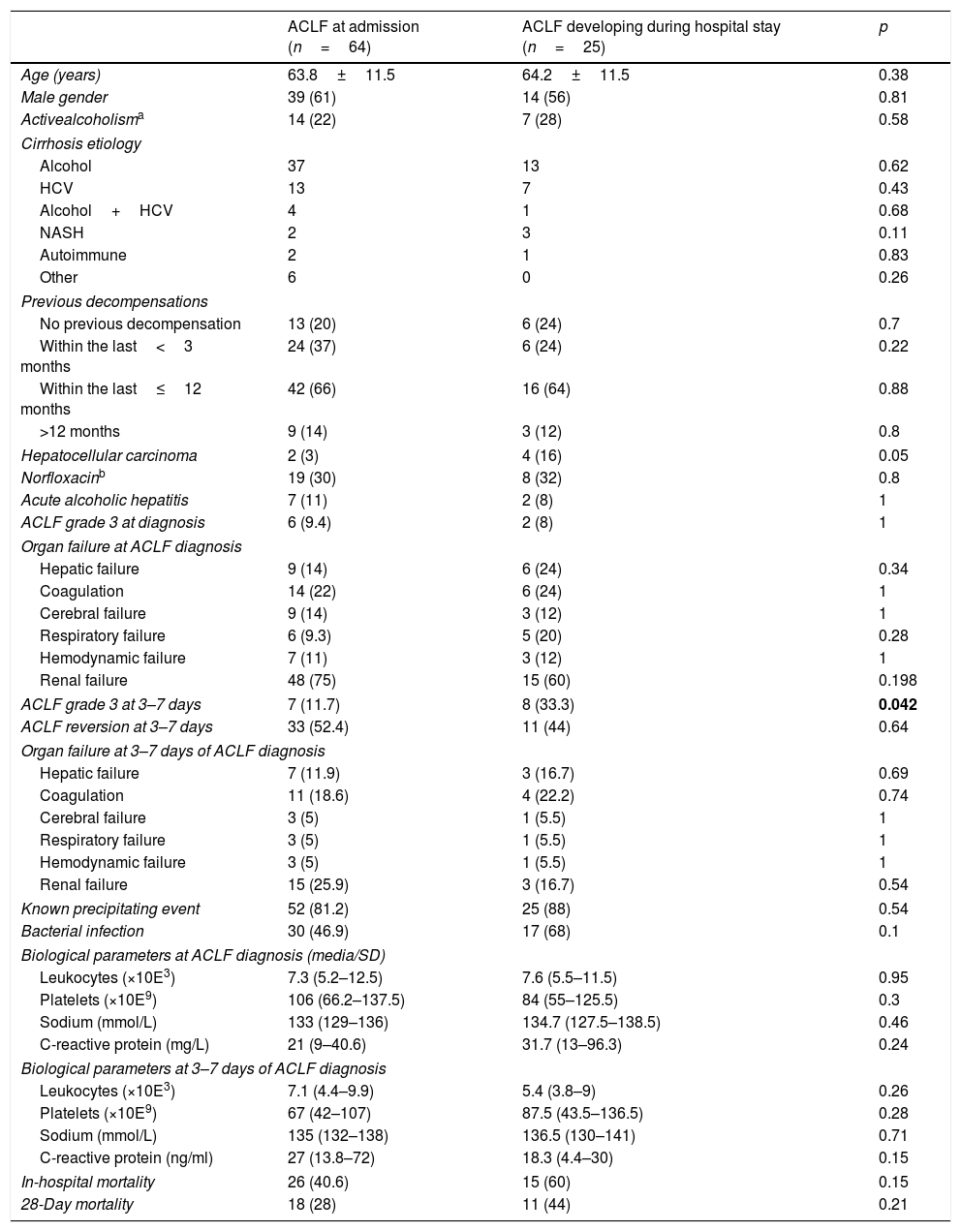
ResultsA total of 354 admissions, corresponding to 233 patients, were identified during the study period. ACLF occurred in 101 admissions but 12 of them were excluded because of underlying HCC outside Milan criteria. Therefore, 89 episodes (28%) corresponding to 77 patients, were finally included; ACLF was already present at admission in most cases (72%). The main characteristics of the patients are summarized in Table 2. The majority of episodes of ACLF (72%) occurred in patients with decompensated cirrhosis (Type C) and 79% of ACLF cases had previous acute decompensations of cirrhosis (ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, variceal bleeding, hepatorenal or hepatopulmonary syndrome). Regarding ACLF severity, 61% were Grade 1 and 30% were Grade 2 at the time of diagnosis. Renal failure was the most prevalent organ failure (71%), followed by coagulation (22%). Table 3 summarizes the baseline characteristics and outcomes of patients with ACLF at admission and those who developed ACLF during their hospital stay. Although these patients were rather similar, we observed a significantly higher proportion of patients with Grade 3 ACLF at 3–7 days among those developing ACLF during admission and this resulted in a non-significant trend toward a higher in-hospital and 28-day mortality in the patients who developed ACLF during admission as compared to those who already presented with ACLF at admission (60% vs 41% p=0.079 and 44% vs 28% p=0.2 respectively). Seventeen cases (19%) required admission into the intensive care unit, and 88% (n=15) of ACLF were already present at admission. In this subgroup, 6 cases (35%) of ACLF improved or resolved after 3–7 days and 9 cases remained unchanged.
Clinical and biological characteristics of acute-on-chronic liver failure episodes (n=89).
| Age (years) | 67 (55–72.5) |
| Male gender | 60 (67) |
| Etiology of liver disease | |
| Alcohol | 50 (56) |
| HCV | 20 (22.5) |
| Alcohol+HCV | 5 (5.6) |
| NASH | 5 (5.6) |
| Autoimmune | 3 (3.4) |
| Other | 6 (6.7) |
| Active alcoholisma | 21 (23.6) |
| Rifaximinb | 13 (14.6) |
| Betablockersb | 25 (28.1) |
| Norfloxacinb | 27 (30.3) |
| Cause ofadmissionc | |
| Gastrointestinal bleeding | 17 (19.1) |
| Spontaneous bacterial peritonitis | 7 (7.9) |
| Bacterial infection | 17 (19.1) |
| Ascites | 24 (27) |
| Hepatic encephalopathy | 15 (16.9) |
| Other | 9 (10.1) |
| Previous decompensations | 70 (78.7) |
| Within the last<3 months | 30 (33.7) |
| Within the last≤12 months | 58 (65.2) |
| >12 months | 12 (13.5) |
| Previous acute alcoholic hepatitis | 9 (10.1) |
| Hepatocellular carcinoma (inside Milan criteria) | 6 (6.7) |
| ACLF at admission | 64 (71.9) |
| Child–Pugh score at admission | 9 (7–10) |
| MELD score at admission | 22 (17.2–27) |
| Biological parameters at admission | |
| Leukocytes (×10E9/L) | 7.2 (5–11.4) |
| Platelets (×10E3/L) | 113.2 (67–135) |
| C-reactive protein (mg/L) | 20 (9.5–39.6) |
| Creatinine (mg/dL) | 2 (1.2–2.7) |
| INRd | 2 (1.2–2.2) |
| Prothrombin time (%) | 53 (36–73.7) |
| Bilirubin (mg/dL) | 2.2 (0.9–5.9) |
| Plasmatic sodium (mmol/L) | 134 (129–137.1) |
| TypeACLFe | |
| A | 3 (3.4) |
| B | 22 (24.7) |
| C | 64 (71.9) |
| ACLF severity at diagnosis | |
| Grade 1 | 54 (60.7) |
| Grade 2 | 27 (30.3) |
| Grade 3 | 8 (9) |
| Organ involvement | |
| Hepatic failure | 15 (16.9) |
| Kidney failure | 63 (70.8) |
| Coagulation | 20 (22.5) |
| Hemodynamic | 10 (11.2) |
| Cerebral failure | 12 (13.5) |
| Respiratory failure | 11 (12.4) |
| Identified precipitating event | 74 (83.1) |
| ACLF severity at 3–7 days | |
| No ACLF | 44 (49) |
| Grade 1 | 20 (22.5) |
| Grade 2 | 9 (10) |
| Grade 3 | 5 (5.6) |
| Death | 11 (12.4) |
| Outcome after 3–7 days of ACLF diagnosis (n/%)f | |
| Unchanged | 30 (33.7) |
| Resolved | 44 (49.4) |
| Improved | 3 (3.4) |
| Worsened | 2 (2.2) |
| Death | 11 (12.4) |
Abbreviations: ACLF acute on chronic liver failure; HCV hepatitis C virus; MELD model for end stage liver disease; NASH non alcoholic steatohepatitis.
Clinical and biological characteristics of patients.
| ACLF at admission (n=64) | ACLF developing during hospital stay (n=25) | p | |
|---|---|---|---|
| Age (years) | 63.8±11.5 | 64.2±11.5 | 0.38 |
| Male gender | 39 (61) | 14 (56) | 0.81 |
| Activealcoholisma | 14 (22) | 7 (28) | 0.58 |
| Cirrhosis etiology | |||
| Alcohol | 37 | 13 | 0.62 |
| HCV | 13 | 7 | 0.43 |
| Alcohol+HCV | 4 | 1 | 0.68 |
| NASH | 2 | 3 | 0.11 |
| Autoimmune | 2 | 1 | 0.83 |
| Other | 6 | 0 | 0.26 |
| Previous decompensations | |||
| No previous decompensation | 13 (20) | 6 (24) | 0.7 |
| Within the last<3 months | 24 (37) | 6 (24) | 0.22 |
| Within the last≤12 months | 42 (66) | 16 (64) | 0.88 |
| >12 months | 9 (14) | 3 (12) | 0.8 |
| Hepatocellular carcinoma | 2 (3) | 4 (16) | 0.05 |
| Norfloxacinb | 19 (30) | 8 (32) | 0.8 |
| Acute alcoholic hepatitis | 7 (11) | 2 (8) | 1 |
| ACLF grade 3 at diagnosis | 6 (9.4) | 2 (8) | 1 |
| Organ failure at ACLF diagnosis | |||
| Hepatic failure | 9 (14) | 6 (24) | 0.34 |
| Coagulation | 14 (22) | 6 (24) | 1 |
| Cerebral failure | 9 (14) | 3 (12) | 1 |
| Respiratory failure | 6 (9.3) | 5 (20) | 0.28 |
| Hemodynamic failure | 7 (11) | 3 (12) | 1 |
| Renal failure | 48 (75) | 15 (60) | 0.198 |
| ACLF grade 3 at 3–7 days | 7 (11.7) | 8 (33.3) | 0.042 |
| ACLF reversion at 3–7 days | 33 (52.4) | 11 (44) | 0.64 |
| Organ failure at 3–7 days of ACLF diagnosis | |||
| Hepatic failure | 7 (11.9) | 3 (16.7) | 0.69 |
| Coagulation | 11 (18.6) | 4 (22.2) | 0.74 |
| Cerebral failure | 3 (5) | 1 (5.5) | 1 |
| Respiratory failure | 3 (5) | 1 (5.5) | 1 |
| Hemodynamic failure | 3 (5) | 1 (5.5) | 1 |
| Renal failure | 15 (25.9) | 3 (16.7) | 0.54 |
| Known precipitating event | 52 (81.2) | 25 (88) | 0.54 |
| Bacterial infection | 30 (46.9) | 17 (68) | 0.1 |
| Biological parameters at ACLF diagnosis (media/SD) | |||
| Leukocytes (×10E3) | 7.3 (5.2–12.5) | 7.6 (5.5–11.5) | 0.95 |
| Platelets (×10E9) | 106 (66.2–137.5) | 84 (55–125.5) | 0.3 |
| Sodium (mmol/L) | 133 (129–136) | 134.7 (127.5–138.5) | 0.46 |
| C-reactive protein (mg/L) | 21 (9–40.6) | 31.7 (13–96.3) | 0.24 |
| Biological parameters at 3–7 days of ACLF diagnosis | |||
| Leukocytes (×10E3) | 7.1 (4.4–9.9) | 5.4 (3.8–9) | 0.26 |
| Platelets (×10E9) | 67 (42–107) | 87.5 (43.5–136.5) | 0.28 |
| Sodium (mmol/L) | 135 (132–138) | 136.5 (130–141) | 0.71 |
| C-reactive protein (ng/ml) | 27 (13.8–72) | 18.3 (4.4–30) | 0.15 |
| In-hospital mortality | 26 (40.6) | 15 (60) | 0.15 |
| 28-Day mortality | 18 (28) | 11 (44) | 0.21 |
Abbreviations: ACLF acute on chronic liver failure; HCV hepatitis C virus; NASH non alcoholic steatohepatitis.
A precipitating event was identified in the majority of patients (83%), with bacterial infection being the most frequent (53%)—particularly spontaneous bacterial peritonitis and urinary infections in 27% and 20%, respectively—, followed by gastrointestinal bleeding (19%), dehydration (4%), alcoholic hepatitis (2%), and acute pancreatitis (1%). Among all the episodes of bacterial infection, the responsible bacteria were identified in 26 episodes (55%), 15 (32%) of which were due to a multidrug resistant microorganism (MDR). Although without reaching a statistical significance a higher prevalence of bacterial infection was observed in the group that developed ACLF during admission (Table 3). All the episodes of bacterial infection due to MDR had at least one risk factor for MDR infection, 46.7% received prophylactic treatment with norfloxacine and 73% were associated to health system.
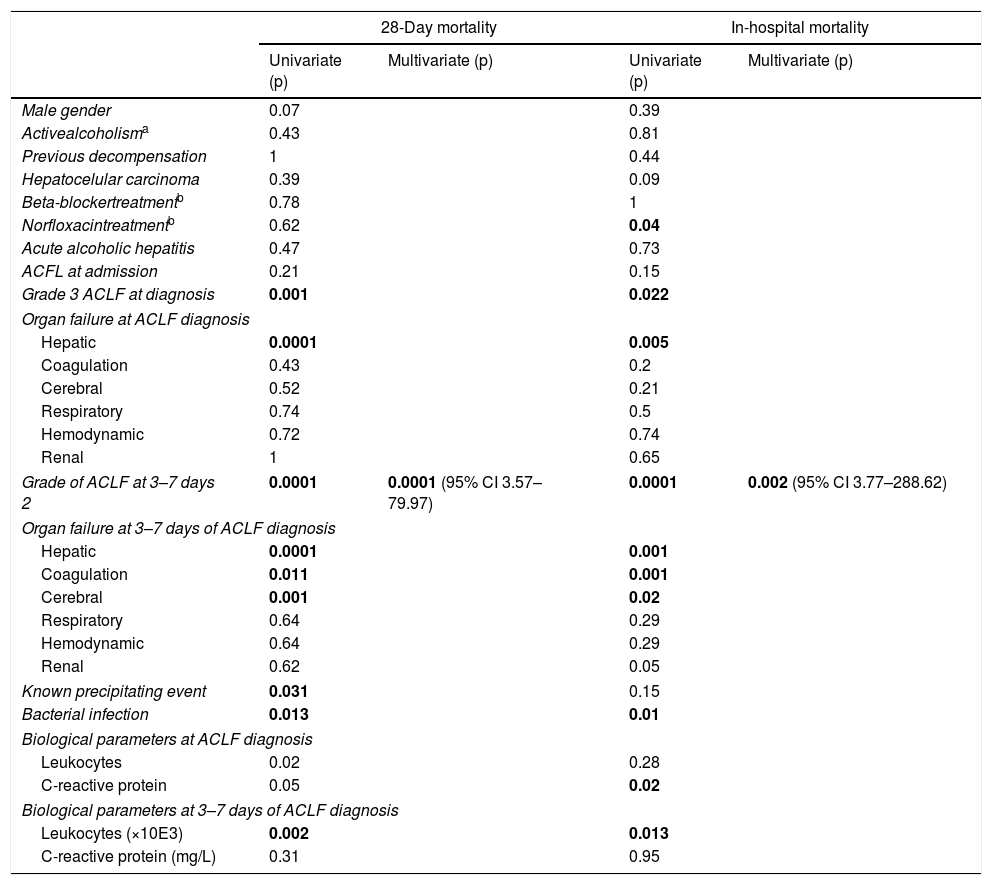
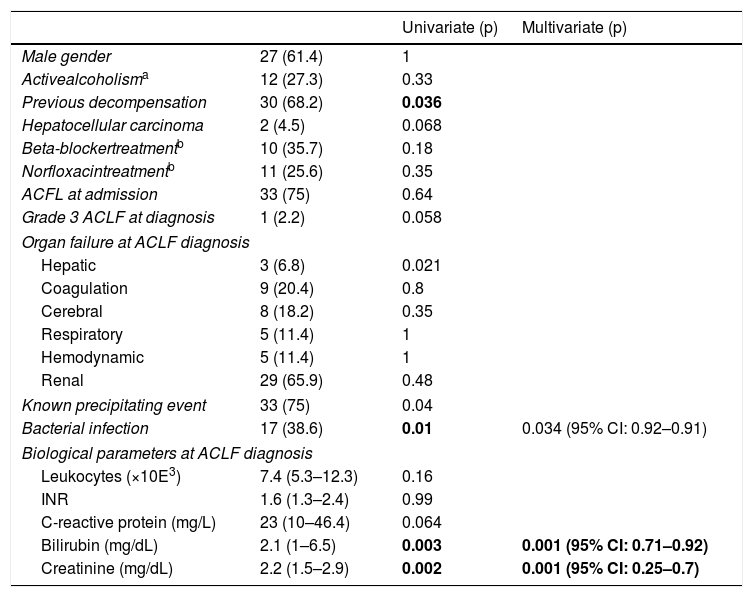
In all, the in-hospital mortality rate was 46% and 28-day mortality rate was 33%. Most deaths (88%) were due to complications of cirrhosis. The mortality rate paralleled the degree of ACLF (39%, 52% and 87%, in Grades 1, 2 and 3, respectively). The same occurred at 3–7 days after ACLF diagnosis (37% mortality in IHCA Grade 1, 87% in Grade 2 and 100% in Grade 3). Similarly, a progressive increase in white cell count and CRP was observed which paralleled the ACLF degree, with the average white cell count being 8.8×103+5.7 in ACLF Grade 1, 9.6×103+6.2 in Grade 2 and 15.2×103+15.4 in Grade 3 (p=0.073), together with a CRP level of 33.2±17.1mg/L in ACLF Grade 1, 43.3±38.9mg/L in Grade 2 and 58.8±60.6mg/L in Grade 3 (p=0.42). Table 4 summarizes the univariate and multivariate analyses of the factors associated with mortality. ACLF grade at 3–7 days was the only predictor of in-hospital and 28-days mortality (OR 33 95% CI 3.74–288.6, p=0.002 and OR 17 95% CI 3.6–79.9, p=0.0001, respectively). On the other hand, ACLF was completely reverted during admission in 44 patients (49.4%). The factors associated with ACLF resolution are summarized in Table 5. In the multivariate analysis, only lower creatinine and bilirubin levels at the time of ACLF diagnosis and a precipitant factor other than bacterial infection were associated with ACLF reversion at 3–7 days. In the subgroup of patients requiring admission into the intensive care unit, mortality was 59%, with no statistical difference with those patients who did not require admission into a critical care unit (43%) (p=0.24). Additionally, the admission into the intensive care unit wasn’t related with mortality.
Predictive factors of in hospital mortality and 28 days mortality.
| 28-Day mortality | In-hospital mortality | |||
|---|---|---|---|---|
| Univariate (p) | Multivariate (p) | Univariate (p) | Multivariate (p) | |
| Male gender | 0.07 | 0.39 | ||
| Activealcoholisma | 0.43 | 0.81 | ||
| Previous decompensation | 1 | 0.44 | ||
| Hepatocelular carcinoma | 0.39 | 0.09 | ||
| Beta-blockertreatmentb | 0.78 | 1 | ||
| Norfloxacintreatmentb | 0.62 | 0.04 | ||
| Acute alcoholic hepatitis | 0.47 | 0.73 | ||
| ACFL at admission | 0.21 | 0.15 | ||
| Grade 3 ACLF at diagnosis | 0.001 | 0.022 | ||
| Organ failure at ACLF diagnosis | ||||
| Hepatic | 0.0001 | 0.005 | ||
| Coagulation | 0.43 | 0.2 | ||
| Cerebral | 0.52 | 0.21 | ||
| Respiratory | 0.74 | 0.5 | ||
| Hemodynamic | 0.72 | 0.74 | ||
| Renal | 1 | 0.65 | ||
| Grade of ACLF at 3–7 days 2 | 0.0001 | 0.0001 (95% CI 3.57–79.97) | 0.0001 | 0.002 (95% CI 3.77–288.62) |
| Organ failure at 3–7 days of ACLF diagnosis | ||||
| Hepatic | 0.0001 | 0.001 | ||
| Coagulation | 0.011 | 0.001 | ||
| Cerebral | 0.001 | 0.02 | ||
| Respiratory | 0.64 | 0.29 | ||
| Hemodynamic | 0.64 | 0.29 | ||
| Renal | 0.62 | 0.05 | ||
| Known precipitating event | 0.031 | 0.15 | ||
| Bacterial infection | 0.013 | 0.01 | ||
| Biological parameters at ACLF diagnosis | ||||
| Leukocytes | 0.02 | 0.28 | ||
| C-reactive protein | 0.05 | 0.02 | ||
| Biological parameters at 3–7 days of ACLF diagnosis | ||||
| Leukocytes (×10E3) | 0.002 | 0.013 | ||
| C-reactive protein (mg/L) | 0.31 | 0.95 | ||
Abbreviations: ACLF acute on chronic liver failure.
Predictive factors of acute-on-chronic liver failure resolution at 3–7 days (n=44).
| Univariate (p) | Multivariate (p) | ||
|---|---|---|---|
| Male gender | 27 (61.4) | 1 | |
| Activealcoholisma | 12 (27.3) | 0.33 | |
| Previous decompensation | 30 (68.2) | 0.036 | |
| Hepatocellular carcinoma | 2 (4.5) | 0.068 | |
| Beta-blockertreatmentb | 10 (35.7) | 0.18 | |
| Norfloxacintreatmentb | 11 (25.6) | 0.35 | |
| ACFL at admission | 33 (75) | 0.64 | |
| Grade 3 ACLF at diagnosis | 1 (2.2) | 0.058 | |
| Organ failure at ACLF diagnosis | |||
| Hepatic | 3 (6.8) | 0.021 | |
| Coagulation | 9 (20.4) | 0.8 | |
| Cerebral | 8 (18.2) | 0.35 | |
| Respiratory | 5 (11.4) | 1 | |
| Hemodynamic | 5 (11.4) | 1 | |
| Renal | 29 (65.9) | 0.48 | |
| Known precipitating event | 33 (75) | 0.04 | |
| Bacterial infection | 17 (38.6) | 0.01 | 0.034 (95% CI: 0.92–0.91) |
| Biological parameters at ACLF diagnosis | |||
| Leukocytes (×10E3) | 7.4 (5.3–12.3) | 0.16 | |
| INR | 1.6 (1.3–2.4) | 0.99 | |
| C-reactive protein (mg/L) | 23 (10–46.4) | 0.064 | |
| Bilirubin (mg/dL) | 2.1 (1–6.5) | 0.003 | 0.001 (95% CI: 0.71–0.92) |
| Creatinine (mg/dL) | 2.2 (1.5–2.9) | 0.002 | 0.001 (95% CI: 0.25–0.7) |
Abbreviations: ACLF acute on chronic liver failure.
As reported in previous studies,1 we found that over a quarter of patients with cirrhosis developed ACLF when admitted for an acute decompensation; moreover, in most cases ACLF was already present at the time of admission. Similarly to Canonic study1 kidney failure was the most prevalent organ failure but with the difference that our series reports a lower prevalence of lung failure than previous studies and these fact did not demonstrated impact on prognosis, probably due to a relatively small sample size. In-hospital mortality rate was 46%, and most deaths were associated with major cirrhosis complications. When looking at the factors associated with mortality, ACLF severity assessed at 3 to 7 days after ACLF diagnosis seemed to be the most important predictor of mortality, highlighting the relevance of the current ACLF classification. Despite this high mortality rate, it is also important to highlight that almost half of the patients reverted ACLF, particularly those patients with lower bilirubin and creatinine values at the time of ACLF diagnosis, as well as, with a precipitating factor different than bacterial infection. These, together with the fact that an advanced ACLF grade within the first 3–7 days from diagnosis was associated with mortality, suggests that a timely diagnosis and therapeutic support might improve the evolution and prognosis of these patients. Mortality might also have been influenced by the fact a specific critical care unit for patients with gastrointestinal and hepatic diseases is not available in our center, and cirrhotic patients are seldom admitted to intensive care unit. In our series, despite the only available treatment was hemodynamic support, only a low percentage of cases were admitted to a critical care unit. We want to emphasize that improving the knowledge of this syndrome and implementing futility rules should be of outstanding interest to facilitate the access of these patients to critical care units. In addition, in smaller hospitals in which intensive care units are not available, the availability of semi-critical care units may allow a more tight monitoring of these patients.
In the absence of a specific therapy, prevention or early identification and treatment of the situations triggering ACLF emerge as potentially relevant measures. Suspected precipitating events of ACLF were identified in most of the patients in our cohort. Of note, bacterial infection was the most frequent identified precipitating factor, particularly among those patients developing ACLF during admission, and this could explain a non-significant trend (probably due to the small sample size) toward a worse outcome among patients who did not meet ACLF criteria at the time of admission but who went on to meet the criteria during their hospital stay. Similarly to the Mücke et al. study, in which bacterial infection was independently associated with short and long-term mortality,13 we observed that patients with bacterial infection were more prone to have an advanced grade of ACLF 3–7 days after diagnosis and infection was associated with higher in-hospital and 28-day mortality rates in the univariate analysis, although statistical significance was not reached in the multivariate analysis probably due to our small sample size. Interestingly, more than half of the infections in our cohort were culture-positive and one third of them were caused by MDR, a higher percentage than previously reported.13 The growing number of bacterial infections caused by these difficult-to-treat bacteria, represent a challenge that should stimulate the search for prevention, early diagnosis and treatment.8,15–19
The retrospective design and relatively small sample size are the main limitations of our study, but the fact that is comes from a single center with more homogeneous clinical management may serve to strengthen our results. Given the retrospective design of our study, some relevant data were not available and preclude the assessment of CLIF-C acute decompensation and CLIF-C ACLF scores that might be of interest in this clinical setting. Our results highlight the need for an early access for these patients to a semicritical care or a intensive care units to improve their management.
In conclusion, the present study confirms that ACLF is a frequent complication among cirrhotic patients admitted for acute decompensations and reproduces the results of previous European studies in which ACLF was associated with a high mortality in the short-term.1 We also found that bacterial infections were the most frequent trigger for ACLF (particularly in patients developing ACLF while admitted) and probably entail a worse prognosis.
Conflicts of interestNone to declare.