At present only monoclonal EIA (enzyme-immunoassay) stool antigen-tests have obtained optimal accuracy in the diagnosis of Helicobacter pylori. Our aim was to evaluate the accuracy of two stool antigen-tests, the validated Premier Platinum HpSA PLUS (EIA test) and the newly available ImmunoCard STAT! HpSA HD (rapid test) for the initial diagnosis and the confirmation of eradication of H. pylori infection.
Patients and methodsPatients with indication of H. pylori diagnosis, or confirmation after treatment were included. Data were coded to protect personal data and ensure blindness between tests. Accuracy was considered as coincident diagnosis with the gold standard (13C-urea breath test, UBT). The EIA was used as a bench standard. All stool tests were performed in duplicate.
Results264 patients completed the protocol (100 naïve, 164 post-eradication). Average age was 52 years, 61% women, 11% ulcer. Positive diagnoses by UBT were 41% for naïve and 17% for post-eradication. Overall ImmunoCard and EIA accuracies were respectively 91% (95%C.I.=88–94%) and 89% (86–93%), sensitivities 72% (67–78%) and 72% (67–78%), and specificities 98% (96–100%), and 95% (92–97%). Concordance between ImmunoCard and EIA was 95% (93–98%).
DiscussionOur results indicate that the newly available ImmunoCard rapid stool antigen-test achieves 90% accuracy, with high specificity but suboptimal sensitivity. The ImmunoCard attained equivalent accuracies as the EIA bench standard, with 95% concordance.
En la actualidad, únicamente los métodos de detección de antígenos en heces monoclonales basados en enzimoinmunoanálisis (ELISA) han obtenido una adecuada precisión para el diagnóstico de la infección por Helicobacter pylori. Nuestro objetivo fue evaluar la exactitud (sensibilidad y especificidad) de 2 métodos de antígenos en las heces, el previamente validado Premier Platinum HpSA® PLUS (ELISA) y el nuevo ImmunoCard® STAT! HpSA® HD (test rápido), para el diagnóstico inicial y la confirmación de la erradicación de la infección por H. pylori.
Pacientes y métodosSe incluyeron pacientes en los que estaba indicado el diagnóstico inicial de la infección por H. pylori o su confirmación tras el tratamiento. Los datos fueron codificados y los evaluadores de ambos test fueron ciegos para los resultados de las pruebas diagnósticas. El resultado principal fue la coincidencia con el resultado del patrón oro (prueba del aliento con 13C-urea). Los test en heces se realizaron por duplicado.
ResultadosDoscientos sesenta y cuatro pacientes completaron el protocolo (100 naïve, 164 posterradicación). La edad media fue de 52 años, el 61% fueron mujeres y el 11% tenían úlcera péptica. La prueba del aliento fue positiva en el 41% de los pacientes naïve y en el 17% posterradicación. La exactitud global del método rápido y del ELISA fue, respectivamente, 91% (IC 95%: 88-94%) y 89% (86-93%), la sensibilidad 72% (67-78%) y 72% (67-78%), y la especificidad 98% (96-100%) y 95% (92-97%). La concordancia entre el método ImmunoCard® y ELISA fue del 95% (93-98%).
DiscusiónEl nuevo método rápido de antígenos en heces (ImmunoCard® STAT! HpSA® HD) tiene una exactitud diagnóstica del 90%, con una elevada especificidad, pero una sensibilidad insuficiente. El método ImmunoCard® tiene una exactitud equivalente al método ELISA estándar, con una concordancia del 95%.
Helicobacter pylori infection is the main cause of chronic gastritis, peptic ulcer disease and gastric cancer (adenocarcinoma and lymphoma).1H. pylori eradication prevents gastric and duodenal ulcer recurrence and improves dyspepsia in a small but relevant percentage of patients, therefore it is a cost-effective strategy.2,3
In order to treat the H. pylori infection in those cases in which eradication is indicated, an accurate diagnosis is required. H. pylori diagnostic methods have been traditionally classified as invasive and non-invasive.4 The first group requires the use of biopsies and therefore an upper gastrointestinal endoscopy, and includes methods such as histology, rapid urease test and culture. Non-invasive methods are based in the study and detection of certain bacterial characteristics, such as urease hydrolization in the 13C-urea breath test, or serology in the blood, or antigens in the stools, and do not require an endoscopy.3
The high prevalence of H. pylori infection, the complexity of its indications and the price of endoscopy make invasive methods not the most cost-effective approach in the management of H. pylori infection in Spain.5,6 Actually, invasive methods are not the recommended method to diagnose the infection unless other factors need to be studied (i.e. risk of gastric cancer or bacterial susceptibility to antibiotics).1,7 Moreover, invasive methods may pose a small but significant risk of complications. Therefore, the most cost-effective approach in the management of dyspepsia, which accounts for over 40% of gastroenterology consultations in primary care, is the ‘Test & Treat’ strategy, a strategy based on performing a non-invasive test and prescribing eradication therapy to the infected patients.5
Several non-invasive tests have been developed for the diagnosis of H. pylori infection: serology, 13C-urea breath test and stool antigen test. Serology has been discarded due to its low accuracy especially as a test for the confirmation of H. pylori eradication.1,4 Urea breath test has demonstrated high sensibility and specificity both for the diagnosis and the eradication confirmation, for which is generally considered as the gold standard8; however, it is an expensive test, and requires preparation and a 30–45min stay in the clinic by the patient. Several different approaches and brands of stool antigen tests have been developed with different results regarding their accuracy. Stool antigen tests are divided into polyclonal or monoclonal, and as EIA (enzyme-immunoassay) or rapid-kit methods.9 Polyclonal tests have been deemed inaccurate and are not recommended by consensus conferences and only monoclonal EIA tests have so far been able to obtain optimal accuracy.9–11 EIA methods require processing by a trained lab technician, what makes this method expensive and difficult to implement in most clinical settings, especially in primary care. A highly accurate and rapid stool antigen test would be of great interest in the management of H. pylori.
The development of novel stool antigen tests requires the assessment of their accuracy in order to re-evaluate the consensus recommendations regarding H. pylori diagnosis. In this context, the aim of the present study was to evaluate the accuracy of two different stool antigen tests (Premier Platinum HpSAEIA and the novel rapid-kit ImmonoCard STAT! method) for the diagnosis and confirmation of eradication of H. pylori infection, comparing them to the 13C-urea breath test as gold standard.
MethodsDesignProspective, comparative, multicenter study aiming to evaluate the accuracy (sensitivity and specificity) of two stool antigen tests: an EIA (Premier Platinum HpSAPLUS, Meridian Bioscience, Inc.) and novel ImmunoCard STAT! HpSA HD (Meridian Bioscience, Inc.), a rapid one-step lateral flow immunoassay that utilizes a monoclonal anti-H pylori antibody for the diagnosis and confirmation of eradication of H. pylori infection. After applying the sample, the ImmunoCard STAT! HpSA is read after 5min of incubation. Protocol was approved by the Ethics Committee of the participant hospitals.
Consecutive adult patients attending digestive services with dyspepsia (non-investigated or functional) or gastroduodenal peptic ulcer, with indication of H. pylori diagnosis (100 patients), and patients that required eradication confirmation after treatment (166 patients) were included. Patients could be included for both pre- and post-treatment tests, but signing an informed consent for each test was required.
Inclusion and exclusion criteriaInclusion criteria were: indication to perform H. pylori infection diagnosis, able and willing to give written informed consent, having a 13C-urea breath test prescribed and if the patient was included for post eradication treatment diagnosis (confirmation of eradication), the test had to be performed at least 4 weeks after the treatment was discontinued. Exclusion criteria were: age below 18 years, advanced chronic disease that would not allow the patient to complete follow up or attend to visits, previous gastric surgery, alcohol or drug abuse, antibiotic or bismuth salts consumption 4 weeks prior to testing, proton pump inhibitor intake two weeks prior to testing.
To avoid interference with the diagnostic result, proton pump inhibitor treatment was withdrawn at least two weeks prior to urea breath test, and antibiotics were withdrawn at least a month before the test.
Study proceduresMedical history of the patient was reviewed to check inclusion and exclusion criteria. Patients were interviewed for confirmation of medical history, informed of the study, invited to participate and signed informed consent.
Patients were given a collection tube to deposit enough fresh stool sample (over 3g, 3cm3) to perform the two different tests (Premier Platinum HpSA and the novel ImmunoCard STAT!). They were asked to store the samples at a range from 2 to 6°C and bring them to their center in the 36h after collection, where they were stored at −20°C until final processing in a central Lab. In the sample collection, visiting patients were interrogated about sample collection, storage, medication intake and other relevant information disclosed by the patient. Patients and samples were coded to protect patients’ personal data and to maintain blindness between tests.
Study data were collected and managed using REDCap electronic data capture tools at the Spanish Platform for Collaborative Research in Gastroenterology (AEG-REDCap) promoted by the Asociación Española de Gastroenterología (AEG; www.aegastro.es). AEG is a non-profit Scientific and Medical Society focused on Gastroenterology, and it provided this service free of charge, with the sole aim of promoting independent investigator driven research. REDCap (Research Electronic Data Capture)12 is a secure, web-based application designed to support data capture for research studies.
Assessment of accuracyAccuracy was considered as coincident diagnosis between the study test and the gold standard. Gold standard was defined as 13C-urea breath test. As recommended by Maastricht V Consensus Report,1 the tests were performed with the administration of citric acid solution prior to basal breath sample collection. For standardization reasons, all included centers used mass spectrometer method.
Stool specimens were processed by batches. Stool Antigen Tests were evaluated independently by two qualified professionals (LL and MDG, supervised by TA) blind to the clinical and demographic characteristics of the patients and to the results of all the different diagnostic tests. Laboratory personnel were trained by the stool tests manufacturer in the hospital's facilities.
Results from all urea breath tests were recorded as positive(delta>5), negative (delta between<4) or unclear (delta between 4 and 5). Results from stool antigen tests were recorded as positive or negative only, as there is no indeterminate range associated with these tests (EIA cut-off for positive result was set at ≥0.100 by manufacturer instructions); however, a secondary variable classified ImmunoCard positive results as either weak or strong positivity based on the clarity/intensity/brightness of the color band. Inconsistency and agreement between evaluators was also recorded. Patients with borderline urea breath test results (delta between 4 and 5) were asked to repeat the urea breath test and stool sample at least four weeks after testing. These final results were the ones valid for the analyses. Invalid ImmunoCard STAT! HpSA results were solved by immediately repeating STAT on another sample from the same specimen using a kit from a different batch. This final result was the one valid for the analyses. All repetition of tests were recorded for quality control, registering the reason for repetition. A random subset of 300ImmunoCards were photographed after testing and reviewed by the supervisor to check for inconsistencies.
Statistical analysisContinuous variables were presented as arithmetic mean and standard deviations. Qualitative variables were presented as percentage and 95% confidence intervals. All statistical tests used considered a signification level of p<0.05.
The percentage of agreement between test evaluators was estimated by the Kappa statistic. Its interpretation was defined as follows: values lower than 0.39 showed a low agreement; 0.40–0.59 was a fair agreement; 0.60–0.74 reflected good agreement; and 0.75 or more meant an excellent agreement.
Area under the Receiver Operating Characteristics (ROC) curve was used to evaluate the accuracy of EIA numeric result versus the gold standard and to identify the best cut-off point for higher sensitivity and specificity. Logistic binary regression modeling was used to identify potential factors associated with accuracy for both stool tests, including as covariates: gender, age, hospital, indication (ulcer vs. dyspepsia), storage time before lab analysis and patient group (naïve vs. post-eradication).
Primary outcomeThe primary outcome was the overall coincidence of results between the stool antigen diagnostic tests (positive or negative) with the 13C-urea breath test.
Secondary outcomesCoincidence of results between the stool antigen diagnostic tests (positive or negative) with the 13C-urea breath test pre-treatment.
Coincidence of results between the stool antigen diagnostic tests (positive or negative) with the 13C-urea breath test post-treatment.
Sample size calculationSample size was calculated based on the formula by Jones et al. (Emergency Medical Journal 2003;20:453–458). Two different sample size calculations were performed: one for the pre-treatment analysis and one for the post-treatment one. Calculations were performed using an expected (desired) sensitivity and specificity of 97%, a precision of 5%, and a confidence interval of 95%. Based on published data and previous experience in our country, the expected prevalence of H. pylori infection pre-treatment was 45% (in dyspeptic patients attending a gastroenterology outpatient clinic), and post-treatment H. pylori prevalence ranged from 20 to 30% depending on the treatment given (as the effectiveness in clinical practice ranges from 70 to 80%). Under these conditions, the estimated sample size required was 100 pre-treatment and 166 post-treatment.
ResultsA total of 394 patients were screened in 8 centres; of these, 130 were excluded. Reasons for exclusion were: 48 patients did not bring any sample and 19 insufficient amount, 32 samples were discarded due to cold chain violation and one was lost, 22 patients collected the sample out of accepted time range, 7 patients did not meet inclusion criteria, one sample was lost and another was mislabelled, and one patient did not perform urea breath test. No patient had to repeat urea breath test due to unclear results. Two hundred and sixty four patients were included and completed the study protocol, 100 for diagnosis prior to eradication treatment and 164 for post-treatment eradication confirmation.
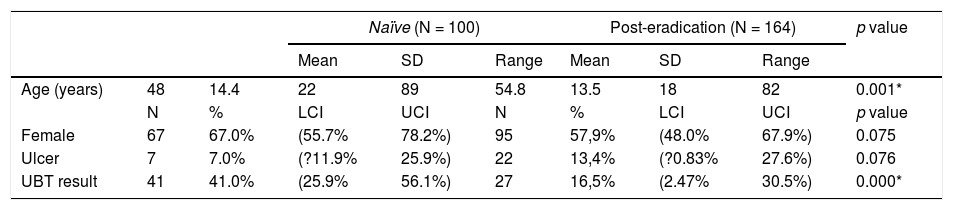
Baseline characteristicsOf the 264 patients, 161 were women (61%), average age was 52±14 years (range 18–89 years). Peptic ulcer disease was diagnosed in 29 patients. Urea breath test classified 68 patients (26%; 95%C.I.=23–28%) as positive and 196 (74%; 72–76%) as negative. Baseline data per patient group (naïve or post-eradication) are shown in Table 1.
Patients’ demographics.
| Naïve (N = 100) | Post-eradication (N = 164) | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||||
| Age (years) | 48 | 14.4 | 22 | 89 | 54.8 | 13.5 | 18 | 82 | 0.001* |
| N | % | LCI | UCI | N | % | LCI | UCI | p value | |
| Female | 67 | 67.0% | (55.7% | 78.2%) | 95 | 57,9% | (48.0% | 67.9%) | 0.075 |
| Ulcer | 7 | 7.0% | (?11.9% | 25.9%) | 22 | 13,4% | (?0.83% | 27.6%) | 0.076 |
| UBT result | 41 | 41.0% | (25.9% | 56.1%) | 27 | 16,5% | (2.47% | 30.5%) | 0.000* |
N, number of patients; LCI, lower 95% confidence interval; SD, standard deviation; UCI, upper 95% confidence interval.
All patients were evaluated by both stool tests in duplicate. EIA's diagnoses were coincident and correlated (r2=0.958, Kappa=0.987) between duplicates in all but one case that scored 0.083 (negative) by technician one and 0.163 (positive) by technician two, and was negative by urea breath test. This patient was excluded from EIA accuracy tests.
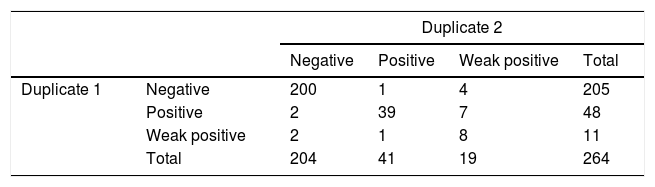
ImmunoCard duplicates coincided in 255/264 cases (96.6%; 94–99%, Kappa=0.829). Technician one classified 11 cases as weak positive and technician two classified 19 cases as weak positive (8 coincident); contingency table is shown in Table 2. Sub-analyses were performed excluding weak positive results and considering them as negative but did not improve results. A random subset of 300 ImmunoCards were photographed during reading and showed to the supervisor who in blind found 0 discordances.
Inter-stool test coincidenceEIA agreed in 249/264 (94.3%; 92–98%) diagnoses with duplicate 1 of ImmunoCard and in 250/264 (94.7%; 93–98%) with duplicate 2. If weak results were excluded, agreement occurred in 242/253 (95.6%; 94–99%) with duplicate 1 and in 236/245 (96.3%; 95–99%) with duplicate 2. If weak results were considered negative, coincidence was 246/264 (93.2%; 91–97%) and 241/264 (91.3%; 89–95%), respectively.
Overall accuracy of stool testsFor the manufacturer's cut-off point, EIA correctly diagnosed 240 patients (90.9%), with sensitivity of 72% (67–78%), specificity of 98% (96–100%), positive predictive value of 93% (89–96%) and negative predictive value of 91% (87–94%). Evaluating the areas under the ROC curves of duplicate tests, technician one achieved 90% (84–95%) and technician two 87% (80–93%). ROC curve identified0.0215 as the best potential cut-off point for the sample, improving results: sensitivity 81% (76–86%) and specificity 97% (96–99.%).
The composite ImmunoCard correctly classified 88.8% of cases, with a sensitivity of 72% (67–78%), specificity of 95% (92–97%), positive predictive value of 82% (78–87%), and negative predictive value of 91% (87–94%). Excluding weak results or considering them as negative, sensitivity decreased to 68% and 55% respectively, without a clear increase in specificity (96% in both).
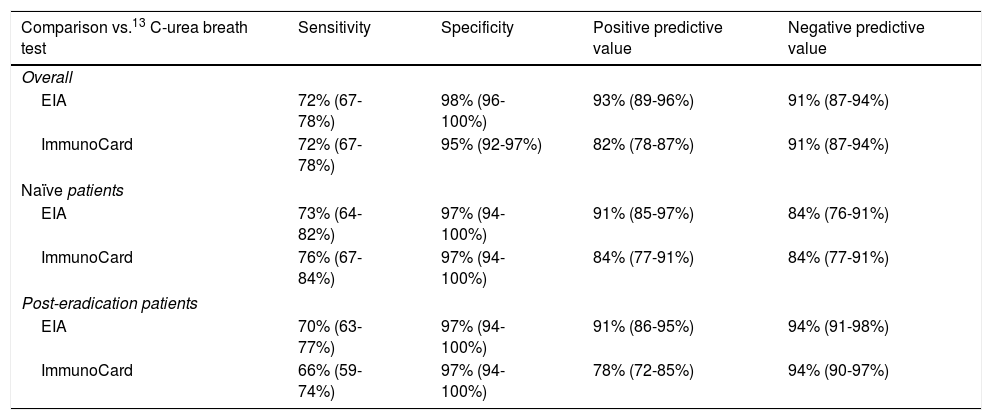
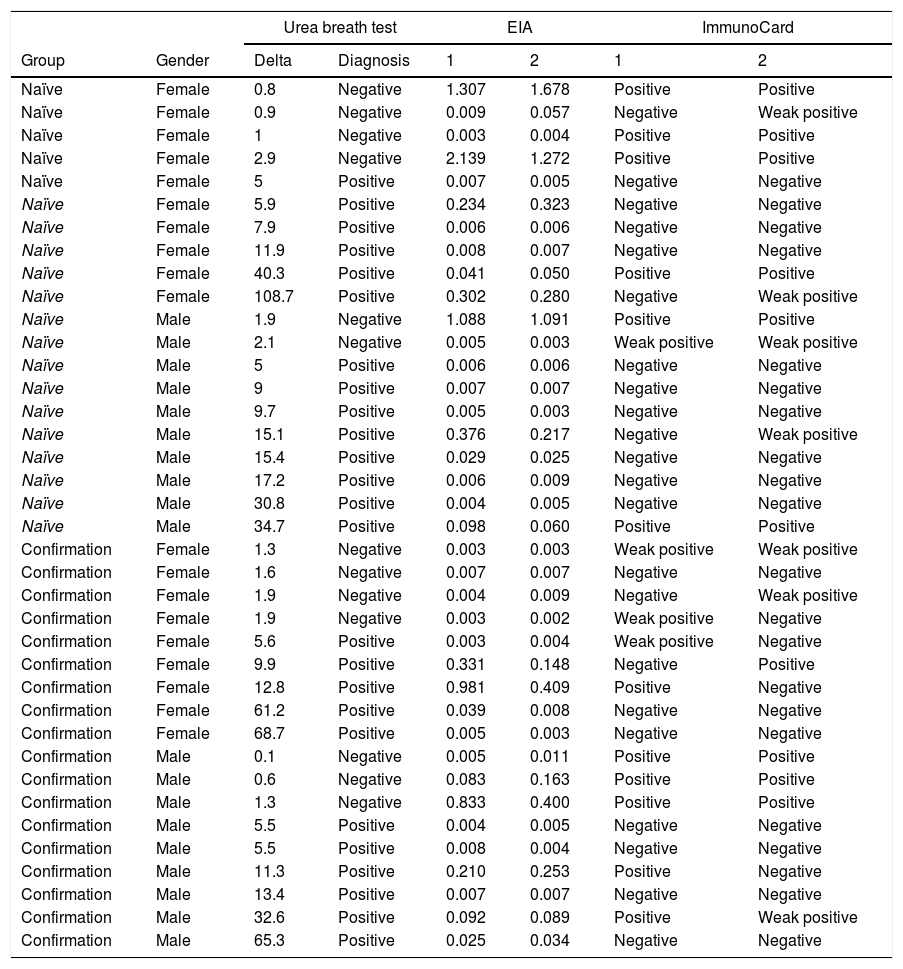
Accuracy pre- and post-treatmentEIA correctly classified 86 naïve patients (86%; 79–93%) and 153 post-treatment patients (93.3%; 90–98%). Composite ImmunoCard correctly classified 84 naïve patients (84%; 77–91%) and 159 post-treatment (97%; 94–100%). Respective sensitivities, specificities, positive and negative predictive values are shown in Table 3. Short description of non-agreeing results is shown in Table 4.
Accuracy scores of ImmunoCard and EIA for Helicobacter pylori diagnosis.
| Comparison vs.13 C-urea breath test | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| Overall | ||||
| EIA | 72% (67-78%) | 98% (96-100%) | 93% (89-96%) | 91% (87-94%) |
| ImmunoCard | 72% (67-78%) | 95% (92-97%) | 82% (78-87%) | 91% (87-94%) |
| Naïve patients | ||||
| EIA | 73% (64-82%) | 97% (94-100%) | 91% (85-97%) | 84% (76-91%) |
| ImmunoCard | 76% (67-84%) | 97% (94-100%) | 84% (77-91%) | 84% (77-91%) |
| Post-eradication patients | ||||
| EIA | 70% (63-77%) | 97% (94-100%) | 91% (86-95%) | 94% (91-98%) |
| ImmunoCard | 66% (59-74%) | 97% (94-100%) | 78% (72-85%) | 94% (90-97%) |
Data in parenthesis represent 95% confidence intervals.
Short description of discrepancies between tests.
| Urea breath test | EIA | ImmunoCard | |||||
|---|---|---|---|---|---|---|---|
| Group | Gender | Delta | Diagnosis | 1 | 2 | 1 | 2 |
| Naïve | Female | 0.8 | Negative | 1.307 | 1.678 | Positive | Positive |
| Naïve | Female | 0.9 | Negative | 0.009 | 0.057 | Negative | Weak positive |
| Naïve | Female | 1 | Negative | 0.003 | 0.004 | Positive | Positive |
| Naïve | Female | 2.9 | Negative | 2.139 | 1.272 | Positive | Positive |
| Naïve | Female | 5 | Positive | 0.007 | 0.005 | Negative | Negative |
| Naïve | Female | 5.9 | Positive | 0.234 | 0.323 | Negative | Negative |
| Naïve | Female | 7.9 | Positive | 0.006 | 0.006 | Negative | Negative |
| Naïve | Female | 11.9 | Positive | 0.008 | 0.007 | Negative | Negative |
| Naïve | Female | 40.3 | Positive | 0.041 | 0.050 | Positive | Positive |
| Naïve | Female | 108.7 | Positive | 0.302 | 0.280 | Negative | Weak positive |
| Naïve | Male | 1.9 | Negative | 1.088 | 1.091 | Positive | Positive |
| Naïve | Male | 2.1 | Negative | 0.005 | 0.003 | Weak positive | Weak positive |
| Naïve | Male | 5 | Positive | 0.006 | 0.006 | Negative | Negative |
| Naïve | Male | 9 | Positive | 0.007 | 0.007 | Negative | Negative |
| Naïve | Male | 9.7 | Positive | 0.005 | 0.003 | Negative | Negative |
| Naïve | Male | 15.1 | Positive | 0.376 | 0.217 | Negative | Weak positive |
| Naïve | Male | 15.4 | Positive | 0.029 | 0.025 | Negative | Negative |
| Naïve | Male | 17.2 | Positive | 0.006 | 0.009 | Negative | Negative |
| Naïve | Male | 30.8 | Positive | 0.004 | 0.005 | Negative | Negative |
| Naïve | Male | 34.7 | Positive | 0.098 | 0.060 | Positive | Positive |
| Confirmation | Female | 1.3 | Negative | 0.003 | 0.003 | Weak positive | Weak positive |
| Confirmation | Female | 1.6 | Negative | 0.007 | 0.007 | Negative | Negative |
| Confirmation | Female | 1.9 | Negative | 0.004 | 0.009 | Negative | Weak positive |
| Confirmation | Female | 1.9 | Negative | 0.003 | 0.002 | Weak positive | Negative |
| Confirmation | Female | 5.6 | Positive | 0.003 | 0.004 | Weak positive | Negative |
| Confirmation | Female | 9.9 | Positive | 0.331 | 0.148 | Negative | Positive |
| Confirmation | Female | 12.8 | Positive | 0.981 | 0.409 | Positive | Negative |
| Confirmation | Female | 61.2 | Positive | 0.039 | 0.008 | Negative | Negative |
| Confirmation | Female | 68.7 | Positive | 0.005 | 0.003 | Negative | Negative |
| Confirmation | Male | 0.1 | Negative | 0.005 | 0.011 | Positive | Positive |
| Confirmation | Male | 0.6 | Negative | 0.083 | 0.163 | Positive | Positive |
| Confirmation | Male | 1.3 | Negative | 0.833 | 0.400 | Positive | Positive |
| Confirmation | Male | 5.5 | Positive | 0.004 | 0.005 | Negative | Negative |
| Confirmation | Male | 5.5 | Positive | 0.008 | 0.004 | Negative | Negative |
| Confirmation | Male | 11.3 | Positive | 0.210 | 0.253 | Positive | Negative |
| Confirmation | Male | 13.4 | Positive | 0.007 | 0.007 | Negative | Negative |
| Confirmation | Male | 32.6 | Positive | 0.092 | 0.089 | Positive | Weak positive |
| Confirmation | Male | 65.3 | Positive | 0.025 | 0.034 | Negative | Negative |
Naïve patients are those who have not been previously prescribed Helicobacter pylori eradication treatment and are being subjected to an initial diagnosis of the infection. Confirmation refers to patients that require testing after treatment to confirm eradication or failure.
The binary logistic regression showed that several factors were associated with higher odds of coincident results between EIA and urea breath test: post-treatment eradication confirmation diagnosis (OR=3.98; 95% C.I.=1.56–10.2; p=0.004), female gender (OR=2.75; 1.13–6.71; p=0.05); and others showed a tendency towards non-coincident diagnosis: storage time before lab analysis (OR=1.11 per month; 1.00–1.22; p=0.043) and patient's age (OR=1.04 per year; 1.00–1.07; p=0.020). No other factor seemed to be associated.
Similar results were found for ImmunoCard, showing higher accuracy for post-treatment confirmation (OR=3.53; 1.43–7.85; p=0.005), and lower for longer storage times (OR=1.10 per month; 1.01–1.21; p=0.037) and older patients (OR=1.04 per year; 1.01–1.07; p=0.02).
DiscussionThe present study evaluated the H. pylori diagnostic accuracy – versus 13C-urea breath test as gold standard – of two different monoclonal stool antigen tests: the novel near-patient ImmunoCard rapid and the commercially available bench standard Premier Platinum HpSA(EIA). Our results showed that there was high stability of results between duplication with the EIA method (Kappa=0.987) and moderate with the ImmunoCard (Kappa=0.829). Low inter-observer or inter-duplicate agreement strongly hampers the validity of a diagnostic test as it reduces the diagnostic precision. It has been described that the most common reasons for this lack of precision can be easily explained in methods based on subjective/unclear outcomes, experienced skills, or easily affected by mishandling. Although EIA protocol is relatively complex and requires trained personnel,13 it seems to maintain high level of precision coincident with other reports on EIA based stool tests.2 In contrast, the ImmunoCard kit evaluated in our study, that, in theory, should be less affected by these nuances, offers a limited precision. One of the reported issues by the technicians was that the consistency of the fecal sample could affect the processing and clot (hard stools) or stain (liquid stools) the card and reduce precision, as has been previously reported.9 Both evaluated stool tests had a high level of inter-test agreement (>95%). This<5% deviation fells on the range of potential stochastic bias probably due to the moderate precision of the ImmunoCard kit.
The studied tests were compared against a locally validated gold standard, 13C-urea breath test,14 following Maastricht consensus recommendations.1 Although high levels of accuracy (91% EIA and 89% Immunocard) and specificity (98% and 95% respectively) were shown, the sensitivity was sub-optimal (72% and 72%) both in naïve diagnostic testing (≈75%) and in post-treatment eradication confirmatory testing (≈70%). Although results are lower than expected, they are inside the range of variability found in the most recent meta-analysis of stool antigen tests which described a high heterogeneity of performances depending on the brand, or even the same brand in different populations.2 Results with the same EIA test used in this study show a high variability in sensitivity ranging from 76% to 97%.15–17 A previous study performed in Turkey with 198patients compared the accuracy of both the EIA and immunoCard methods used in our study, showing sensitivities of 92% and 79% respectively.11 Published results with the previous version immunoCard are even more diverse, ranging from 58% in an Italian study18 to 91% in a Spanish study.19 A meta-analysis from 2008 provided a pooled sensitivity of 93% for that rapid test,20 and in previous experience in Spain sensitivities ranged from an acceptable 90% for eradication confirmation to a disappointing 69–80% for “test and treat” diagnosis of H. pylori.21–23 Even though the three aforementioned studies were performed by the same research team and collaborators, results are highly disperse, coinciding with the lack of precision for ImmunoCard identified in our study.
Sub-analyses performed trying to improve the sensitivity of the methods (excluding weak positives, or controlling for other factors) were unsuccessful with the ImmunoCard; but an adapted cut-off point for positive (>0.025) was able to improve the performance of the EIA method up to 81% without reducing specificity.
Several limitations should be taken in consideration when extracting conclusions of our results. First, the lack of a composite diagnostic test may hide the existence of correct stool classifications misdiagnosed by the urea breath test. The urea breath test and the antigen tests are focused on different aspects of the infection: the breath test focuses on a omnipresent capacity of H. pylori infective strains, the urease activity, while the stool tests focus on the presence of antigens that may be strain specific. Although urea breath test has been strongly validated in the area of study8,14 and is the method of choice recommended by consensus1,7 all around the world, it can rarely cause misdiagnoses: false negatives, generally caused by low H. pylori activity due to low bacterial density, recent use of antibiotics, bacteriostatic agents or proton pump inhibitor consumption; and false positives, which are extremely rare and may be caused by other non-H. pylori urease positive bacteria in the stomach.8 To control for these potential errors, all patients took citric acid prior to urea breath testing to reduce false negative results as recommended in guidelines,1 patients were explained to withdraw antibiotics and proton pump inhibitors prior to testing, that were used as exclusion criteria, and were interrogated about it during testing and during the stool sample reception.
In conclusion, the results from our study show that stool antigen tests have an overall high concordance with urea breath test in Spanish population; however, the studied EIA and ImmunoCard tests evaluated in our protocol showed insufficient sensitivity for a widespread recommendation for its use in clinical practice.
Author's contributionAG McNicholl: Coordinated the project, designed the protocol, planned and coordinated the study, designed and programmed the electronic case report form, analyzed the data, wrote the manuscripts draft, and approved the submitted manuscript.
A. Garre, M. Ramas, J. Perez and M.G. Donday: Supervised, coordinated and monitored data collection, interpreted data, critically reviewed the manuscripts’ drafts, and approved the submitted manuscript.
L. Llorca and M.D. Guerrero: Performed the stool tests and laboratory analyses, interpreted data, critically reviewed the manuscripts’ drafts, and approved the final submitted manuscript.
T. Alarcon: Coordinated the laboratory work and the microbiology part of the project, collaborated in the design the protocol, interpreted the data, critically reviewed the manuscripts’ drafts, and approved the submitted manuscript.
J.P. Gisbert: Directed the project, obtained funding, designed the protocol and planned the study, analyzed and interpreted the data, collected patients, critically reviewed the manuscripts’ drafts, and approved the final submitted manuscript.
The remaining authors collected and helped interpreting data, critically reviewed the manuscripts’ drafts, and approved the submitted manuscript.
Conflict of interestDr. Gisbert has served as a speaker, a consultant and advisory member for or has received research funding from Almirall, Nycomed, AstraZeneca, Casen Recordati, and Allergan. Dr. McNicholl has received retribution from Allergan for formative actions. Dra Pérez-Aisa has served as a speaker and has received retribution from Allergan, Norgine, Casen-Recordati for formative actions and advisory for Shionogi. Dr. Molina-Infante has served as a speaker and has received retribution from Allergan, Almirall and Casen Recordati for formative actions. Dr. Calvet has received grants for research from Abott, MSD, Vifor fees for advisory boards form Abott, MSD, Takeda AND VIFOR and has given lectures for Abott, MSD, Takeda, Shire and Allergan.
The rest of authors disclosed no conflict of interest.
We want to thank the Spanish Association of Gastroenterology (Asociación Española de Gastroenterología, AEG) and the Scientific Director of AEG-REDCap (Adrian G. McNicholl) for providing the e-CRF service free of charge. This project was an investigator initiated study funded by Meridian Bioscience who acted as promoter but took no part in the design, development, analysis or interpretation of this manuscript.