The leucine-rich repeat-containing G-protein-coupled receptor 4 (LGR4) plays an important role in stem cell differentiation, organ development and cancer. Whether LGR4 affects the progression of hepatocellular carcinoma (HCC) remains unknown. This study aimed to reveal the role of LGR4 in HCC.
MethodsClinical samples of HCC were collected to assess the expression of LGR4 and its correlation with patients’ clinical characteristics. The expression level of LGR4 in HCC cells was altered by pharmacological and genetic methods, and the role of LGR4 in HCC progression was analyzed by in vivo and in vitro assays. HCC was induced by diethylnitrosamine (DEN) and carbon tetrachloride (CCl4) in wild-type and LGR4 deficient mice, the effect of LGR4 on HCC was examined by histopathological evaluation and biochemical assays.
ResultsLGR4 expression was up-regulated in HCC samples, and its expression level was positively correlated with tumor size, microvascular invasion (MVI), TNM stage and pathological differentiation grade of HCC patients. In the mouse HCC model induced by DEN+CCl4, knockdown of LGR4 effectively inhibited the progression of HCC. Silencing of LGR4 inhibited the proliferation, migration, invasion, stem cell-like properties and Warburg effect of HCC cells. These phenotypes were promoted by R-spondin2 (Rspo2), an endogenous ligand for LGR4. Rspo2 markedly increased the nuclear translocation of β-catenin, whereas IWR-1, an inhibitor of Wnt/β-catenin signaling, reversed its effect. Deficiency of LGR4 significantly reduced the nuclear translocation of β-catenin and the expression of its downstream target genes cyclinD1 and c-Myc.
ConclusionsLGR4 promotes HCC progression via Wnt/β-catenin signaling pathway.
El receptor de acoplamiento de proteínas G de secuencia repetida 4 (LGR4), rico en leucina, juega un papel importante en la diferenciación de células madre, el desarrollo de órganos y el cáncer. Se desconoce si LGR4 afecta la progresión del carcinoma hepatocelular (HCC). El objetivo de este estudio es revelar el papel de LGR4 en el HCC.
MétodosSe recolectaron muestras clínicas de HCC para evaluar la expresión de LGR4 y su correlación con los resultados clínicos de HCC. Alterar los niveles de expresión de LGR4 en las células de HCC mediante métodos farmacológicos y genéticos y analizar el papel de LGR4 en la progresión del cáncer de hígado mediante mediciones in vivo e in vitro. El HCC fue inducido en ratones de tipo salvaje y con defectos de LGR4 con Nitrosamina de dietilo (DEN) y cloruro de carbono (CCl4), y los efectos de LGR4 sobre el HCC fueron detectados por evaluación histopatológica y determinación bioquímica.
ResultadosLa expresión de LGR4 está regulada en HCC, y su nivel de expresión está positivamente relacionado con el tamaño tumoral, la infiltración microvascular (MVI), la etapa de TNM y el grado de diferenciación patológica en pacientes con HCC. En el modelo de HCC de ratón inducido por DEN+CCl4, golpear bajo LGR4 inhibió efectivamente la progresión del HCC. El silencio de LGR4 inhibe la proliferación, migración, invasión, propiedades similares a las células madre y el efecto Warburg de las células HCC. Estos fenotipos son promovidos por el ligando endógeno roof slab-specific sponge 2 (Rspo2)de LGR4. El Rspo2 aumentó significativamente la translocación nuclear de la proteína beta-catenina, mientras que el inhibidor de la señalización Wnt/beta-cateninaIWR-1 revirtió su acción. La deficiencia de LGR4 redujo significativamente la translocación nuclear de la proteína beta-catenina y su expresión de los genes Diana aguas abajo, cyclinD1 y C-myc.
ConclusiónLGR4 promueve la progresión del HCC a través de la vía de señalización Wnt/beta-catenina.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. The onset of HCC is insidious, and most patients are already in the middle or late stage when they are diagnosed. Even with radical resection therapy, HCC still has a high recurrence rate, making it difficult for patients to recover effectively after surgery.1 Despite of its serious threat on people's life and health due to its high incidence, high mortality and high recurrence rate,2,3 current intervention of HCC is limited. A better understanding of the molecular mechanisms underlying its pathogenesis is crucial for the novel strategy of effective intervention of HCC.
The leucine-rich repeat-containing G-protein-coupled receptor 4 (LGR4) belongs to the superfamily of G protein-coupled receptors (GPCRs), which are characterized by a large leucine-rich extracellular structural domain that recognizes ligands and regulates many cellular processes.4 LGR4 is widely expressed in mammals, with high expression activity in liver, bone, pancreas, skin, placenta, and heart.5 Current research on LGR4 is focused on stem cell differentiation, tissue and organ development, and cancer.6 LGR4 has been shown to be overexpressed in a wide range of tumors such as breast, gastric, colorectal, tongue and skin cancers.7–11 By regulating cell proliferation, migration and invasion, LGR4 is associated with poor patient prognosis.12 LGR4 also enhances the properties of cancer stem cells (CSCs) in various types of tumors, affecting stem cell behavior and tumor progression.7,13,14 Whether LGR4 is involved in the progression of hepatocellular carcinoma remains unclear.
In this study, we investigated the expression of LGR4 in clinical HCC specimens and its relationship with clinicopathological parameters of HCC. Loss or gain of function of LGR4 was achieved by deletion of LGR4 in hepatocytes or treatment with R-spondin2 (Rspo2). And the effects of Rspo2-LGR4 on the growth, metastasis, stem cell-like properties, and Warburg effect of HCC cells were assessed. Further, the role of LGR4 on HCC progression was evaluated in mouse model induced by diethylnitrosamine (DEN) and carbon tetrachloride (CCl4).15,16 In addition, the association of Rspo2-LGR4 with Wnt/β-catenin signaling in HCC was examined. Our results suggest that Rspo2-LGR4 promotes HCC cell proliferation, migration, invasion, stem cell-like properties and Warburg effect. This effect likely occurs via activation of Wnt/β-catenin signaling.
Patients and methodsPatient tissue specimensNinety HCC patients from the First Hospital of Sun Yat-sen University between June 2020 and December 2021 were enrolled in this study. Among these patients, hepatitis B patients accounted for 92.2%, 42.2% had cirrhosis. All patients underwent radical surgery and did not receive any form of preoperative adjuvant therapy. The main clinical features of HCC patients are shown in Table 1. The study protocol was approved by the ethics committee of Sun Yat-sen University (No. [2021]170). Paraffin-embedded HCC samples and adjacent tissues were sectioned and used for immunohistochemical staining.
Association of LGR4 expression with the clinical characteristics from HCC patients.
| Characteristic | Subcharacteristic | n | LGR4 expression | P value | |
|---|---|---|---|---|---|
| Low (27) | High (63) | ||||
| Age (years) | ≤55 | 40 | 12 | 28 | 1.00 |
| >55 | 50 | 15 | 35 | ||
| Gender | Male | 78 | 25 | 53 | 0.457 |
| Female | 12 | 2 | 10 | ||
| Tumor size (cm) | ≤5 | 45 | 19 | 26 | 0.011 |
| >5 | 45 | 8 | 37 | ||
| Microvascular invasion | M0 | 23 | 12 | 11 | 0.027 |
| M1 | 36 | 8 | 28 | ||
| M2 | 31 | 7 | 24 | ||
| Liver fibrosis | S1 | 28 | 8 | 20 | 0.405 |
| S2 | 24 | 5 | 19 | ||
| S3 | 36 | 14 | 22 | ||
| S4 | 2 | 0 | 2 | ||
| AJCC TNM stage | I | 21 | 10 | 11 | 0.028 |
| II | 44 | 14 | 30 | ||
| III–IV | 25 | 3 | 22 | ||
| Differentiation grade | Well | 25 | 12 | 13 | 0.047 |
| Moderately | 33 | 6 | 27 | ||
| Poor | 32 | 9 | 23 | ||
| BCLC stage | A | 35 | 15 | 20 | 0.066 |
| B | 39 | 10 | 29 | ||
| C | 16 | 2 | 14 | ||
| Child–Pugh score | A | 85 | 27 | 58 | 0.317 |
| B | 5 | 0 | 5 | ||
Bold values indicate statistical significance by the Chi-squared test. Differences were considered significant at P<0.05.
Abbreviations: AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer.
Six-week-old male BALB/c nude mice were purchased, housed and cared in the SPF environment at the Experimental Animal Center of Peking University Health Science Center (Beijing, China). The nude mice were randomly divided into two groups: LV-sh-LGR4 (n=6) and LV-sh-NC (n=6). Each mouse was subcutaneously injected with 2×106 HepG2 cells expressed LV-sh-LGR4 or LV-sh-NC into the right flank. Six weeks later, all mice were sacrificed and the tumors were dissected for further evaluation.
Lgr4flox/flox mice generated as described previously17 were housed in the SPF environment at the Experimental Animal Center of Peking University Health Science Center. LGR4 in parenchymal hepatic cells was knocked down by administration of serotype 8 AAV-thyroxine-binding globulin (TBG)-Cre (4×1010 gene copies per mouse) (Weizhen Biosciences Inc., Shandong, China) into four-week-old male Lgr4flox/flox mice by caudal vein injection. AAV8-TBG-GFP was used as a control. Mice were then injected with DEN (25mg/kg, i.p.) at the age of 6 weeks, followed by 25 weekly injections of CCl4 (0.5mL/kg, i.p.) to induce HCC animal model according to the published procedure.16 Mice were randomly assigned to the control (n=6), Lgr4flox/flox+HCC (n=8), and Lgr4lko+HCC (n=8) groups. Mice were euthanized by exsanguination under deep anesthesia. Tissues were harvested two days after the last injection. All protocols were approved by the Institutional Animal Care and Use Committee of the Peking University Health Science Center (No. LA2019141), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell lines, reagents and primersHuman HCC cell lines HepG2 and SMMC-7721 were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). HepG2 cells were cultivated in Dulbecco's modified Eagle's medium (DMEM). SMMC-7721 cells were maintained in RPMI-1640. Both media were supplemented with 10% fetal bovine serum (FBS), 100units/mL of penicillin, and 100μg/mL of streptomycin. All cells were incubated at 37°C in a humid atmosphere containing 5% CO2.
The antibodies used in immunoblotting and immunohistochemistry/immunofluorescence are shown in online supplemental Table 1. The primers used in real-time PCR are listed in online supplemental Table 2, and all other reagents are summarized in online supplemental Table 3.
Transient transfectionLGR4 siRNA (50nM, Synbio-Tech, Suzhou, China), and the negative controls (si-NC) were transfected into HCC cells using the Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, CA, USA) according to the manufacturer's instructions. The efficiency of transfection was evaluated by quantitative real-time PCR (qRT-PCR) and Western blotting.
Viral vector constructs and stable infectionThe LGR4 shRNA lentiviral vector (pHBLV-U6-MCS-CMV-ZsGreen-PGK-Puro LGR4), and control vector were constructed by Hanbio (Shanghai, China). To establish stable cell line, relevant vectors packaged as pseudoviral particles (2×106TU/mL) were infected into HepG2 cells. Cells were selected with 0.5μg/mL of puromycin (Sigma, St. Louis, MO, USA) for four weeks.
CCK-8 and plate colony formation assaysCCK-8 assay was used to detect cell viability. Trypsin digestion of HepG2 and SMMC-7721 cells at the logarithmic growth phase and cell counting were performed. The siRNA of LGR4 and negative control (NC), as well as Rspo2 (100ng/mL) and solvent control PBS were added to the cell suspension, then cells were inoculated in 96-well plates at 1×103/well. Cell viability was assessed at 1–6 days by measuring absorbance at 450nm with a microplate reader.
For the plate colony formation assay, 5×102 HepG2 or SMMC-7721 cells were inoculated onto 6-well plates. After 10 days of culture, unattached cells were washed off with PBS, fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet. Colonies of more than 50 cells were counted.
Wound healing, transwell and tumor sphere formation assaysCells were prepared as described above. Wound healing, transwell and tumor sphere formation assays were performed as previously described.18
Measurement of representative metabolites contents and metabolic enzyme activitiesThe contents of glucose (Applygen, Beijing, China), lactate (Jiancheng Bio, Nanjing, China), and GCK (Solarbio, Beijing, China) activity were measured referring to the manufacturer's instructions.
Immunohistochemical (IHC) stainingAfter obtained tissue samples, paraffin embedding was completed according to standard procedures. The paraffin block were made into slices (6μm) and incubated with primary antibody at 4°C overnight and HRP-linked secondary antibody for 1h. After that, sections were stained with 3,3′-diaminobenzidine and hematoxylin.
LGR4 immunohistochemical staining scoreThe percentage of positive cells under the microscope and the staining intensity were scored as follows: 1) Number of positive staining cells: 5 high-power fields (×200) were observed in each section, and the percentage of positive cells were counted (a) 0, 0–5%; (b) 1, 6–25%; (c) 2, 26–50%; (d) 3, 51–75%; and (e) 4, 76–100%. 2) Staining intensity: (a) colorless, 0; (b) weak, 1; (c) moderate, 2; and (d) intense, 3. 3) Multiply the two points to get the total score. A score of 0–6 was marked as low expression and a score of 7–12 was marked as high expression.
Statistical analysisAll statistical analyses were conducted with GraphPad Prism version 8.0 (GraphPad Software Inc.). Student's t-test was used to compare two groups. One-way ANOVA was used to assess multiple group comparisons. All data were expressed as mean±standard deviation (Mean±SD). P<0.05 was considered statistically different.
ResultsLGR4 is highly expressed in HCC and correlated with the malignant degree of HCCTo investigate the role of LGR4 in HCC, we examined the expression of LGR4 in liver tissues from HCC patients. Relevant to adjacent liver tissues, HCC tissues demonstrated a significant disturbance in lobular structure, obvious hepatocyte degeneration and necrotic foci, increased cellular heterogeneity and irregular mitosis (Fig. 1a). Levels of LGR4 immunoreactivity were up-regulated in HCC tissues compared with adjacent tissues. At the same time, we use immunoglobulin IgG of the same species origin, subtype and dose as LGR4 antibody as the negative control (Isotype control) (Fig. 1b, c). Sixty-three of the 90 HCC patients demonstrated high levels of LGR4 immunoreactivity (Fig. 1d). In addition, levels of LGR4 immunoreactivity were strongly correlated with tumor size, microvascular invasion (MVI), TNM stage and pathological differentiation grade of HCC patients (Table 1). However, there was no significant relationship between LGR4 expression and the degree of liver fibrosis, BCLC stage and Child–Pugh score (Table 1).
LGR4 is highly expressed in HCC and correlated with the degree of malignancy of HCC. (a) Representative H&E staining of human HCC tissues and adjacent tissues. The rectangular image in the left panel was magnified in the middle and right panels. (b) Representative immunohistochemical staining of LGR4 in human HCC tissues and adjacent tissues. The rectangular image in the left panel was magnified in the middle and right panels. Isotype control: The use of the same species source, same subtype, same dose, and same immunoglobulin and subtype of the primary antibody is used to eliminate background staining resulting from non-specific binding of the antibody to the cell surface. (c) Intensity of LGR4 immunoreactivity. (d) LGR4 immunoreactivity is up-regulated in 63 out of 90 HCC tissues. Data was expressed as mean±SD and analyzed by two-tailed Student's t-test. *P<0.05. LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; HCC, hepatocellular carcinoma; SD, standard deviation.
Because the HCC specimens collected were from patients whose prognosis was subject to further follow-up, we used the GEPIA database (http://gepia.cancer-pku.cn) to analyze the relationship between LGR4 and patient prognosis. HCC patients with higher LGR4 expression shows worse overall survival and disease-free survival than those with lower expression despite of no statistical difference (Fig. 1e). Overall, the results suggest that LGR4 is likely associated with hepatocarcinogenesis and its poor prognosis.
LGR4 promotes the proliferation of HCC cellsWe next investigated whether LGR4 affects the proliferation of HCC. Immunoreactivity of proliferating cell nuclear antigen (PCNA) or Ki67 in HCC tissues was increased relevant to adjacent tissues. In contiguous sections, cells with high levels of LGR4 also showed increased levels of PCNA and Ki67 immunoreactivity (Fig. 2a). This observation suggests that LGR4 may be related to the proliferation of HCC cells.
LGR4 promotes HCC cell proliferation. (a) Immunoreactivity of PCNA and Ki67 in human HCC tissues and adjacent tissues. (b–d) Quantitative real-time PCR and Western blotting analysis of LGR4 in HepG2 and SMMC-7721 cells treated with si-LGR4 or si-NC. (e–h) CCK-8 assays (e, f) and plate colony formation assays (g, h) in HepG2 and SMMC-7721 cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4. Control used PBS or scramble siRNA (si-NC). (i, j) Cell cycle analysis of HepG2 (i) and SMMC-7721 (j) cells treated with si-LGR4 or si-NC. (k) Subcutaneous xenograft tumor of HepG2 cells with stable silencing of LGR4, and the weight and volume of xenografts (n=6 mice per group). At least three independently repeated experiments were conducted. Data was expressed as mean±SD and analyzed by two-tailed Student's t-test. *P<0.05, **P<0.01, ***P<0.001. LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; HCC, hepatocellular carcinoma; PCNA, proliferating cell nuclear antigen; CCK-8, cell counting kit-8; Rspo2, R-spondin2; PBS, phosphate buffer saline; SD, standard deviation.
To confirm the effect of LGR4 on the proliferation of HCC cells, following in vitro assays were performed. First, LGR4 expression was knocked down with small interfering RNA (si-LGR4) in HepG2 and SMMC-7721 cells. Quantitative real-time PCR (qRT-PCR) and Western blotting analysis showed that the expression of LGR4 in HCC cells transfected with LGR4 siRNA was significantly lower than that in the si-NC group (Fig. 2b–d). Deficiency of LGR4 significantly suppressed the growth ability of HCC cells evidenced by the CCK-8 assay (Fig. 2e, f) and colony formation assay (Fig. 2g, h). Treatment of HCC cells with Rspo2, an endogenous ligand of LGR4, markedly increased the growth ability of HCC cells compared with the control group (Fig. 2e–h). Knockdown of LGR4 significantly decreased the proportion of S-phase cells (Fig. 2i, j), suggesting that LGR4 promotes the proliferation of HCC cells by regulating the cell cycle.
To further demonstrate that knockdown of LGR4 inhibits the growth of HCC cells, we constructed a xenograft tumor model using a stable HepG2 cell line with knockdown of LGR4. HepG2 cells with significant deficiency of LGR4 (LV-sh-LGR4 group) were inoculated into the axilla of nude mice by subcutaneous injection. As shown in Fig. 2k, the LV-sh-LGR4 group showed a slower growth rate of the xenograft tumor and a smaller final tumor volume and weight relevant to the LV-sh-NC group. These observations further confirm that LGR4 can promote the growth of HCC cells.
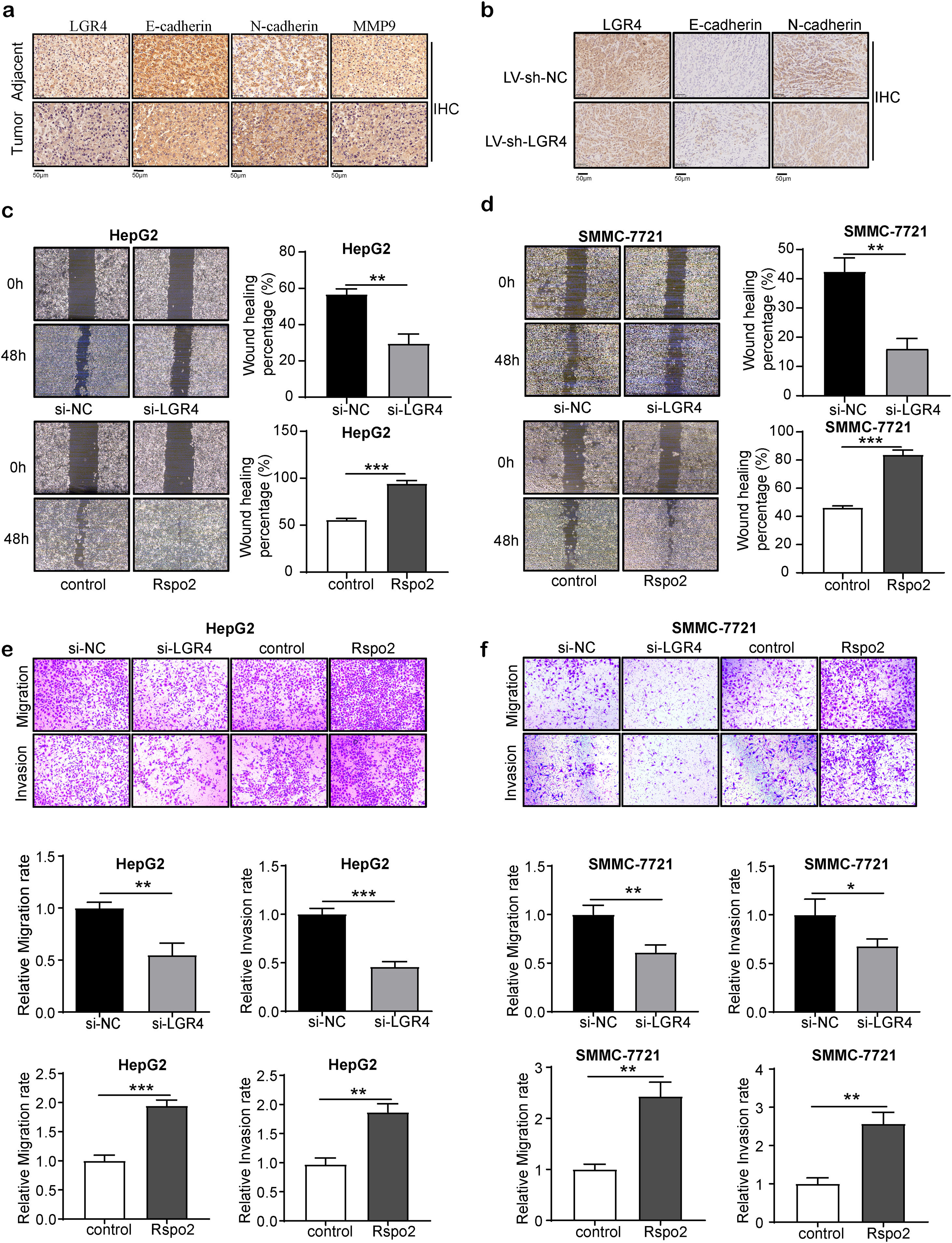
LGR4 increases the invasion and migration of HCC cellsNext, we analyzed whether LGR4 is related to the invasion and migration of hepatocellular carcinoma. We first examined the key molecules relevant to epithelial–mesenchymal transition (EMT). E-cadherin, an epithelial cell marker, was reduced in HCC tissues relevant to adjacent normal tissues. HCC cells with high levels of LGR4 also showed a significantly lower expression of E-cadherin. On the other hand, levels of the mesenchymal phenotypic marker N-cadherin and matrix metalloproteinase 9 (MMP9) increased in HCC cells with high expression of LGR4 (Fig. 3a). Knockdown of LGR4 (LV-sh-LGR4 group) obviously elevated the expression of E-cadherin while reduced the expression of N-cadherin (Fig. 3b). These findings suggest that LGR4 is involved in the EMT of HCC cells.
LGR4 promotes migration, invasion and EMT of HCC cells. (a) Representative immunohistochemical staining of E-cadherin, N-cadherin and MMP9 in human HCC tissues and adjacent tissues. (b) Representative immunohistochemical staining of E-cadherin and N-cadherin in LV-sh-NC and LV-sh-LGR4 group of xenograft tumor model. (c, d) Wound healing assay on HepG2 (c) and SMMC-7721 (d) cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4. Control used PBS or scramble siRNA (si-NC). (e, f) Transwell assays on HepG2 (e) and SMMC-7721 (f) cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4 with (lower panel) or without (upper panel) matrigel, scale bar: 200μm. At least three independently repeated experiments were conducted. Data was expressed as mean±SD and analyzed by two-tailed Student's t-test. *P<0.05, **P<0.01, ***P<0.001. LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; EMT, epithelial–mesenchymal transition; HCC, hepatocellular carcinoma; MMP9, matrix metalloproteinase 9; Rspo2, R-spondin2; PBS, phosphate buffer saline; SD, standard deviation.
Next, we examined the effect of LGR4 on the migration and invasion of HCC cells. The wound healing assay and transwell migration assay showed that si-LGR4 significantly decreased the migration of HepG2 and SMMC-7721 cells than that of si-NC group. Next, cell invasion was examined by using a matrix gel-coated transwell invasion assay, si-LGR4 significantly reduced the invasion in HCC cells than that of si-NC group. In contrast, Rspo2 increased cell migration and invasion in HepG2 and SMMC-7721 cells (Fig. 3c–f). The above results indicate that knockdown of LGR4 inhibits the invasion and migration of HCC cells.
LGR4 enhances the stem cell-like properties of HCC cellsSubsequently, we analyzed whether LGR4 is associated with the stem cell-like properties of hepatocellular carcinoma. CD133 and CD44, markers of cancer stem cells (CSCs), were highly expressed in HCC tissues relevant to adjacent tissues. And their expression levels were higher in HCC cells with enhanced immunoreactivity of LGR4 (Fig. 4a). The effect of LGR4 on the stemness of HepG2 and SMMC-7721 cells were then explored using the sphere formation assay. Deficiency of LGR4 significantly reduced the sphere-forming efficiency of HepG2 and SMMC-7721 cells (Fig. 4b, c). These alterations were associated with a significant reduction in the expression of CD133, CD44 and aldehyde dehydrogenase 1 (ALDH1) (Fig. 4d). Exogenous recombinant Rspo2 increased the sphere-formation efficiency of HepG2 and SMMC-7721 cells (Fig. 4b, c). Consistently, Rspo2 increased the expression of CD133, CD44 and ALDH1 (Fig. 4d). These observations suggest that LGR4 promotes the stem cell-like properties of HCC cells.
LGR4 promotes cancer stem cell-like properties of HCC cells. (a) Representative immunohistochemical staining of CD133 and CD44 in human HCC tissues and adjacent tissues. (b, c) Sphere formation assay of HepG2 (b) and SMMC-7721 (c) cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4 (scale bar: 200μm). Control used PBS or scramble siRNA (si-NC). (d) Quantitative real-time PCR analysis of CD133, CD44 and ALDH1 expression in HepG2 cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4. Control used PBS or scramble siRNA (si-NC). At least three independently repeated experiments were conducted. Data was expressed as mean±SD and analyzed by two-tailed Student's t-test. *P<0.05, **P<0.01, ***P<0.001. LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; HCC, hepatocellular carcinoma; Rspo2, R-spondin2; PBS, phosphate buffer saline; ALDH1, aldehyde dehydrogenase 1; SD, standard deviation.
The Warburg effect of LGR4 was next examined. Expression levels of glucose kinase (GCK) and pyruvate kinase M (PKM), two key enzymes of glycolysis, were increased in HCC tissues relevant to adjacent tissues. And their levels were higher in regions with high LGR4 expression (Fig. 5a). Further experiments using HepG2 cells with deficiency of LGR4 showed that knockdown of LGR4 by its siRNA significantly reduced the expression of genes relevant to glycolysis such as GCK, phosphofructokinase (PFKL), pyruvate kinase 2 (PKM2), glucose 6-phosphate dehydrogenase (G6PD) and lactate dehydrogenase A (LDHA). On the other hand, Rspo2 significantly increased the expression of these enzyme genes (Fig. 5b). Moreover, knockdown of LGR4 markedly decreased glucose consumption and lactate production, while Rspo2 increased these metabolisms (Fig. 5c, d). These results suggest that LGR4 can enhance the Warburg effect in HCC cells.
LGR4 promotes the Warburg effect of HCC cells. (a) Representative immnunohistochemical staining of GCK and PKM in human HCC tissues and adjacent tissues. (b) Quantitative real-time PCR analysis of GCK, PFKL, PKM2, G6PD and LDHA expression in HepG2 cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4. Control used PBS or scramble siRNA (si-NC). (c, d) Glucose (c) and lactate (d) concentration in the supernatant of cultured HepG2 cells treated with exogenous Rspo2 (100ng/mL) or si-LGR4. Control used PBS or scramble siRNA (si-NC). At least three independently repeated experiments were conducted. Data was expressed as mean±SD and analyzed by two-tailed Student's t-test. *P<0.05, **P<0.01, ***P<0.001. LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; HCC, hepatocellular carcinoma; GCK, glucose kinase; PKM, pyruvate kinase M; PFKL, phosphofructokinase; G6PD, glucose 6-phosphate dehydrogenase; LDHA, lactate dehydrogenase A; Rspo2, R-spondin2; PBS, phosphate buffer saline; SD, standard deviation.
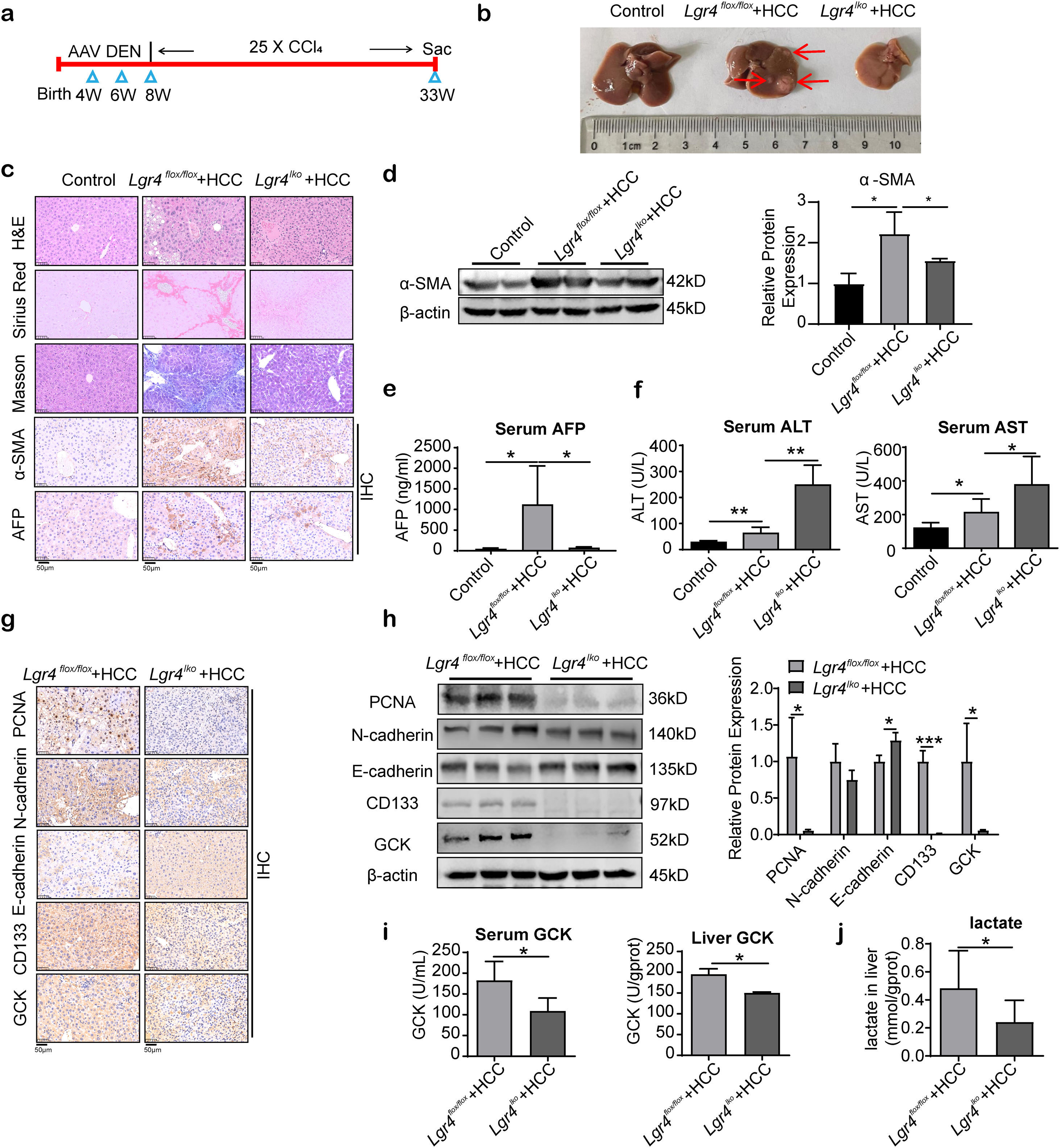
To further determine the role of LGR4 in hepatocellular carcinoma formation, we constructed a transgenic mouse in which LGR4 was specifically knocked down in liver parenchymal cells. HCC was then induced by DEN and CCl4 in these transgenic mice (Fig. 6a). In Lgr4flox/flox mice, DEN and CCl4 successfully induced HCC in liver. Multiple cancer foci with various sizes were detected (Lgr4flox/flox+HCC group). In mice with knockdown of LGR4 (Lgr4lko+HCC group), livers were significantly smaller and no obvious cancer foci were observed (Fig. 6b). H&E staining showed that liver steatosis and fibrosis were obviously reduced in Lgr4lko+HCC mice relevant to Lgr4flox/flox+HCC animals. In the Lgr4flox/flox+HCC group, polygonal cancer cells exhibited abundant eosinophilic cytoplasm and heterogeneous nuclei (Fig. 6c). Sirius Red staining, Masson staining and immunoreactivity of alpha smooth muscle actin (α-SMA) revealed an increased deposition of collagen fibers in the Lgr4flox/flox+HCC mice compared with Lgr4lko+HCC and control animals, indicating that deficiency of LGR4 rendered the mice of Lgr4lko+HCC group resistant to fibrosis (Fig. 6c, d). Alpha-fetoprotein (AFP), a marker for primary hepatocellular carcinoma, was significantly increased in the Lgr4flox/flox+HCC group compared to the control and Lgr4lko+HCC groups (Fig. 6c, e). Levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly increased in Lgr4flox/flox+HCC and Lgr4lko+HCC groups relevant to the control group, with the highest levels detected in the Lgr4lko+HCC group (Fig. 6f). The above results confirm the concept that LGR4 could promote HCC formation in mouse model induced by DEN and CCl4.
LGR4 promotes the HCC formation in mice. (a) Protocol on HCC model induced by DEN combined with CCl4. (b) Representative images of livers from each group of mice. Mice were randomly assigned to the control, Lgr4flox/flox+HCC, and Lgr4lko+HCC groups (n=6–8). (c) Representative images of H&E, Sirius Red staining, Masson staining, immunohistochemical staining of α-SMA and AFP of livers from each group of mice. (d) Western blotting analysis of α-SMA in liver tissues from each group of mice. (e) Determination of serum AFP in each group of mice. (f) Measurement of ALT and AST in the serum of each group of mice. (g) Representative images of immunohistochemical staining of PCNA, N-cadherin, E-cadherin, CD133 and GCK in livers of each group of mice. (h) Representative Western blotting of PCNA, N-cadherin, E-cadherin, CD133 and GCK in liver tissues from each group of mice. (i) GCK enzyme activity in serum and liver of each group of mice. (j) Lactate concentration in liver tissues. At least three independently repeated experiments were conducted. Data was expressed as mean±SD. Significance tested using: One-Way ANOVA (d–f), and two-tailed Student's t-test (h–j). * P<0.05, **P<0.01. LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; HCC, hepatocellular carcinoma; DEN, diethylnitrosamine; CCl4, carbon tetrachloride; α-SMA, alpha smooth muscle actin; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PCNA, proliferating cell nuclear antigen; GCK, glucose kinase; ANOVA, analysis of variance; SD, standard deviation.
Further analysis using IHC staining and Western blotting showed that PCNA expression was significantly increased in the Lgr4flox/flox+HCC group compared with the Lgr4lko+HCC group, suggesting that deficiency of LGR4 could reduce the hepatocyte proliferation. N-cadherin expression was increased, and E-cadherin expression decreased in the Lgr4flox/flox+HCC group compared with the Lgr4lko+HCC group, suggesting that LGR4 could promote EMT in mouse hepatoma cells. CD133, a CSCs marker, was significantly increased in the Lgr4flox/flox+HCC group compared with the Lgr4lko+HCC group, indicating that deficiency of LGR4 could reduce the stemness of mouse HCC cells (Fig. 6g, h). GCK, a key enzyme of glycolysis, as well as GCK activity in serum and liver tissues were significantly increased in the Lgr4flox/flox+HCC group compared with the Lgr4lko+HCC group (Fig. 6g–i). Consequently, increase in the production of lactate, a glycolytic metabolite, was significantly attenuated in the Lgr4lko+HCC mice relevant to Lgr4flox/flox animals (Fig. 6j), suggesting that deficiency of LGR4 could reduce the Warburg effect of mouse HCC cells.
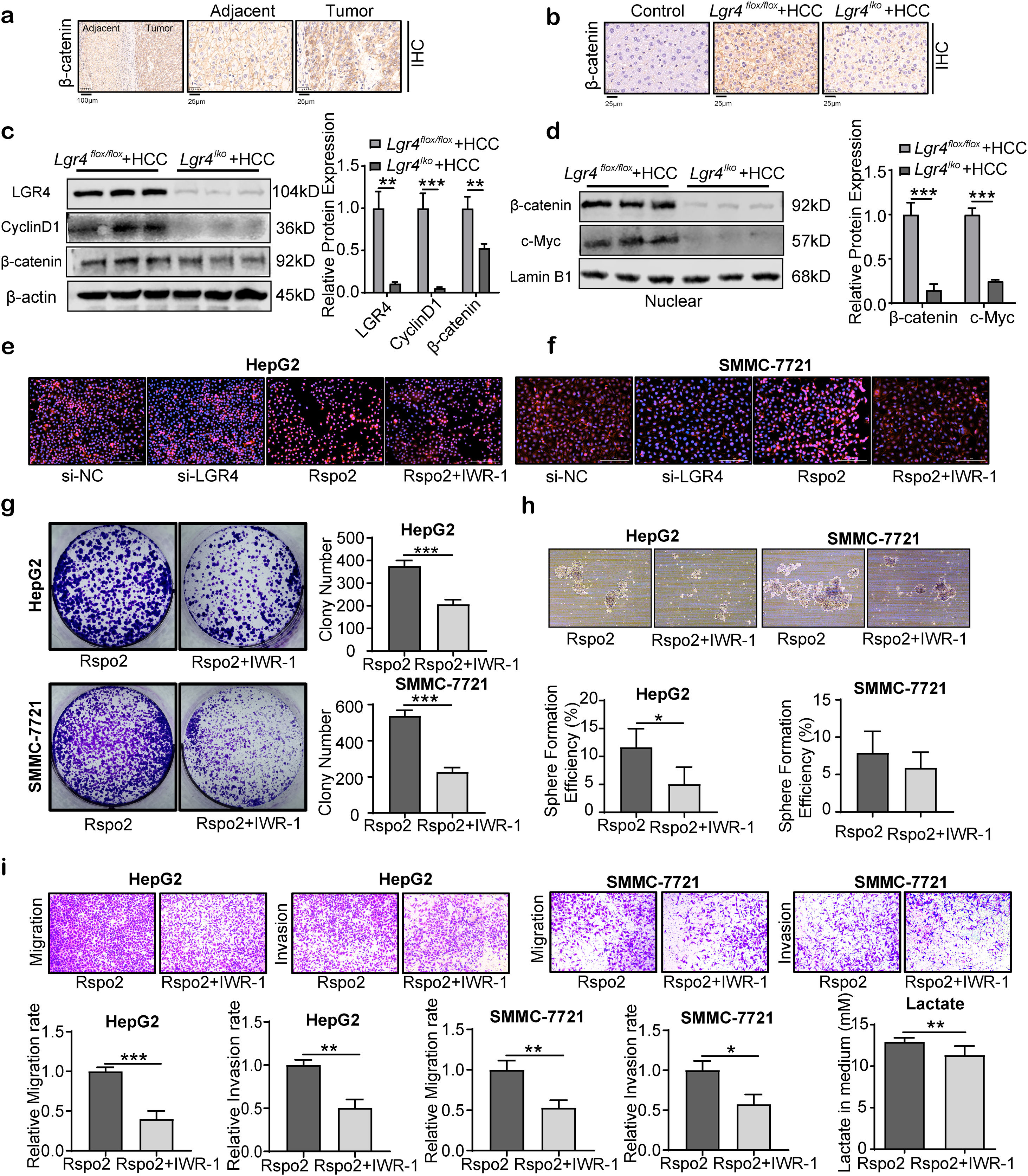
Rspo2-LGR4 exacerbates HCC progression via Wnt/β-catenin signaling pathwayActivation of LGR4 by R-spondins has been recognized to potentiate the Wnt/β-catenin signaling.19 We thus examined this signaling pathway in HCC cells. Firstly, β-catenin immunoreactivity was significantly increased in patients’ HCC tissues compared to adjacent tissues (Fig. 7a). Increased β-catenin expression was also observed in mouse HCC specimens (Fig. 7b). Deficiency of LGR4 (Lgr4lko+HCC group) obviously reduced β-catenin expression compared with the Lgr4flox/flox+HCC group (Fig. 7b). Knockdown of LGR4 significantly decreased β-catenin expression and its nuclear translocation. This alteration was associated with the down-regulation of c-Myc and cyclinD1 expression, the downstream targets of Wnt/β-catenin signaling pathway (Fig. 7c, d).
Rspo2-LGR4 exacerbates HCC progression via Wnt/β-catenin signaling pathway. (a) Representative immunohistochemical staining of β-catenin in human HCC tissues and adjacent tissues. The rectangular image in the left panel was magnified in the right panels. (b) Representative images of β-catenin immunohistochemical staining in the liver tissues of each group of mice. (c) Representative Western blotting of LGR4, cyclinD1 and β-catenin in liver tissues from each group of mice. (d) Representative Western blotting of nuclear β-catenin and c-Myc in liver tissues from each group of mice. (e, f) Immunofluorescent images for β-catenin in HepG2 (e) and SMMC-7721 (f) cells treated with si-LGR4, exogenous Rspo2 (100ng/mL) or Rspo2+IWR-1 (10μM). Scale bar: 200μm. (g–i) Plate colony-formation, sphere formation and transwell assays on HepG2 and SMMC-7721 cells treated with exogenous Rspo2 (100ng/mL) or Rspo2+IWR-1 (10μM). Scale bar: 200μm. The lactate concentration in medium in HepG2 cells treated with exogenous Rspo2 (100ng/mL) or Rspo2+IWR-1 (10μM). At least three independently repeated experiments were conducted. Data was expressed as mean±SD and analyzed by two-tailed Student's t-test. *P<0.05, **P<0.01, ***P<0.001. Rspo2, R-spondin2; LGR4, the leucine-rich repeat-containing G-protein-coupled receptor 4; HCC, hepatocellular carcinoma; SD, standard deviation.
We next examined the nuclear translocation of β-catenin in HCC cell lines by cellular immunofluorescence. Knockdown of LGR4 obviously decreased the translocation of β-catenin to the nucleus, while Rspo2 increased its nuclear translocation (Fig. 7e, f). This effect was blocked by IWR-1, an inhibitor of Wnt/β-catenin signaling pathway. Blockade of Wnt/β-catenin signaling by IWR-1 significantly attenuated the up-regulation of colony formation, migration, invasion and sphere formation, as well as production of lactate in HepG2 and SMMC-7721 HCC cells induced by Rspo2 (Fig. 7g–i). These observations suggest that LGR4 promotes HCC formation through the Wnt/β-catenin signaling pathway.
DiscussionEmerging evidence has suggested that LGR4 is associated with the occurrence, development and metastasis of various cancers such as breast, ovarian, lung and intestinal cancers.7,20–24 The relation between LGR4 and hepatocellular carcinoma, one of the most common types of malignant tumors worldwide, remains largely unknown. In this study, we demonstrate that LGR4 could promote the progression of HCC via increasing proliferation, migration, invasion, stemness and Warburg effect of HCC cells.
The development of HCC cancer involves genetic and epigenetic alterations that free cells from mechanisms limiting proliferation and survival. Majority of these alterations involves intracellular molecules such as oncogenes and nuclear transcription factors. Our present study has identified LGR4, a G protein-coupled receptor, as a novel molecule critical for the development of HCC. This concept is supported by following observations. LGR4 expression is up-regulated in HCC cells. Its level is correlated with the malignant degree of HCC. Further, loss of LGR4 decreases tumorigenic capacity and cell proliferation in xenograft tumor and chemical induced mouse liver cancer. Consistently, high level of LGR4 has been shown to promote the proliferation of glioma and gastric cancer cells,25,26 as well as to reduce overall survival and recurrence-free survival in breast and ovarian cancers.7,21 All these findings indicate that LGR4 is critical for the progression of cancer cells.
Cancer stem cells, a group of cells able to self-renew and unlimitedly produce tumor cells, are critical for tumor survival, proliferation, metastasis and recurrence.27,28 Our study suggests that LGR4 promotes stem cell-like properties in HCC cells. Deficiency of LGR4 reduces the expression of CSCs markers CD133, CD44, ALDH1 and decreases the sphere-forming ability of HCC cells in suspension culture.29 These results suggest that LGR4 promotes stem cell-like properties in HCC cells. Consistently, LGR4 has been shown to express preferentially in cancer stem cell subpopulations compared to non-cancer stem cells.6 Further, down-regulation of LGR4 decreases the self-renewal potential of breast CSCs and disrupts the EMT process by modulating the Wnt/β-catenin signaling cascade.7 Together, these evidence indicate that LGR4 may function through the CSCs to promote the growth of various cancers.
EMT, the transformation of epithelial cells into mesenchymal cells, is the most common cause of tumor invasion and metastasis initiation.30 Our results suggest that interfering with LGR4 expression reduces the invasion and migration of HCC cells. This observation is further confirmed with the xenograft tumor models and mouse HCC models. Consistent with our study, loss of LGR4 reduces migration, invasion and metastasis of breast cancer cells.7,25 Knockdown of LGR4 expression decreases the invasive potential of HeLa cells and Lewis lung cancer cells. In contrast, over-expression of LGR4 increases the invasive activity and lung metastatic potency of HCT116 cells in vitro.22 Rspo3/LGR4 signaling enhances cell migration and invasion by promoting EMT in lung cancer cells.23 Suppression of LGR4 by over-expression of mir-449b reduces the proliferation and invasion ability of lung cancer cell lines.31 These studies suggest a significant role for LGR4 in tumor migration and invasion.
Warburg effect, discovered by Otto Warburg and colleagues, defines the shift to use glycolysis instead of oxidative phosphorylation as the main energy supply even under conditions of adequate oxygen supply in tumor cells.32 It is one of the important features of energy metabolism in cancer cells. By consuming large amount of glucose, Warburg effect provides material basis for tumor cells to synthesize biological macromolecules such as nucleic acid, amino acid and fatty acid.33 Further, the lactic acid produced by glycolysis of tumor cells forms a local acidic microenvironment which favors the invasion of tumor cells.34 In addition, increased production of NADPH and glutathione during the Warburg effect enhances ability of tumor cells to resist oxidative damage.35 Thus, Warburg effect is critical for growth and invasion of various cancers. The relation between LGR4 and tumor metabolism remains unknown. Our study reveals that LGR4 could promote metabolic reprogramming in HCC cells. Down-regulation of LGR4 reduces the expression and enzyme activity of enzymes relevant to glycolysis, leading to subsequent decrement in the production of lactate. These results suggest that LGR4 may promote HCC progression by facilitating the Warburg effect.
The intracellular signaling for LGR4 remains largely unknown. Previous studies have suggested that activation of LGR4 by any of its four ligands R-spondin1-4 could potentiate the Wnt/β-catenin signaling.36 Upon binding with R-spondins, LGR4 functions to inhibit the activity of the cell-surface transmembrane E3 ubiquitin ligases ZNRF3 and its homolog ring finger 43 (RNF43), leading to subsequent reduction in the degradation of the Frizzled/LRP5-6 complex. The Frizzled/LRP5-6 receptor complex then interacts with Wnt ligands to increase cytoplasmic accumulation of β-catenin and its subsequent nuclear translocation.19 The role of Rspo2 in HCC progression remains controversial.37–39 Our studies extend the knowledge on the potentiation of Wnt/β-catenin signaling by Rspo2-LGR4 to HCC cells. Activation of LGR4 increases nuclear translocation of β-catenin, leading to subsequent increment in cyclinD1 and c-Myc expression in HCC cells. Blockade of Wnt/β-catenin signaling by IWR-1 attenuates the proliferation, invasion, migration, stemness, and Warburg effect of HCC cells induced by Rspo2. These results suggest that Rspo2-LGR4 regulates HCC progression through Wnt/β-catenin signaling. However, it is worth of noting that mechanism other than Wnt/β-catenin signaling may mediate the effect of LGR4 on cancer cells. Yue and colleagues have reported that LGR4 levels are closely associated with the epidermal growth factor receptor (EGFR) signaling instead of the recognized Wnt/β-catenin pathway.20
In our collected HCC patient samples, there was no significant correlation between LGR4 and liver fibrosis, probably due to the small amount of patient specimens and individual patient differences. However, in the mouse HCC model, we observed that LGR4 significantly inhibited the formation of liver fibrosis in mice. We also demonstrated in a pre-test that LGR4 inhibits the progression of liver fibrosis in a mouse model of liver fibrosis, suggesting that intervention with LGR4 at the stage of liver fibrosis can delay or prevent the progression of liver disease. What's more, in the mouse HCC model, although knockdown of LGR4 inhibited hepatocellular carcinoma formation, compared with the Lgr4flox/flox+HCC group, the Lgr4lko+HCC group showed reduced liver volume and increased levels of ALT and AST. Previous studies have shown that LGR4 deletion reduces hepatocyte proliferation and affects liver size by decreasing Wnt zonation.40 This suggests that LGR4 also plays an important role in the regulation of liver size and liver function.
In conclusion, our study demonstrates that Rspo2-LGR4 promotes proliferation, migration, invasion, stem cell-like properties and Warburg effect of HCC cells by Wnt/β-catenin signaling pathway. Our findings provide evidence that LGR4 is activated and acts as a tumor promoter in HCC. LGR4 may serve as a biomarker for predicting HCC progression and a potential therapeutic target for HCC.
Authors’ contributionsConception, design and supervision: Yanghui Bi, Liping Zhang, Yue Yin and Weizhen Zhang.
Data acquisition: Yanghui Bi.
Analysis and interpretation of data: Yanghui Bi, Yue Yin and Weizhen Zhang.
Writing, review, and/or revision of the manuscript: Yanghui Bi, Michael W. Mulholland, Yue Yin and Weizhen Zhang.
Technical support: Yue yin, Yan Song, Lijun Sun.
Ethical statementThe study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Sun Yat-sen University (No. [2021]170). Experiments were performed under a research protocol with approval of the Institutional Animal Care and Use Committee of the Peking University Health Science Center (No. LA2019141), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
FundingThis research was supported by grants from the National Natural Science Foundation of China (81730020, 81930015, and 82070592) and National Institutes of Health Grant R01DK112755, 1R01DK129360 and 1R01DK110273 (USA). Weizhen Zhang provided financial support for the conduct of the research and participated in the study design, analysis and interpretation of the data.
Conflict of interestNo potential conflict of interest was disclosed.