Helicobacter pylori resistance to antimicrobial agents is on the rise and it is thus imperative to be aware of local resistance rates. The main objective of the present study was to describe the evolution of primary antimicrobial resistance in H. pylori, analysing its antibiotic susceptibility over a 13-year period in a region of northern Spain, as well as host-related factors.
Patients and methodsBetween 2004 and 2016 a total of 3426 patients who met the H. pylori eradication criteria underwent gastroscopy. The gastric biopsies were processed and those testing positive for H. pylori were identified and tested for clarithromycin, metronidazole and levofloxacin susceptibility using E-test.
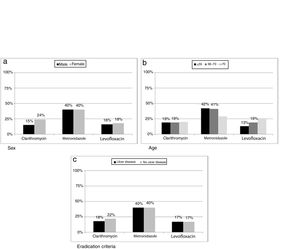
ResultsH. pylori was isolated in 1604 (47%) patients, ranging from 63% (133/212) in 2004 to 39% (137/347) in 2016. Primary resistances to clarithromycin, metronidazole and levofloxacin were on average 19% (278/1116), 40% (572/865) and 17% (137/669), respectively. Clarithromycin resistance was 24% (167/686) in females and 15% (11/753) in males (p=0.0002); metronidazole resistance was 29% (72/246) in patients over 70 years compared to 42% (499/1190) in younger patients (p=0.0396); levofloxacin resistance increased with age, being 13% (57/439) in patients ≤55 years, 19% (46/236) for those between 56 and 70, and 26% (34/130) in patients >70 years (p=0.0087).
DiscussionA decline in the prevalence of H. pylori infection was observed over the years, along with relatively high rates of primary resistance to clarithromycin, metronidazole and levofloxacin. Variations in resistance rates were found with sex and age.
El aumento de la resistencia de Helicobacter pylori a los antibióticos hace indispensable conocer las tasas de resistencia locales. El principal objetivo de este estudio fue describir la evolución de la resistencia primaria de H. pylori a los antibióticos, analizando su sensibilidad durante un período de tiempo de 13 años en una región del norte de España, así como los factores asociados del huésped.
Pacientes y métodosEntre 2004 y 2016 se realizaron gastroscopias a 3.426 pacientes que cumplían criterios de erradicación de la infección por H. pylori. En las biopsias gástricas en las que se detectó crecimiento compatible con H. pylori se identificó este microorganismo y se testó la sensibilidad a claritromicina, metronidazol y levofloxacino mediante Etest®.
ResultadosSe aisló H. pylori en 1.604 (47%) pacientes, desde el 63% (133/212) en 2004 hasta el 39% (137/347) en 2016. Las resistencias primarias a claritromicina, metronidazol y levofloxacino fueron del 19% (278/1.116), 40% (572/865) y 17% (137/669), respectivamente. La resistencia a claritromicina fue mayor en mujeres: 24% (167/686) vs. 15% (11/753) (p=0,0002); la resistencia a metronidazol fue mayor en jóvenes: 29% (72/246) en >70 años vs. 42% (499/1.190) en ≤70 años (p=0,0396); la resistencia a levofloxacino aumentó con la edad de los pacientes: 13% (57/439) en <55 años, 19% (46/236) entre 56 y 70 años y 26% (34/130) en >70 años (p=0,0087).
DiscusiónSe observa una disminución en la prevalencia de la infección por H. pylori a lo largo de los años, con tasas relativamente altas de resistencia primaria a claritromicina, metronidazol y levofloxacino. Se encuentran variaciones en estas tasas de resistencia en función del sexo y de la edad de los pacientes.
Helicobacter pylori (H. pylori) colonizes the gastric mucosa mainly at the antrum, producing gastric inflammation. Patients may remain asymptomatic for their whole life, or develop multiple gastric pathologies such as gastritis, peptic ulcer disease or gastric cancer,1 and an association with non-digestive diseases has also been described.2,3H. pylori eradication is also a challenge for physicians in so much as it improves the clinical outcome of patients with duodenal ulcer, prevents recurrence and decreases the risk of gastric cancer in infected patients.4 Since the positive results of a randomized control trial in 1996,5 the therapy for H. pylori eradication has been based on a combination of two antibiotics and a proton pump inhibitor (PPI). Clarithromycin or metronidazole, with amoxicillin plus a proton pump inhibitor is the recommended first line therapy if local resistance rate is below 15–20%.1 However, the increasing rates of antibiotic resistance reported worldwide, especially in western, central and southern European countries, has meant that these recommendations have had to change.6,7 The 2016 update from the Spanish Consensus conference on the management of H. pylori proposes a quadruple regimen as fist-line therapy.8
What is more, there is wide geographical variation regarding the prevalence of antibiotic resistance, with differences in clarithromycin resistance rates being greater than 10% between different regions of Europe.9 Since empirical treatment with clarithromycin is no longer recommended if local primary resistance rates exceed 15–20%,10 knowledge of local resistance rates is crucial to provide the most appropriate first-line and second-line treatment regimes.
The main objective of the present study was to describe the evolution of primary resistance to antibiotics in H. pylori strains isolated in this region of Spain over a 13-year period, as well as to identify possible associated factors. The evolution of the H. pylori isolates was also described according to various criteria (age, gender and eradication criteria).
Materials and methodsPatientsIn this retrospective study we considered all patients older than 18 years attending the Gastroenterology Unit of the San Agustín University Hospital (Avilés, Spain) between January 2004 and December 2016 with dyspeptic symptoms where upper endoscopy was employed to take antral biopsies for microbiological culture. Patients were excluded if they had been previously treated for H. pylori infection or had used either PPI or antibiotics in the 4 weeks preceding the procedure.
A total of 3426 patients (mean age 55.7±16.9 years old (range 15–94); 1740 of whom were female) met the inclusion criteria. According to the H. pylori eradication criteria,11–13 the patients were classified as shown in Table 1.
Clinical characteristics of patients included in the study.
| Total | Sex | Age | ||||
|---|---|---|---|---|---|---|
| (n=3426) | Male | Female | ≤55 | 55–70 | >70 | |
| (n=1686) | (n=1740) | (n=1670) | (n=999) | (n=755) | ||
| Ulcer disease | 1895 (55%) | 1099 (65%) | 796 (46%) | 853 (51%) | 567 (57%) | 475 (63%) |
| Duodenal ulcer | 616 (18%) | 388 | 228 | 301 | 179 | 136 |
| Ulcer (unknown localization) | 357 (10%) | 188 | 169 | 138 | 106 | 113 |
| Gastric ulcer | 333 (10%) | 178 | 155 | 114 | 112 | 107 |
| Erosive duodenitis | 232 (7%) | 150 | 82 | 142 | 60 | 30 |
| Erosive gastritis | 188 (5%) | 101 | 87 | 83 | 58 | 47 |
| Healed ulcer | 169 (5%) | 94 | 75 | 75 | 52 | 42 |
| No ulcer disease | 1531 (45%) | 587 (35%) | 944 (54%) | 817 (49%) | 432 (43%) | 280 (37%) |
| Dyspepsia | 957 (28%) | 360 | 597 | 569 | 253 | 133 |
| Iron deficiency anaemia | 263 (8%) | 101 | 162 | 95 | 84 | 84 |
| Family history of gastric carcinoma | 155 (5%) | 55 | 100 | 75 | 53 | 27 |
| Other criteria/unknown | 122 (4%) | 61 | 61 | 51 | 37 | 34 |
| Preoperative bariatric surgery | 26 (1%) | 7 | 19 | 23 | 3 | |
| B12 deficit | 8 (0%) | 3 | 5 | 4 | 2 | 2 |
The biopsies collected were transported in Portagerm pylori (BioMèrieux, France) to the Microbiology laboratory of the same hospital. Homogenized tissue was streaked onto both non-selective and selective media (Columbia agar with 5% sheep blood and Pylory agar, respectively, both from BioMèrieux, France) and then incubated for 10 days at 37°C under microaerophilic conditions (5% O2, 10% CO2 and 85% N2). Isolates were identified as H. pylori based on colony morphology, positive biochemical reactions for urease, catalase and oxidase test, and characteristic Gram staining. The strains identified as H. pylori were subcultured in non-selective media (Columbia agar with 5% sheep blood) for 48–72h in order to perform susceptibility studies. The minimal inhibitory concentrations (MICs) of clarithromycin, metronidazole and levofloxacin (the latter only employed after 2010) were determined by the Epsilometer test (E-test; BioMèrieux, France): a suspension with turbidity equivalent to 3.0 McFarland standards was used to inoculate plates of Mueller Hinton sheep blood agar (BioMèrieux, France) with sterile cotton swabs, E-test strips were applied and incubated at 37°C for 3–5 days under microaerophilic conditions. MIC was considered as the lowest concentration of drug which inhibited visible growth and was read as the intercept of the elliptical zone of inhibition with the graded E-test strip. Based on EUCAST criteria,14 strains were resistant if MIC ≥0.5mg/L for clarithromycin, MIC ≥8mg/L for metronidazole and MIC ≥1mg/L for levofloxacin. Below these thresholds, strains were considered susceptible.
EthicsThe study was performed in accordance with the corresponding sections of the World Medical association declaration of Helsinki-ethical principles for medical research involving human subjects. Approval of the institutional review board was not required because of the retrospective nature of our study, which analyzed medical treatments that were already carried out. All patients gave written informed consent before endoscopic interventions and before their samples were submitted to data analyses.
Analysis of resultsFor statistical analysis, Student's t-test, Mann–Whitney and Fisher's exact test were used. The statistical software package SPSS v.23.0 (IBM Corp., Released 2011, IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY: IBM Corp.) was used. All p-values were two sided and considered significant when below 0.05.
ResultsH. pylori isolatesH. pylori strains were isolated in 1604 (47%) patients (mean age 54.1±15.9 years (range 15–94), 790 female).
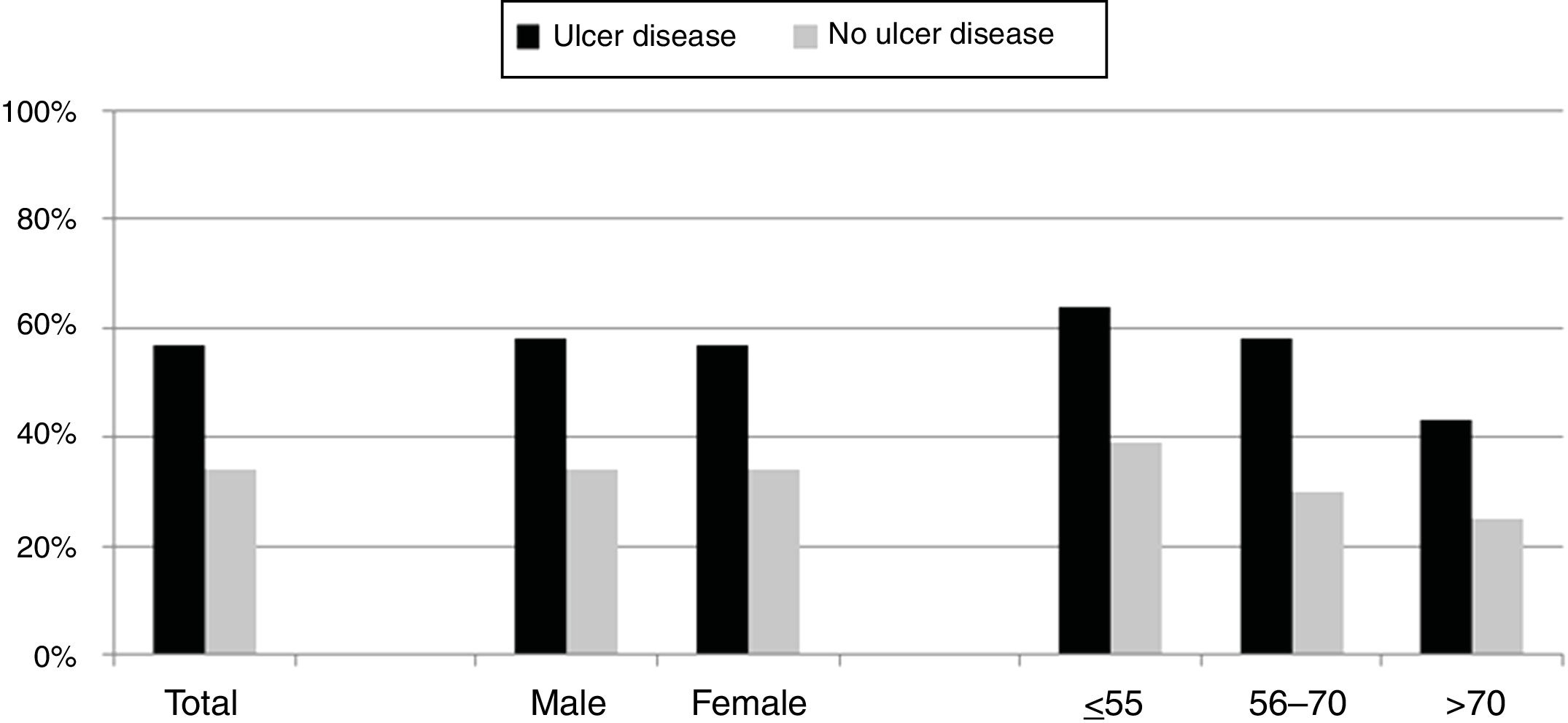
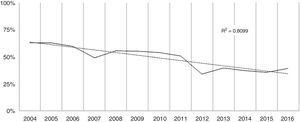
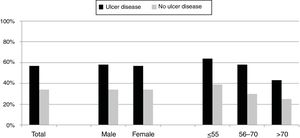
Over the study period the annual percentage of isolates of H. pylori declined from 63% in 2004 to 41% in 2016 as shown in Table 2 and Fig. 1. From the 1604 patients testing H. pylori positive, 1084 (57%) had ulcer disease and 520 (34%) no ulcer disease (p<0.0001). Data of the positive cultures according to sex, age and eradication criteria of H. pylori are shown in Table 3.
Evolution of H. pylori isolations and resistance over the period studied.
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | 133/212 | 151/241 | 105/176 | 100/204 | 100/180 | 116/210 | 123/228 | 146/288 | 91/266 | 129/326 | 146/390 | 127/358 | 137/374 |
| (63%) | (60%) | (49%) | (56%) | (55%) | (54%) | (51%) | (34%) | (40%) | (37%) | (35%) | (39%) | (63%) | |
| CLH | 26/122 | 26/131 | 19/104 | 27/89 | 20/87 | 20/109 | 20/115 | 22/131 | oct-81 | 17/113 | 18/124 | 26/112 | 27/121 |
| (21%) | (20%) | (18%) | (30%) | (23%) | (18%) | (17%) | (17%) | (12%) | (15%) | (15%) | (23%) | (22%) | |
| MTZ | 55/122 | 54/132 | 4/104 | 45/87 | 34/86 | 37/108 | 45/115 | 59/131 | 28/81 | 43/114 | 51/124 | 34/112 | 41/121 |
| (45%) | (41%) | (43%) | (52%) | (40%) | (34%) | (40%) | (45%9 | (35%) | (38%) | (41%) | (30%) | (34%) | |
| LEV | oct-79 | 19/131 | 14/81 | 20/116 | 21/124 | 25/112 | 21/121 | ||||||
| (13%) | (15%) | (17%) | (17%) | (17%) | (22%) | (17%) |
CHL: clarithromycin, MTZ: metronidazole, LEV: levofloxacin.
H. pylori isolates classified by sex, age and eradication criteria. N=total number of samples (H. pylori positive and negative).
| Total | Male | Female | ≤55 | 56–70 | >70 | |
|---|---|---|---|---|---|---|
| Ulcer disease (n=1895) | 1084* (57%) | 633 (58%) | 451 (57%) | 550 (64%) | 330 (58%) | 204 (43%) |
| Duodenal ulcer | 427 (69%) | 260 | 167 | 225 | 126 | 76 |
| Ulcer (unknown localization) | 153 (43%) | 80 | 73 | 74 | 52 | 27 |
| Gastric ulcer | 180 (54%) | 101 | 79 | 70 | 62 | 48 |
| Erosive duodenitis | 147 (63%) | 92 | 55 | 91 | 37 | 19 |
| Erosive gastritis | 104 (55%) | 62 | 42 | 46 | 34 | 24 |
| Healed ulcer | 73 (43%) | 38 | 35 | 44 | 19 | 10 |
| No ulcer disease (n=1531) | 520* (34%) | 201 (34%) | 319 (34%) | 318 (39%) | 130 (30%) | 70 (25%) |
| Dyspepsia | 319 (33%) | 122 | 197 | 207 | 74 | 37 |
| Iron deficiency anaemia | 79 (30%) | 32 | 47 | 36 | 24 | 19 |
| Family history of gastric carcinoma | 65 (42%) | 21 | 44 | 43 | 18 | 4 |
| Other criteria/unknown | 48 (39%) | 25 | 22 | 26 | 12 | 10 |
| Preoperative bariatric surgery | 7 (27%) | 1 | 7 | 5 | 2 | |
| B12 deficit | 2 (25%) | 2 | 1 | 1 | ||
Differences between eradication criteria were maintained when population was classified by sex or age (Fig. 2).
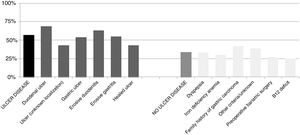
The percentage of H. pylori isolates according to the different symptoms within the ulcer and no ulcer disease groups is shown in Fig. 3. Incidence of each condition varies between 43% and 69% in patients with ulcer disease, and from 25% to 42% in patients with no ulcer disease.
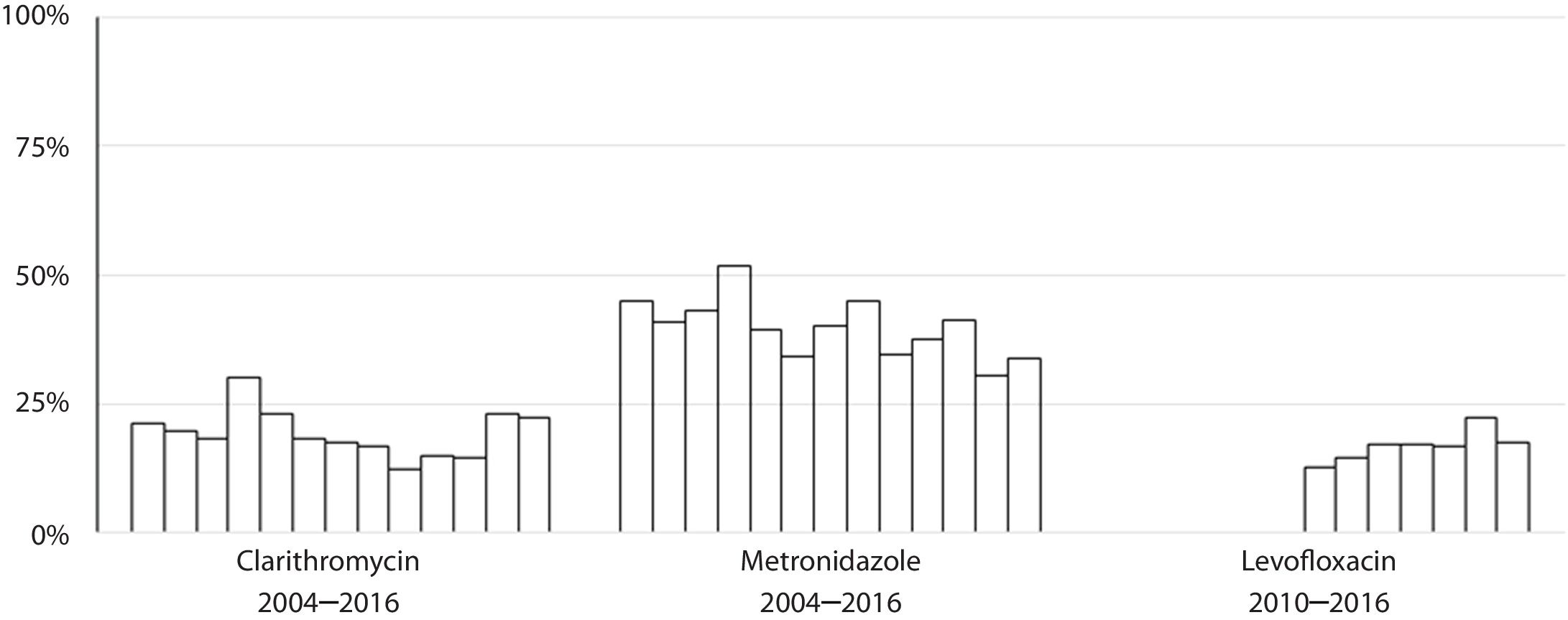
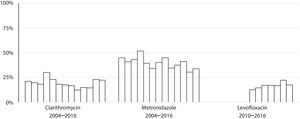
Susceptibility dataThe overall prevalence of resistance was 19% (278/1439) for clarithromycin, 40% (572/1437) for metronidazole and 17% (137/806) for levofloxacin. Clarithromycin resistance varied annually between 12% and 30%, metronidazole between 30% and 52% and levofloxacin between 13% and 22% (Table 2 and Fig. 4).
Resistance data when patients were classified by sex and age are shown in Table 4.
Rate of H. pylori resistance classified by sex and age.
| ≤55 years old | 56–70 years old | >70 years old | Total | |
|---|---|---|---|---|
| Clarithromycin | ||||
| Male | 61/406 (15%) | 32/229 (14%) | 18/117 (15%) | 111/753 (15%) |
| Female | 91/384 (24%) | 46/175 (26%) | 30/127 (24%) | 167/686 (24%)* |
| Total | 152/790 (19%) | 78/404 (19%) | 48/244 (20%) | 278/1439 (19%) |
| Metronidazol | ||||
| Male | 168/404 (42%) | 96/230 (42%) | 35/118 (30%) | 300/753 (40%) |
| Female | 166/383 (43%) | 69/173 (40%) | 37/128 (29%) | 272/684 (40%) |
| Total | 334/787 (42%) | 165/403 (41%) | 72/246 (29%)* | 572/1437 (40%) |
| Levofloxacin | ||||
| Male | 20/197 (10%) | 25/137 (18%) | 16/54 (30%) | 71/389 (16%) |
| Female | 37/242 (15%) | 21/99 (21%) | 18/79 (24%) | 76/417 (18%) |
| Total | 57/439 (13%) | 46/236 (19%) | 34/130 (26%)* | 147/806 (18%) |
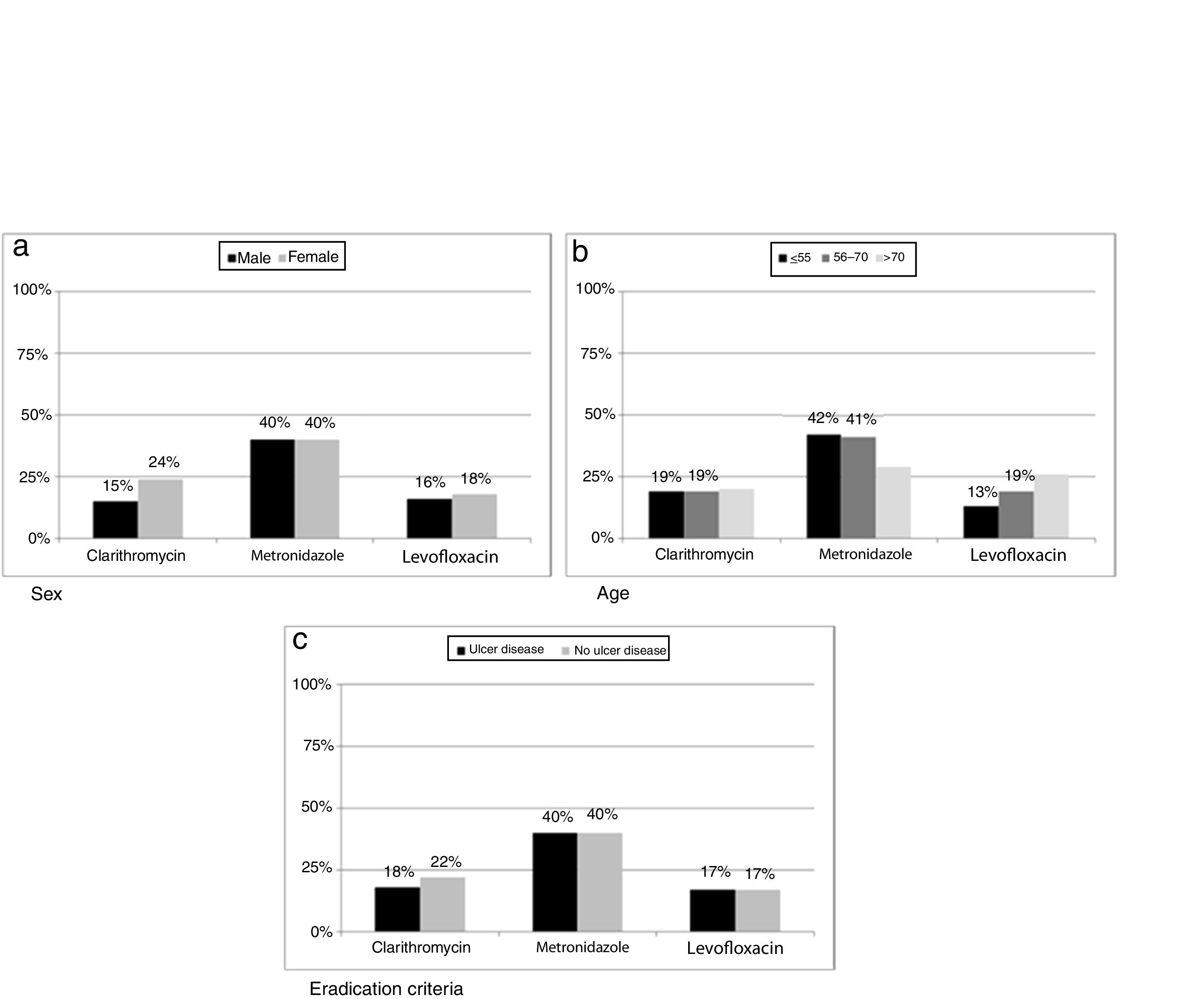
Resistance to clarithromycin was found in 167/686 (24%) women as compared to 111/753 (15%) men (p=0.0002). Moreover, metronidazole resistance was higher in isolates from patients below seventy years old (499/1190 vs 72/246, p=0.0396) while levofloxacin showed an increase in resistance rate with increasing age of patient (p=0.0087). When data were classified as ulcer or no ulcer disease, the rates of resistance were, respectively, 176/970 (18%) vs 102/469 (22%) for clarithromycin, 387/970 (40%) vs 185/467 (40%) for metronidazol, and 82/474 (17%) vs 55/332 (17%) for levofloxacin (Fig. 5).
A total of 223 strains showed multiple resistances. The most common type of multiresistance detected was to clarithromycin plus metronidazole (127/1306, 9%), followed by metronidazole plus levofloxacin (54/750, 7%) and clarithromycin plus levofloxacin (30/773, 4%). Resistance to all three antibiotics was observed in 12/806 (1%) isolates.
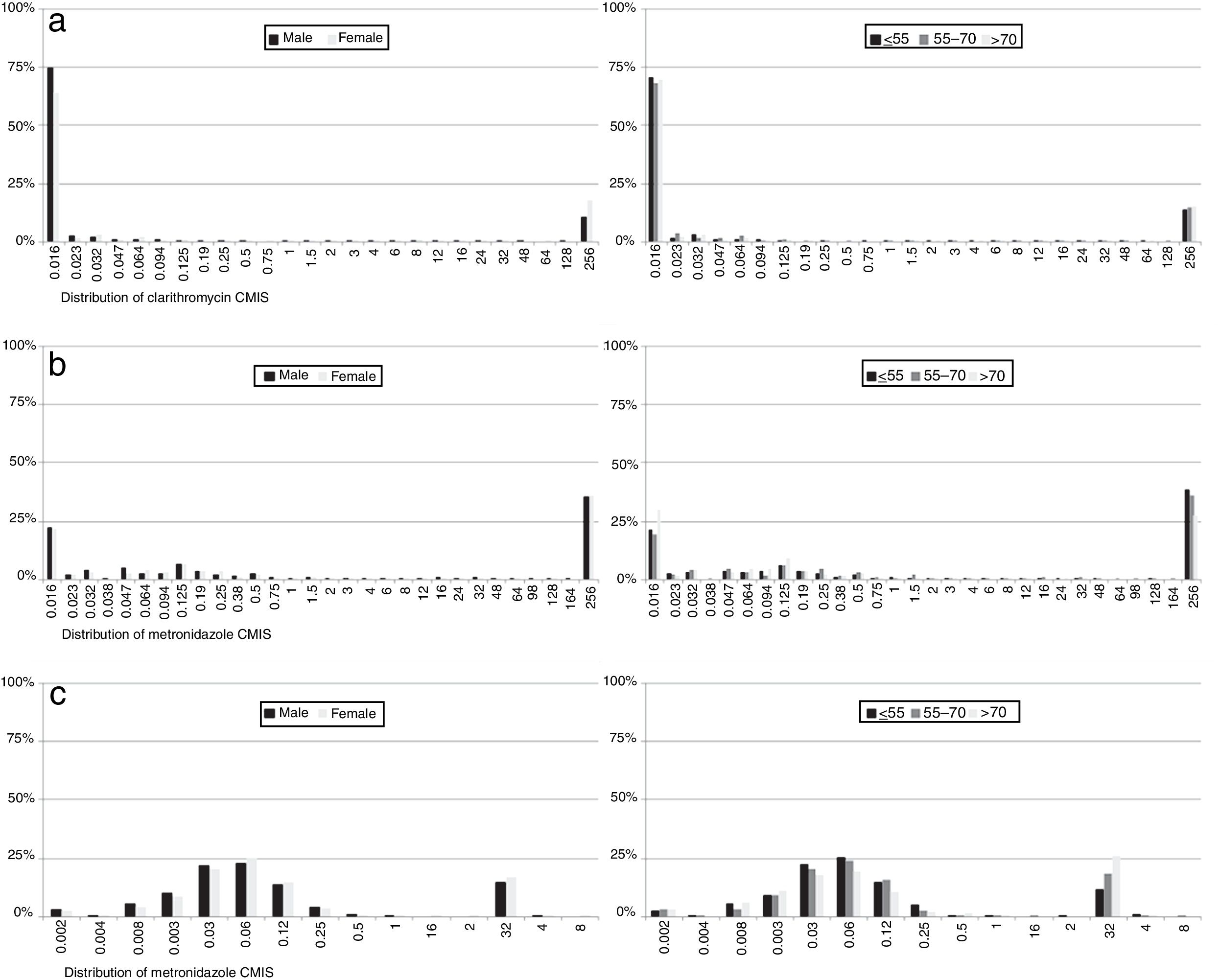
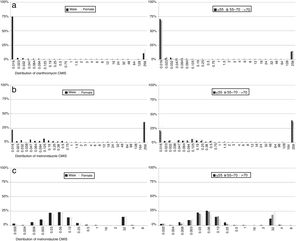
With regard to MIC data, it was observed that in resistant strains most MIC values for each of the antibiotics were above the highest concentration of the E-test strips, although there were differences in susceptible isolates: 87% of clarithromycin non-resistant strains had an MIC value below the lowest E-test concentration, while 37% of metronidazole susceptible strain MICs had values below the lowest concentration. In contrast, 96% of levofloxacin susceptible isolates had an MIC value close to the cut-off. No differences were observed by sex or age (Fig. 6).
DiscussionAt the present study, H. pylori was isolated in 1604 patients ranging from 63% in 2004 to 39% in 2016. Primary resistances rates found to clarithromycin, metronidazole and levofloxacin was on average 19%, 40% and 17% respectively.
This study shows, therefore, a high overall primary resistance rate to the three antibiotics tested. This data are similar to other studies across Europe, although rates are variable between countries.7 In fact, a recent systematic review and meta-analysis in World Health Organization regions between 2006 and 2016 showed a prevalence of primary resistance to clarithromycin and metronidazole of more than 15% in Europe.15
The rate of resistance to clarithromycin remained more or less stable during the period studied, although an increase was observed with respect to a previous Spanish multicentre study carried out in 2009 in which this hospital took part, where clarithromycin resistance rate was 14%.16 This data were included in a multicentre European study were average resistance rate for clarithromycin was 17.5%.7 Other Spanish studies described resistance to clarithromycin of 18%.16 The stability in the clarithromycin resistance rate described in this study is in agreement with the data extracted from the meta-analysis mentioned above, where the clarithromycin resistance rate remained around 28% between 2006 and 2016.15 Also, the results presented here highlight the fact that women have more resistance to clarithromycin than men, also something demonstrated in other studies.17,18 This finding could possibly be explained by the use of macrolides to treat gynaecological and urinary infections.19
With respect to levofloxacin, recent data from Europe describe increasing resistance rates, albeit with wide variability among countries, ranging from 4% in Croatia to 28% in Belgium, while in Spain a resistance rate of 14% has been reported20,21 and, interestingly, these results show an association with consumption of quinolones.7 Levofloxacin-based triple therapy is currently recommended as a second-line therapy in several guidelines, but taking into consideration the resistance rate found in the area of this study, which is over 15%, the importance of controlling the evolution of this resistance should be emphasized. Levofloxacin, which was started to be tested in our hospital in 2010 had 15% resistance in the first two tears, but this had increased to 22% by 2015, something which should probably be more widely advertised within the regional health service. This data, however, does not match with the results reflected by Savoldi et al.,15 where the rate of resistance to levofloxacin decreases throughout the study period. The current study also registered an increased risk of levofloxacin resistance with age, something which could maybe be expected due to the extensive use of quinolones for respiratory infections, which are more common in older people. In the latest programme concerning outpatient antibiotic use in Europe, Spain was one of the countries with the highest consumption of quinolones, with a defined daily dose (DDD) per 1000 inhabitants per day of between 2.3 and 2.92.22 A multicentre cross sectional study carried out in Andalucía20 detected variability in clarithromycin and levofloxacin resistance rates between different treatment centres, which could only be explained by the existence of differences in the use of macrolides and quinolones in the different centres. Further multicentre studies of resistance rates together with data on the consumption of these antibiotics should be performed.
Resistance to metronidazole in this work is at a comparably high level to that reported in other studies conducted in Europe, which has remained stable for 14 years.7 The rate of resistance to metronidazole varies between different countries because it is widely used to treat parasitic diseases and anaerobic or gynaecological infections. Its use in gynaecological infections is reflected in the higher resistance in female patients found in some studies.23 This, however, is not the case in the current study. The impact of the high resistance rate to metronidazole found (40%) on eradication success is, however, limited and can be overcome by increasing the length of treatment or adding bismuth salts in quadruple therapies.24
The most recent Spanish Consensus Conference (2016) on the treatment of H. pylori infection8 recommends as a first line empirical treatment a quadruple therapy containing clarithromycin and metronidazole. In this study the combined resistance rate for both drugs was 9%, as has been observed in other Spanish studies25 but not in Savoldi meta-analysis15 where this rate was 1%. Anyway, this resistance rate is not too high at the moment, in spite of the high resistance to each drug separately, making the empiric use of quadruple therapy including them a good option.
Therefore, although the eradication treatments used over the last 20 years has led to a decrease in the prevalence of the infection itself and H. pylori-related diseases, the success of these treatments is now compromised by the increasing resistance that H. pylori is developing to antibiotics.26 Although antimicrobial resistance is not the only reason for treatment failure, this increase in resistance has reached such a level that the latest Spanish Consensus on H. pylori infection no longer recommends the use of classical empiric triple therapy, but rather a quadruple therapy comprising clarithromycin plus metronidazole plus amoxicillin plus IBP.8 The European consensus, however, still recommends triple therapy as the first line empiric treatment in areas with low clarithromycin resistance.13 This is one of the major reasons why H. pylori resistance needs to be assessed in surveillance programmes. A recent meta-analysis showed that culture guided triple therapy was more effective than empiric therapy, as well as it being more cost efficient.27 In addition, there are other studies that have tested a culture-based strategy in naïve patients which have shown higher efficacy and better cost-effectiveness than standard therapy. Tailored treatment thus seems to represent the most logical next step.28,29
Regarding MIC distributions, they showed a normal distribution with a clear separation between susceptible and resistant strains for clarithromycin and levofloxacin, but nor for metronidazole, which had a continuous distribution, the same as in other similar studies.5,7,30
With regard to prevalence, H. pylori is a very common infection that affects about 50% of the world's population, though over the last decades there has been a decline in its prevalence in most developed countries. This decrease could be explained by increased knowledge of H. pylori disease, improvements in non-invasive diagnostic techniques and the better use of the test and treat strategy. In the present study we analyzed the population attending the Gastroenterology Unit with symptoms that justified a treatment for H. pylori eradication. The overall positive rate was 47%, with a declining trend over the studied period as has been described in other studies.31 It should however be recognized that during this time period there has been a 64% increase in the number of samples received at the laboratory due to less restrictive inclusion criteria when looking for H. pylori, a fact which also contributes to the declining tendency described.
As expected, patients with ulcer disease showed a higher rate of H. pylori isolations than patients with no ulcer disease, and within the ulcer group, those patients with duodenal ulcer or erosive duodenitis were more likely to have a positive culture for H. pylori. It is worth noting the high percentage of patients with a positive culture from the group of patients where the eradication criteria registered for H. pylori was family history of gastric cancer. Probably these patients presented with dyspepsia or some other intestinal symptoms in addition to this family history although these symptoms have not been recorded. Nevertheless, we can confirm in this work that the prevalence of H. pylori is as high as other studies have shown.
Taking into account that resistance to antibiotics is the most important reason for therapeutic failure, it is easy to see the importance of the periodic determination of antibiotic resistance patterns in each region in order to establish what the best therapy combination is to eradicate H. pylori. Hence it is essential to carry out microbiological studies to see if the downward trend in infection continues, as well as its association with new pathologies and, above all, the resistance profile. Furthermore, faster and more sensitive methodologies for the detection of resistance mechanisms are necessary, as well as studies relating the rate of resistance with the consumption of antibiotics.
FundingThe author(s) received no specific funding for this work.
Conflict of interestsThe authors declare no conflict of interests.
We thank Ronnie Lendrum for her help with correcting the English.