Large serrated polyps (SP), proximal SP, SP with dysplasia and the presence of multiple sessile serrated adenomas/polyps (SSA/P), which we refer to as SP with increased risk of metachronous lesions (SPIRML), have been associated with an increased risk of advanced colon lesions on follow-up. It is unclear, however, whether SPIRML are also associated with an increased risk of synchronous advanced colorectal neoplasia (ACN).
AimThe aim of this study was to estimate the prevalence of SPIRML and to evaluate the association between SPIRML and synchronous ACN.
MethodsA cross-sectional population-based study in all patients (1538) with histological diagnosis of SP obtained from colonoscopies, sigmoidoscopies and colonic surgery performed in Navarra Health Service hospitals (Spain) in 2011. Demographic parameters and synchronous colonic lesions (adenomas, advanced adenomas [AA] and ACN) were analysed.
ResultsOne fourth of the sample (384 patients) presented SPIRML. These were older patients, with a slight predominance of women, and with no differences in body mass index (BMI) compared to patients without SPIRML. In the univariate analysis, patients with SPIRML showed an increased risk of adenoma, AA and ACN. In the multivariate analysis, the SPIRML group had a higher risk of synchronous AA and ACN (odds ratio [OR]: 2.38 [1.77–3.21] and OR: 2.29 [1.72–3.05], respectively); in the case of ACN, this risk was statistically significant in both locations (proximal or distal), with OR slightly higher for the proximal location. Different subtypes of SPIRML had a higher risk of AA and synchronous NA.
ConclusionSPIRML were common in patients with SP, and their presence was associated with an increased risk of synchronous ACN.
Los pólipos serrados (PS) grandes, PS proximales, PS con displasia y la presencia de múltiples adenomas sésiles serrados (P/ASS), que englobamos bajo el término PS con riesgo aumentado de lesiones metacrónicas (PSRALM), se asocian a un mayor riesgo de presentar dichas lesiones, pero desconocemos si también se asocian a un mayor riesgo de neoplasia avanzada de colon (NA) sincrónica.
ObjetivoEstimar la prevalencia de PSRALM y evaluar la asociación con NA sincrónica.
MétodosSe trata de un estudio transversal de base poblacional que incluyó a todos los pacientes (1.538) con diagnostico histológico de PS de muestras procedentes de colonoscopias, rectosigmoidoscopias e intervenciones quirúrgicas de los hospitales públicos del Servicio Navarro de Salud durante el año 2011. Se analizaron parámetros demográficos y presencia de lesiones sincrónicas de colon (adenomas, adenomas avanzados [AA] y NA)
ResultadosLa cuarta parte de los pacientes (384) presentaron PSRALM, con una edad media más avanzada, un ligero predominio en mujeres y sin diferencias en cuanto al IMC respecto a los pacientes sin PSRALM. En el análisis multivariante el grupo PSRALM presentó un mayor riesgo de AA y NA sincrónicos (OR: 2,38 [1,77-3,21] y OR: 2,29 [1,72-3,05] respectivamente) y en el caso de NA, este riesgo fue estadísticamente significativo en ambas localizaciones (proximal y distal), con OR superior para la proximal. Los distintos subtipos de PSRALM presentaron un mayor riesgo de AA y NA sincrónicos.
ConclusiónLos PSRALM fueron frecuentes entre los pacientes con PS y se asociaron a un mayor riesgo de presentar NA sincrónica.
Colorectal cancer (CRC) is one of the most common cancers in Western countries, and is the second most common cause of cancer death in the United States and Europe. Epidemiological, molecular and genetic studies undertaken in the last 2 decades have broadened our understanding of this disease. These studies have shown that CRC, hitherto considered a homogeneous entity, is in fact heterogeneous in both its oncogenesis and development, and treatment and prevention strategies have changed accordingly.1
CRC usually progresses from known precancerous lesions such as colon polyps. The term colon polyp includes colon adenomas, serrated polyps (SP) and a heterogeneous group of lesions that include different types of polyps – inflammatory, hamartomatous, juvenile, etc. SPs were initially differentiated, from a histological perspective, into hyperplastic polyps (HP) and serrated adenomas (described by Longrace and Fenoglio-Preiser in 1990).2 Later, in 2002, Torlakovic et al.3 sub-classified serrated adenomas into sessile serrated adenomas/polyps (SSA/P) and traditional serrated adenomas (TSA). Finally, the new World Health Organisation (WHO) classification includes these polyps within the denomination SP, and classifies them into 3 histological types: HPs, SSA/Ps (with and without dysplasia) and TSAs.4
Colon adenomas were previously considered to be preneoplastic lesions that could progress to CRC through the adenoma-carcinoma sequence, whilst HPs were considered harmless lesions, with no risk of becoming malignant. However, in the last 2 decades, thanks to improved genetic and molecular understanding of the pathogenesis of CRC, the efforts made by pathologists to standardise diagnostic criteria, and the qualitative and technological improvements made in colonoscopy, the malignization of SPs has been reconsidered, and they are now believed to progress via the serrated pathway to CRC, with this route being responsible for 15–30% of CRCs.5,6 However, not all SPs will progress to CRC. The natural history of SPs and the genetic-epigenetic changes that cause them to progress to CRC are still unclear, so at present, SPs that will follow a benign course cannot be differentiated from those that will evolve to CRC. Furthermore, the consideration of SPs as precancerous lesions has also led to a change in CRC prevention strategies. While there were no specific recommendations for post-polypectomy endoscopic surveillance of SPs prior to 2010, in the last 5 years, some authors and scientific societies have stressed the importance of considering SPs as preneoplastic lesions, and advise taking them into account in screening programmes.
In this respect, several authors and scientific societies7–10 have recently highlighted certain characteristics of SPs that are associated with a higher risk of metachronous lesions of the colon, and that must be taken into account when defining the endoscopic surveillance interval in patients with SPs. These characteristics are the presence of large SPs (≥10mm); presence of proximal SPs (located proximal to the descending colon); presence of SPs with dysplasia (SSA/P with dysplasia or TSA) and the presence of multiple SSA/Ps (3 or more SSA/Ps). In our study, we unified these characteristics under the term serrated polyp with increased risk of metachronous lesions (SPIRML). The recommended endoscopic surveillance interval for patients with SPIRML differs little among authors and scientific societies, and in general, ranges from 1 to 5 years, according to the type of SPIRML.
In the last 7 years, several studies have shown an association between large (≥10mm) or proximal SPs (proximal to the descending colon) and the synchronous presence of CRC11–16 or advanced colorectal neoplasia (ACN).12,13,17–24 The presence of dysplasia in the SPs is considered a marker of a more advanced precancerous lesion, both in cases of SSA/P with dysplasia and TSA.25–28 The presence of multiple SPs has also been associated with a higher risk of neoplastic lesions of the colon, the clearest example of which is serrated polyposis syndrome.9,28 However, no studies to date have investigated the risk of presenting synchronous ACN lesions in patients with SPs with any of the 4 characteristics that define SPIRML.
The aims of this population-based study were to estimate the prevalence of SPIRML in patients diagnosed histologically with SPs in the Navarra region of Spain, and to evaluate the association of SPIRMLs with the synchronous presence of ACN.
Materials and methodsPopulationThe study population comprised all patients diagnosed with SPs by histological study in the records of the histopathology laboratories of 3 public hospitals of the Navarra Health Service in 2011 (Complejo Hospitalario de Navarra, Hospital Reina Sofía and Hospital García Orcoyen, which provide healthcare services to a population of 542,961 individuals aged over 14 years), and included the samples obtained from endoscopic studies (colonoscopy or rectosigmoidoscopy), surgical specimens and clinical autopsies.
Histological findingsThe histological samples from patients included in the study were analysed by 6 pathologists experienced in gastrointestinal pathology. Patient demographic (age, sex, body mass index [BMI]) and morphological characteristics (number, size and location) of the histologically diagnosed polyps were recorded. The open biopsy forceps technique was used to calculate the size of the excised polyps.29 Polyps resected using a polypectomy loop were measured immediately before resection. For polyps from surgical specimens, the size was calculated once the sample had been fixed in formaldehyde. In the cases of endoscopic tests, the location of the lesions was determined during extraction of the endoscope; the lesion was considered to be proximal when it was found proximal to the descending colon or when it was found ≥60cm from the external anal margin.
SPs were classified according to the 2010 WHO classification4 into 3 types of polyps: HP, SSA/P (with or without associated dysplasia) and TSA. Cases of SSA/P and TSA were reviewed by 2 pathologists (MG and MM); additionally, polyps that were difficult to classify into an SP subtype were evaluated by consensus by a group of pathologists.
SPIRML was defined as any SP that presented at least 1 of the characteristics associated with a higher risk of presenting ACN during endoscopic surveillance: large size (≥10mm), proximal location (located proximal to descending colon), presence of dysplasia (SSA/P with dysplasia and TSA), or multiple SSA/Ps (3 or more SSA/Ps). Serrated polyp with no increased risk of metachronous lesions (SPNIRML), meanwhile, was defined as any SP that presented none of the characteristics that define SPIRML.
Advanced adenoma (AA) of the colon was defined as any adenoma that presented at least 1 of the following characteristics: size ≥10mm, villous component or high grade dysplasia (HGD). Adenomas that presented none of the characteristics described were classified as non-advanced adenomas (NAA). CRC was described following the 7th revision of the TNM staging system,30 and ACN was defined as the presence of AA of the colon and/or CRC.
The study protocol was approved by the Clínica de Navarra research ethics committee in 2011 (Project 70/11), allowing anonymous collection of the data included in the study.
Statistical studyFor statistical analysis of the data, patients were divided into 2 groups: the SPIRML group and the SPNIRML group. The first group included patients with any SPIRML, while the second group included those with SP who did not present any of the characteristics of SPIRML. Patients who presented SPIRML and synchronously also presented SPNIRML were classified as SPIRML.
The characteristics of the patients and the polyps were expressed as frequencies and percentages for the categorical variables, and as mean and standard deviation (SD) for the continuous variables. The Student t test was used to compare quantitative variables. The association between categorical variables was evaluated using the χ2 test and univariate and multivariate logistics models, calculating the odds ratio (OR) and its 95% confidence interval (95% CI). p values <0.05 were considered statistically significant. Data were analysed using IBM SPSS Statistics 21.0 (IBM Corporation, United States).
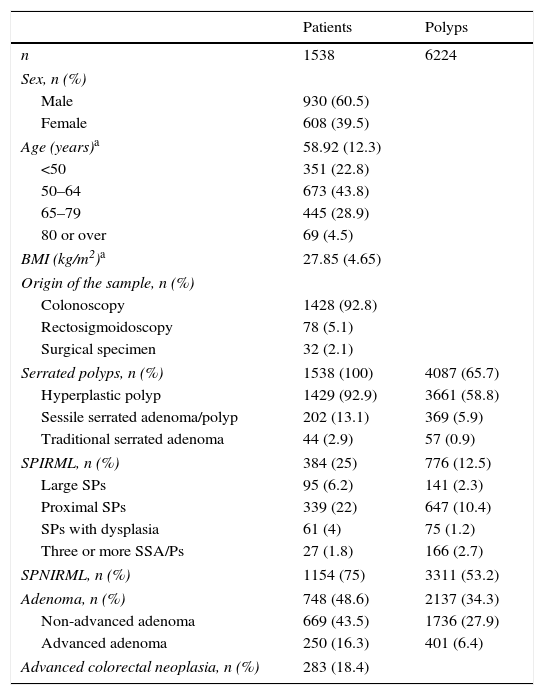
ResultsThe study included a total of 1538 patients histologically diagnosed with at least 1 SP during routine clinical practice in public hospitals in the Navarra region in 2011. The general characteristics of both patients and polyps included are described in Table 1. The histological samples were obtained during colonoscopies (92.8%), rectosigmoidoscopies (5.1%), and surgical interventions (2.1%). The presence of SPs was not reported in any of the clinical autopsies performed in Navarra Health Service hospitals. Mean age and SD at diagnosis was 58.92±12.3years; 60.5% of patients included were men. BMI was calculated in 1305 patients (84.8%), with a mean value (SD) of 27.85kg/m2 (4.65kg/m2) and median (minimum–maximum) of 28kg/m2 (15–51kg/m2).
General characteristics of patients and polyps.
| Patients | Polyps | |
|---|---|---|
| n | 1538 | 6224 |
| Sex, n (%) | ||
| Male | 930 (60.5) | |
| Female | 608 (39.5) | |
| Age (years)a | 58.92 (12.3) | |
| <50 | 351 (22.8) | |
| 50–64 | 673 (43.8) | |
| 65–79 | 445 (28.9) | |
| 80 or over | 69 (4.5) | |
| BMI (kg/m2)a | 27.85 (4.65) | |
| Origin of the sample, n (%) | ||
| Colonoscopy | 1428 (92.8) | |
| Rectosigmoidoscopy | 78 (5.1) | |
| Surgical specimen | 32 (2.1) | |
| Serrated polyps, n (%) | 1538 (100) | 4087 (65.7) |
| Hyperplastic polyp | 1429 (92.9) | 3661 (58.8) |
| Sessile serrated adenoma/polyp | 202 (13.1) | 369 (5.9) |
| Traditional serrated adenoma | 44 (2.9) | 57 (0.9) |
| SPIRML, n (%) | 384 (25) | 776 (12.5) |
| Large SPs | 95 (6.2) | 141 (2.3) |
| Proximal SPs | 339 (22) | 647 (10.4) |
| SPs with dysplasia | 61 (4) | 75 (1.2) |
| Three or more SSA/Ps | 27 (1.8) | 166 (2.7) |
| SPNIRML, n (%) | 1154 (75) | 3311 (53.2) |
| Adenoma, n (%) | 748 (48.6) | 2137 (34.3) |
| Non-advanced adenoma | 669 (43.5) | 1736 (27.9) |
| Advanced adenoma | 250 (16.3) | 401 (6.4) |
| Advanced colorectal neoplasia, n (%) | 283 (18.4) | |
BMI, body mass index; SP, serrated polyps; SPIRML, serrated polyps with increased risk of metachronous lesions; SPNIRML, serrated polyps with no increased risk of metachronous lesions; SSA/P, sessile serrated adenoma/polyp.
Of the 1538 patients included, 384 (25%) presented at least 1 SP with 1 or more of the characteristics that define SPIRML and were included in the SPIRML group; the remaining patients were assigned to the SPNIRML group (75%). When the different SPIRML subtypes were analysed, the most common was found to be the presence of proximal SP in 339 patients (88.3%), followed by the presence of large SP in 95 patients (24.7%), SP with dysplasia in 61 (15.9%), and 3 or more SSA/Ps in 27 (7%). Of the 339 patients who presented at least 1 proximal SP, 124 (36.6%) presented SSA/P; 21 (6.2%) TSA and 194 (57.2%) presented HP. Among the latter, 13 patients (3.8%) presented at least 1 proximal HP ≥10mm, 80 patients (20.8%) presented proximal HP between 5 and 9.9mm and 101 patients (26.3%) had at least 1 proximal HP <5mm.
Almost half of our study patients presented at least 1 synchronous adenoma, 16.3% presented at least 1 AA and 43.5% presented at least 1 NAA. Almost one fifth of our patients (18.4%) presented synchronous ACN. A total of 52 patients (3.4%) presented invasive colon cancer, 17 patients (32.7%) presented colon cancer located proximal to the descending colon and 35 patients (67.3%) presented distal colon cancer.
A total of 6224 polyps were excised from study participants, with a median (minimum-maximum) per patient of 3 polyps (1–102), of which 4087 (65.7%) were SP and 2137 (34.3%) were colorectal adenomas, with medians of 1 (1–50) SP per patient and 2 (1–100) adenomas per patient. A total of 93 patients (6.05%) presented more than 10 colon polyps: 23 patients (1.5%) presented more than 10 adenomas and 40 patients (2.6%) presented more than 10 SPs. In terms of the different SP histological subtypes, most (89.6%) were classified as HP, 9% as SSA/P and only 1.4% as TSA. The median of each of these subtypes was 1 (1–50), 1 (1–17) and 1 (1–6), respectively. One fifth (19%) of the SPs excised presented 1 or more of the characteristics that define SPIRML and the rest (81%) were classified as SPNIRML.
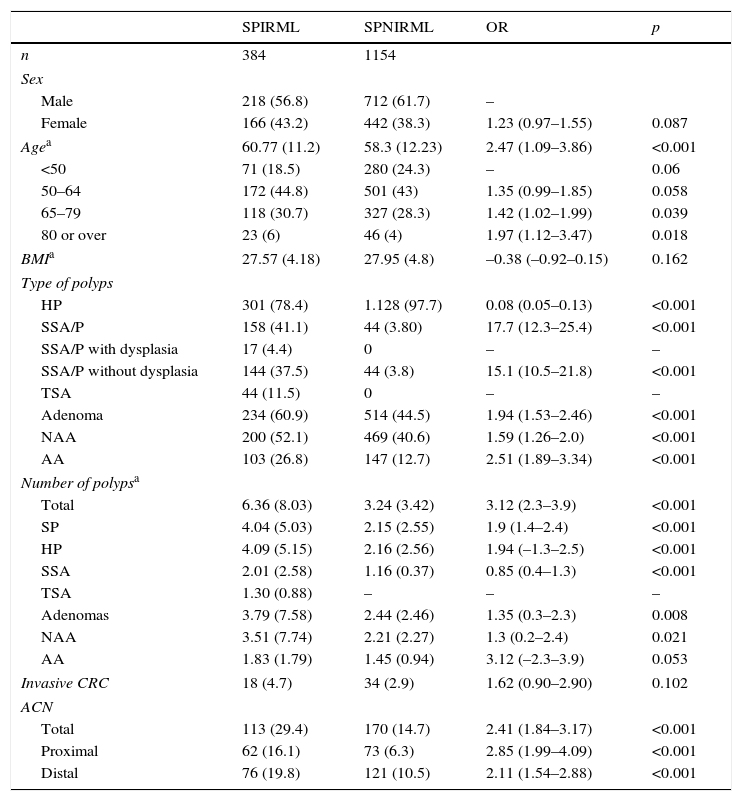
Analysis of the presence of serrated polyps with increased risk of metachronous lesions (SPIRML)We analysed the magnitude or distribution of the study variables according to whether the patient was assigned to the SPIRML group or the SPNIRML group; the most relevant findings are described in Table 2. With respect to the demographic characteristics, patients in the SPIRML group were older at diagnosis (mean age, SD) than patients in the SPNIRML group (60.77±11.2 vs 58.3±12.23; p<0.001). No significant differences were found between groups as regards sex or BMI. Most of the patients included in our study were men (60.5%); there were also more men in both groups studied. The SPIRML group had a higher proportion of women compared to the SPNIRML group (43.2% vs 38.3%); this difference was close to statistical significance (p=0.082). No significant differences were observed between groups in BMI values.
Univariate analysis according to the presence of SPIRML.
| SPIRML | SPNIRML | OR | p | |
|---|---|---|---|---|
| n | 384 | 1154 | ||
| Sex | ||||
| Male | 218 (56.8) | 712 (61.7) | – | |
| Female | 166 (43.2) | 442 (38.3) | 1.23 (0.97–1.55) | 0.087 |
| Agea | 60.77 (11.2) | 58.3 (12.23) | 2.47 (1.09–3.86) | <0.001 |
| <50 | 71 (18.5) | 280 (24.3) | – | 0.06 |
| 50–64 | 172 (44.8) | 501 (43) | 1.35 (0.99–1.85) | 0.058 |
| 65–79 | 118 (30.7) | 327 (28.3) | 1.42 (1.02–1.99) | 0.039 |
| 80 or over | 23 (6) | 46 (4) | 1.97 (1.12–3.47) | 0.018 |
| BMIa | 27.57 (4.18) | 27.95 (4.8) | –0.38 (–0.92–0.15) | 0.162 |
| Type of polyps | ||||
| HP | 301 (78.4) | 1.128 (97.7) | 0.08 (0.05–0.13) | <0.001 |
| SSA/P | 158 (41.1) | 44 (3.80) | 17.7 (12.3–25.4) | <0.001 |
| SSA/P with dysplasia | 17 (4.4) | 0 | – | – |
| SSA/P without dysplasia | 144 (37.5) | 44 (3.8) | 15.1 (10.5–21.8) | <0.001 |
| TSA | 44 (11.5) | 0 | – | – |
| Adenoma | 234 (60.9) | 514 (44.5) | 1.94 (1.53–2.46) | <0.001 |
| NAA | 200 (52.1) | 469 (40.6) | 1.59 (1.26–2.0) | <0.001 |
| AA | 103 (26.8) | 147 (12.7) | 2.51 (1.89–3.34) | <0.001 |
| Number of polypsa | ||||
| Total | 6.36 (8.03) | 3.24 (3.42) | 3.12 (2.3–3.9) | <0.001 |
| SP | 4.04 (5.03) | 2.15 (2.55) | 1.9 (1.4–2.4) | <0.001 |
| HP | 4.09 (5.15) | 2.16 (2.56) | 1.94 (–1.3–2.5) | <0.001 |
| SSA | 2.01 (2.58) | 1.16 (0.37) | 0.85 (0.4–1.3) | <0.001 |
| TSA | 1.30 (0.88) | – | – | – |
| Adenomas | 3.79 (7.58) | 2.44 (2.46) | 1.35 (0.3–2.3) | 0.008 |
| NAA | 3.51 (7.74) | 2.21 (2.27) | 1.3 (0.2–2.4) | 0.021 |
| AA | 1.83 (1.79) | 1.45 (0.94) | 3.12 (–2.3–3.9) | 0.053 |
| Invasive CRC | 18 (4.7) | 34 (2.9) | 1.62 (0.90–2.90) | 0.102 |
| ACN | ||||
| Total | 113 (29.4) | 170 (14.7) | 2.41 (1.84–3.17) | <0.001 |
| Proximal | 62 (16.1) | 73 (6.3) | 2.85 (1.99–4.09) | <0.001 |
| Distal | 76 (19.8) | 121 (10.5) | 2.11 (1.54–2.88) | <0.001 |
AA, advanced adenoma; ACN, advanced colorectal neoplasia; BMI, body mass index; CRC, colorectal cancer; HP, hyperplastic polyps; NAA, non-advanced adenoma; SP, serrated polyps; SPIRML, serrated polyps with increased risk of metachronous lesions; SPNIRML, serrated polyps with no increased risk of metachronous lesions; SSA/P, sessile serrated adenoma/polyp; TSA, traditional serrated adenoma.
Patients with SPIRML had a significantly higher number of colon polyps per patient, both polyps in general as well as adenomas and SPs. The presence of the different histological subtypes of SP differed between groups in relation to the criteria used to define SPIRML. Patients in the SPIRML group had a higher risk of presenting at least 1 SSA/P and TSA with respect to patients in the SPNIRML group, while the latter group had a higher risk of harbouring HP. Similarly, with respect to the total number of polyps of each histological subtype of SP, the SPIRML group was found to have a higher number of SSA/P and TSA and fewer HP with respect to the SPNIRML group. The presence of colon adenomas also differed between groups. Patients in the SPIRML group had a higher risk of synchronously presenting at least 1 adenoma, both NAA and AA, with respect to patients in the SPNIRML group, with OR greater than 1.5 (OR: 1.94; 95% CI: 1.53–2.46; OR: 1.59; 95% CI: 1.26–2.0 and OR: 2.51; 95% CI: 1.89–3.34 respectively, with p<0.001). The mean number of adenomas per patient also differed between study groups. The SPIRML group had a higher mean number of adenomas per patient (mean, SD) than the SPNIRML group; these differences were significant for the total number of adenomas and the number of NAA (3.79±7.58 vs 2.44±2.46; p=0.008 and 3.51±7.74 vs 2.21±2.27; p=0.02, 1 respectively) and close to significance for the mean number of AAs (1.83±1.79 vs 1.45±0.94; p=0.053). Finally, patients in the SPIRML group had a higher risk of synchronously presenting an ACN with respect to the SPNIRML group (OR: 2.41 95% CI: 1.84–3.17; p<0.001), both in the proximal and distal location (OR: 2.85 95% CI: 1.99–4.09 and OR: 2.11 95% CI: 1.54–2.88; p<0.001, respectively).
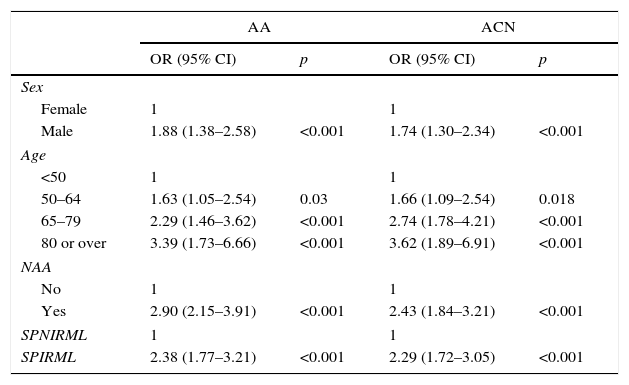
Multivariate analysis of the association between serrated polyps with increased risk of metachronous lesions (SPIRML) and presence of advanced adenoma and advanced colorectal neoplasiaWe performed a multivariate analysis for the synchronous presence of AA and ACN in both groups; results are described in Table 3. The multivariate model included sex, age, presence of NAA and presence of SPIRML. The BMI was not included, as no significant differences were observed in this parameter among patients study patients both with and without AA or ACN. The same analysis was performed to evaluate the association between SPIRML and the synchronous presence of invasive CRC, but no significant differences were observed in the case of CRC, probably due to the low number of invasive CRC in the series (52 cases).
Multivariate analysis of the association between the presence of SPIRML and the synchronous presence of AA and ACN.
| AA | ACN | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.88 (1.38–2.58) | <0.001 | 1.74 (1.30–2.34) | <0.001 |
| Age | ||||
| <50 | 1 | 1 | ||
| 50–64 | 1.63 (1.05–2.54) | 0.03 | 1.66 (1.09–2.54) | 0.018 |
| 65–79 | 2.29 (1.46–3.62) | <0.001 | 2.74 (1.78–4.21) | <0.001 |
| 80 or over | 3.39 (1.73–6.66) | <0.001 | 3.62 (1.89–6.91) | <0.001 |
| NAA | ||||
| No | 1 | 1 | ||
| Yes | 2.90 (2.15–3.91) | <0.001 | 2.43 (1.84–3.21) | <0.001 |
| SPNIRML | 1 | 1 | ||
| SPIRML | 2.38 (1.77–3.21) | <0.001 | 2.29 (1.72–3.05) | <0.001 |
AA, advanced adenoma; ACN, advanced colorectal neoplasia; NAA, non-advanced adenoma; SPIRML, serrated polyps with increased risk of metachronous lesions; SPNIRML, serrated polyps with no increased risk of metachronous lesions.
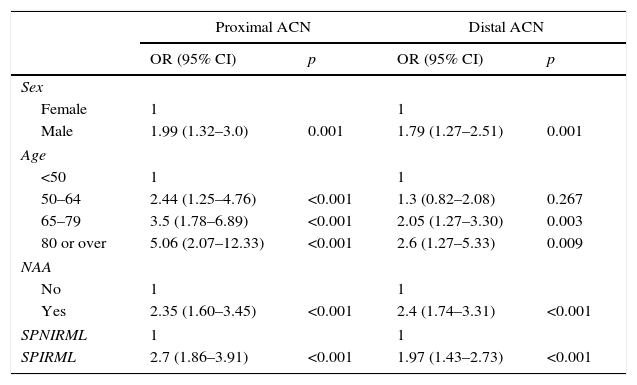
In our analysis, patients in the SPIRML group were found to have twice the risk of presenting synchronous AA and ACN with respect to patients in the SPNIRML group (OR 2.38; 95% CI: 1.77–3.21 and OR 2.29; 95% CI: 1.72–3.05, p<0.001, respectively). We also analysed the association between the presence of SPIRML and the synchronous presence of ACN according to the location of the latter; the results are described in Table 4. Again, we observed that patients in the SPIRML group had a significantly higher risk of presenting NAA in both locations (proximal and distal) with respect to patients in the SPNIRML group, with the OR of the proximal location slightly higher than that of the distal location (OR 2.7; 95% CI: 1.86–3.91 and OR: 1.97; 95% CI: 1.43–2.73, respectively).
Multivariate analysis of the association between the presence of SPIRML and the presence of ACN according to location.
| Proximal ACN | Distal ACN | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.99 (1.32–3.0) | 0.001 | 1.79 (1.27–2.51) | 0.001 |
| Age | ||||
| <50 | 1 | 1 | ||
| 50–64 | 2.44 (1.25–4.76) | <0.001 | 1.3 (0.82–2.08) | 0.267 |
| 65–79 | 3.5 (1.78–6.89) | <0.001 | 2.05 (1.27–3.30) | 0.003 |
| 80 or over | 5.06 (2.07–12.33) | <0.001 | 2.6 (1.27–5.33) | 0.009 |
| NAA | ||||
| No | 1 | 1 | ||
| Yes | 2.35 (1.60–3.45) | <0.001 | 2.4 (1.74–3.31) | <0.001 |
| SPNIRML | 1 | 1 | ||
| SPIRML | 2.7 (1.86–3.91) | <0.001 | 1.97 (1.43–2.73) | <0.001 |
ACN, advanced colorectal neoplasia; NAA, non-advanced adenoma; SPIRML, serrated polyps with increased risk of metachronous lesions; SPNIRML, serrated polyps with no increased risk of metachronous lesions.
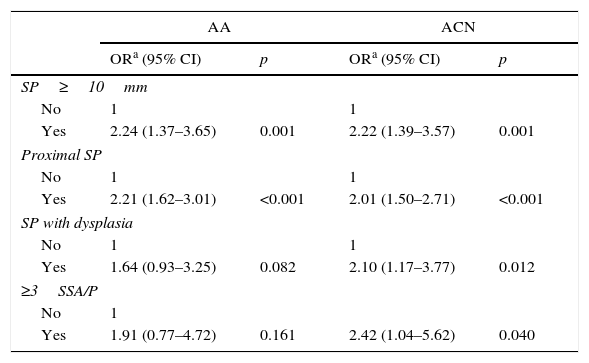
Finally, we also analysed the association of different SPIRML subtypes with synchronous presence of AA and ACN; the results are described in Table 5. In this analysis, we observed that the 4 SPIRML subtypes had twice the risk of presenting synchronous ACN. With respect to the association with the synchronous presence of AA, patients with at least 1 large SP or at least 1 proximal SP were found to have a statistically significant increased risk of presenting synchronous AA; although patients with SP subtypes with dysplasia and multiple SSA/Ps also had an increased risk, this did not reach statistical significance. We also performed a sub-analysis to evaluate the risk of ACN in patients with proximal HP less than 1cm with no other characteristics of SPIRML (no SP >1cm, no SP with dysplasia; no proximal SSA/Ps or not more than 2 SSA/Ps). We identified a total of 181 patients (11.8%) with this type of polyp in our series. In this patient group, we found that a total of 55 patients (30.4%) had synchronous ACN compared to 228 (16.8%) in the remaining patients with SPs, with a significant increase in risk in the univariate study (OR: 2.161; 95% CI: 1.53–3.06) that was maintained after adjusting for age, sex and presence of NAA (OR: 1.975; 95% CI: 1.375–2.836).
Multivariate analysis of the association between the different subtypes of SPIRML and the synchronous presence of AA and ACN.
| AA | ACN | |||
|---|---|---|---|---|
| ORa (95% CI) | p | ORa (95% CI) | p | |
| SP≥10mm | ||||
| No | 1 | 1 | ||
| Yes | 2.24 (1.37–3.65) | 0.001 | 2.22 (1.39–3.57) | 0.001 |
| Proximal SP | ||||
| No | 1 | 1 | ||
| Yes | 2.21 (1.62–3.01) | <0.001 | 2.01 (1.50–2.71) | <0.001 |
| SP with dysplasia | ||||
| No | 1 | 1 | ||
| Yes | 1.64 (0.93–3.25) | 0.082 | 2.10 (1.17–3.77) | 0.012 |
| ≥3SSA/P | ||||
| No | 1 | |||
| Yes | 1.91 (0.77–4.72) | 0.161 | 2.42 (1.04–5.62) | 0.040 |
AA, advanced adenoma; ACN, advanced colorectal neoplasia; SP, serrated polyp; SPIRML, serrated polyps with increased risk of metachronous lesions; SSA/P, sessile serrated adenoma/polyp.
One quarter of patients with SP included in our study presented at least 1 SPIRML, the presence of which was associated with a higher risk of synchronous AA and ACN, especially proximal.
In our study, 25% of patients presented SPIRML; prevalence differed in the different SPIRML subtypes. The most common subtypes were the presence of proximal SP (22%) and presence of large SP (6.2%), while the presence of SP with dysplasia or multiple SSA/Ps was less common (4% and 1.8% respectively). Several studies to date have described the prevalence of large12,13,17–24 and proximal SPs.13,18–24 Most of these studies had major differences as regards design: some were conducted in an asymptomatic population with an average risk for CRC who underwent colonoscopic screening,13,17,19 while others did not include all the histological subtypes12,13 or did not classify SPs according to 2010 WHO criteria.12,13,17 The prevalence of large and proximal SPs was similar in our series, although lower than the 8.6–11.4% (large SPs) and 28.6–33.6% (proximal SP) rates previously described by other authors.18,20–22,24 These studies reported a slightly higher prevalence than that described in our series. This is likely because they included histological samples obtained only in colonoscopies, while our study also included samples obtained in rectosigmoidoscopies and surgical specimens.
A point of note in our study is that it describes the prevalence of SP with dysplasia or the presence of multiple SSA/Ps in the general population seen by the Navarra Health Service. Population-based studies have so far contributed little information about the prevalence of SP with dysplasia or with multiple SSA/Ps, for various reasons. Firstly, they are rare lesions, so they require studies that include a large number of patients. Secondly, the diagnostic criteria for SSA/P with dysplasia were only recently standardised4; many of these lesions had been previously classified as mixed polyps and were occasionally not classified as serrated lesions. Furthermore, the diagnosis of TSA is not always simple and is prone to interobserver variability, so that some pathologists may classify a polyp as TSA while others may classify it as tubulovillous adenoma.
We found only 1 recent population-based study18 that described the presence of 30 SPs with dysplasia out of a total of 493 SPs excised in 309 patients. SPs with dysplasia thus accounted for 6.09% of all SPs. The prevalence of patients with at least 1 SP with dysplasia is not described in this study. In our population, 75 SPs with dysplasia were excised out of a total of 4087 SPs, corresponding to 1.83%. There are several reasons that could explain the differences in these findings. Firstly, differences in the origin of the samples may help explain the differences between the percentages. The inclusion in our study of patients without a complete colonoscopy reduces the likelihood of detecting SPs with dysplasia, thus affecting the numerator of the ratio. Secondly, the overall number of SPs excised differs, and this affects the denominator of the ratio. In our series, 4087 SPs were excised, with an arithmetic mean of 2.66 SPs per patient, while in the study by Rondagh et al.,18 493 SPs were excised, with a mean of 1.59 SPs per patient. Moreover, this difference could also be due to the larger number of small HPs excised in our series, which increases the denominator of the ratio. We were unable to find any population-based study in the literature that described the prevalence of patients with multiple SSA/Ps (≥3SSA/Ps).
Another notable finding in our study was the association between the presence of SPIRML and the synchronous presence of AA or ACN. In our series, we observed that patients with SPIRML had an increased risk of synchronously harbouring ACN, in both the proximal and distal location, although the OR for the proximal location was slightly higher. With respect to the analysis of this association according to SPIRML subtypes, in our study we observed that all presented a higher risk of synchronous ACN. The presence of a proximal SP has been considered as one of the risk factors that could be associated with a higher risk of developing CRC. However, some authors have recently highlighted the importance of size and the presence of dysplasia with respect to location in terms of the risk of developing metachronous lesions of the colon. The strength of this risk most probably differs between SPIRML categories (SP≥10mm, proximal location, with dysplasia or multiple SSA/Ps). It is also likely that this risk differs between histological subtypes of proximal polyps (TSA or SSA/P>HP) and between sizes within the same histological subtype (e.g. proximal HP measuring >10mm with respect to a proximal hyperplastic micropolyp). It should also be taken into account that the presence of molecular alterations that play a role in the progression of SPs to CRC (basically the CpG island methylator phenotype [CIMP] and the presence of mutations in the BRAF gene) are more common in large and proximal SPs.31 Since at present we are unable to confidently discriminate which SPs will progress to CRC and which will have a favourable outcome, and since a centralised review of all HPs was not carried out in our study, we decided to include all SPs in the category of proximal SPs, regardless of their histological subtype and size. The association of large and proximal SPs with the presence of synchronous ACN has recently been described in several studies12,13,17–23 and a meta-analysis.24 Despite the previously mentioned limitations, the association between large or proximal SPs observed in our study (OR: 2.22; 95% CI: 1.39–3.57 and OR: 2.01; 95% CI: 1.50–2.71, respectively) was similar to that reported by these authors.
No study to date has described the association between SPs with dysplasia or multiple SSA/Ps and synchronous presence of ACN. As previously mentioned, one possible explanation for this is the low prevalence of these subtypes of patients with SP and the lack of population-based studies focussing on this association. In our study, approximately one third of patients with SPs with dysplasia (20/61) and patients with multiple SSA/Ps (9/27) presented synchronous ACN. Despite being rare entities (4% and 1.8% respectively), SPs are associated with an increased risk of presenting ACN (with adjusted ORs greater than 2). One possible explanation for this association between SPIRML and ACN could be that both entities originate from a common genetic substrate that is affected by certain environmental factors that contribute to the appearance of different advanced colon lesions via different pathogenic pathways. The results of our study, in which CRC precursor lesions from 2 different pathways synchronously coincide, reflect the heterogeneity of the oncogenesis of CRC; while several different pathways have been described in the pathogenesis of CRC, these are not mutually exclusive, but can often be mixed or overlapping.1 One of the weaknesses of our study was the absence of a molecular profile study of the serrated lesions with increased risk of metachronous lesions (especially aimed at determining the CIMP status and presence of mutations in the BRAF gene) which could help clarify the association between SPIRML and ACN. We believe that further prospective studies that include the description of the molecular pattern of the SPIRMLs and synchronous ACN lesions are needed.
In our study, patients in the SPIRML group had a higher risk of ACN, a higher proportion of synchronous adenomas (both advanced and non-advanced), and a higher number of adenomas per patient with respect to the SPNIRML group. Our findings, similar to those of other authors,32,33 suggest that patients with SPIRML present a more aggressive adenoma phenotype with respect to the SPNIRML group. At present, we do not know if the concurrence of 2 advanced colon lesions (SPIRML and AA/CRC) in the same individual explains the existence of a phenotype associated with a higher risk of presenting CRC or new metachronous advanced lesions in the future. We therefore consider that new longitudinal studies aimed at evaluating this risk during the endoscopic surveillance of patients with synchronous SPIRML and ACN are needed.
Our results suggest that SPIRMLs should be considered not only as risk markers of metachronous development of advanced colon lesions, but also as markers of the presence of synchronous ACN. Therefore, the presence of a SPIRML during a rectosigmoidoscopy or colonoscopy will warn of the importance of carefully checking the entire colon.
From a methodological point of view, one strength of our study was its population-based design, which enabled a large number of patients diagnosed with SPs to be included, and allowed us to evaluate the association between SPIRML and ACN. Foremost among the limitations of our study is the interobserver variability in the classification of the SPs. As this is a multicentre study, in which the histopathology laboratories of 3 public hospitals of the Navarra Health Service participated, and in which no central review of all the histological diagnoses of SP was performed, the presence of an interobserver interpretation bias is inevitable, especially when differentiating between SSA/P and HP.34 In order to reduce the impact of interobserver variability in the classification of different histological subtypes of SP, various training sessions and courses were held in the participating hospitals in 2010–2011 to disseminate and standardise the use of WHO diagnostic criteria for the classification of SPs. Furthermore, borderline cases were evaluated by consensus by a group of pathologists, and all cases diagnosed with SSA/P or TSA were reviewed by 2 experienced pathologists. Another limitation of our study, also linked to its multicentre design, was the presence of a bias in detection of serrated lesions in the endoscopic studies (colonoscopy and rectosigmoidoscopy). Differences in SP detection rates in the different participating hospitals are known to occur in multicentre studies, and there may also be differences between endoscopists from the same endoscopy unit. In an attempt to reduce this risk, several training lectures were organised for participating endoscopists stressing the clinical importance of diagnosing SPs, and highlighting the key factors in the detection and resection of SPs. Taking into account classification and detection bias, some SPs might have gone undetected and/or were erroneously classified, and therefore the prevalence found in our study is most probably underestimated. Another limitation of our study concerns the origin of the samples analysed, obtained from both endoscopic examinations and surgical interventions. Although this design enabled all patients with SPs diagnosed in the Navarra Health System to be analysed, information on synchronous lesions in the entire colon could not be obtained when samples were obtained from rectosigmoidoscopies or surgical interventions in which a complete colonoscopy was not performed.
ConclusionThe presence of SPIRML is common in patients with SPs and is associated with a higher risk of presenting synchronous AA and ACN. Therefore, SPIRML should be considered not only a risk marker for the development of ACN during surveillance, but for the synchronous presence of ACN, and when detected must prompt clinicians to perform a detailed colon examination.
Conflict of interestsThe authors declare that they have no conflict of interests.
We would sincerely like to thank Rebeca Irisarri, Edurne Amorena, Ana Ciáurriz and David Ruiz Clavijo for their help and dedication in carrying out this project.
Please cite this article as: Urman J, Gomez M, Basterra M, Mercado MR, Montes M, Gómez Dorronsoro M, et al. Pólipos serrados y su asociación con neoplasia avanzada de colon. Gastroenterol Hepatol. 2016;39:574–583.