Signet ring cell carcinoma of the gastrointestinal tract is commonly of the stomach or colon origin. Signet ring cell carcinoma of the biliary tract is a very rare disease, with only a few cases reported to date. To the best of our knowledge, this is the second report of signet ring cell cholangiocarcinoma in the hilar region. An appropriate sampling method, yielding sufficient diagnostic materials in suspected cases, is important for the morphological confirmation of this rare type of cancer and ruling out the primary origin other than the biliary tract.1
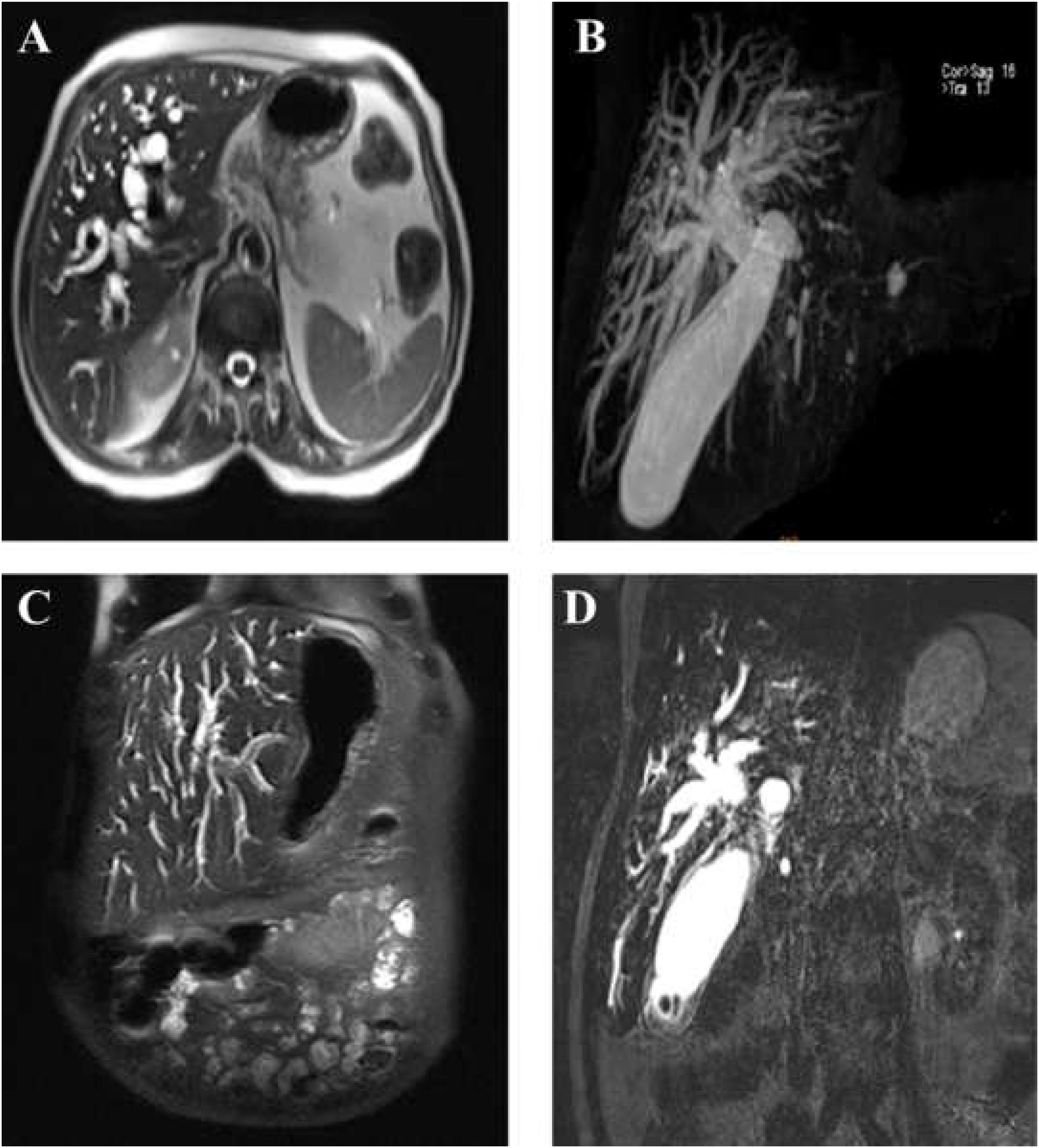
A 68-year-old diabetic woman presented with anorexia, icterus, and pruritus nine months before the final diagnosis of signet ring cell cholangiocarcinoma (CCA). The complete blood count test revealed normal levels of white blood cells and elevated levels of alkaline phosphatase, aspartate amino transferase, alanine transferase and direct bilirubin (Table 1). Dilation of intrahepatic bile ducts was found in abdominal sonography and magnetic resonance cholangiopancreatography. Esophagogastroscopy was normal. On endoscopic retrograde cholangiopancreatography (ERCP), severe stenosis was noted in proximal common bile duct (CBD) (Fig. 1). After sphincterotomy, brush cytology was carried out, and a plastic stent was inserted (10 French×10cm, Endo-Flex-Germany Plastic Biliary Stent). The cytological examination indicated the presence of a few atypical cells. Next, endoscopic ultrasonography (EUS) showed intrahepatic bile duct dilation and narrowing of the common hepatic duct (CHD). A second ERCP was performed, and the plastic stent was removed. Then, biopsy was taken from the CHD, and a metal stent was inserted (10mm×60mm removable Fully Covered Metal Stent, HILZO, S. Korea). The pathological examination of biopsy and the CHD stent content showed no evidence of malignancy. Due to high clinical suspicion of malignancy, the patient was referred to the oncologist. Serum tumor markers were requested, and a significantly elevated level of CA19-9 was observed. These values are summarized in Table 1. Although no definite pathological diagnosis of malignancy was made, based on clinical judgment, the patient received three courses of IV cisplatin and gemcitabine, followed by five courses of oral capecitabine. In the patient follow-up, CA19-9 gradually decreased to the normal range (to 49). The spiral abdominopelvic CT scan showed the moderate dilation of intrahepatic bile ducts. Nine months after the initial presentation, the patient was hospitalized due to weakness and icterus with elevated levels of direct bilirubin and alkaline phosphatase. Abdominal sonography showed echogenic areas around the CHD stent, suggestive of tumoral lesion. The dilation and stricture of intrahepatic bile ducts in the CHD were observed. Brushing and trans papillary direct cold forceps biopsy were performed under fluoroscopy from the CHD stenotic site, and a CBD plastic stent was inserted (see sup Fig. 2).
Laboratory and oncological serum markers values during evaluation.
| Test, Unit | Result | Reference range |
|---|---|---|
| FBS, mg/dL | 105a | 70–99Diabetic: >126 |
| Hb A1c, % | 6 | Non diabetic: 4–6Diabetic: >6.5 |
| Direct bilirubin, mg/dL | 18a | 0–0.3 |
| Indirect-bilirubin, mg/dL | 24a | 0–1.1 |
| Alkaline phosphatase | 2150a | 100–460 |
| SGOT (AST), U/L | 250a | 11–37 |
| SGPT (ALT), U/L | 340a | 13–40 |
| WBC, ×10^3/μL | 7 | 4.1–10.5 |
| Neutrophil, ×10^3/μL | 5.70 | 2–7.7 |
| Platelet, 10^3/μL | 165 | 150–450 |
| Tumor marker (unit) | Result | Normal references (method) |
|---|---|---|
| AFP (IU/ml) | 10a | 0–4 (Clia immulite method) |
| CEA (ng/ml) | 1.5 | Up to 4.7 (Clia immulite method) |
| CA125 (U/ml) | 8 | <35 (EIA) |
| CA19-19 (U/ml) | 680a,b | <37 (EIA) |
Abbreviation: FBS: Fasting Blood Sugar; Hb A1c: Glycosylated Hemoglobin; AST: Aspartate Amino Transferase; ALT: Alanine Transferase; WBC: White Blood Cell; AFP: Alpha Fetoprotein; CEA: Carcinoembryonic Antigen; CA125: Cancer Antigen 125; CA19-19: Carbohydrate Antigen 19-9; EIA: Enzyme Immunoassay.
On cytological evaluation of CHD brush smears; a few clusters of atypical cells with high nucleus/cytoplasm ratio, enlarged and hyperchromatic nuclei with prominent nucleoli, suggestive of malignancy, were noted (see sup Fig. 3A). Sections of materials removed from the brush and fluid sediment also revealed small sheets of malignant cells with pleomorphic and hyperchromatic nuclei, vacuolated cytoplasm, mitotic figures, and apoptotic bodies, compatible with adenocarcinoma (see sup Fig. 3B). Sections of CHD biopsy clearly showed sheets and cords of loosely cohesive malignant epithelial cells with pleomorphic, hyperchromatic, and eccentrically located nuclei and large cytoplasmic vacuoles, containing mucin, as shown by periodic Acid-Schiff stain (see sup Fig. 3C) with a characteristic signet ring morphology (see sup Fig. 3D, E).
The immunohistochemical study of biopsy materials showed strong and diffuse staining for CK7, CK19, and CA19-9, and focal positivity for only CK20 and CDX2 (see sup Fig. 4A–E), suggesting the biliary tract origin of carcinoma. Also, the level of Ki67, a proliferation marker, was high (about 80%), and P53, a tumor suppressor gene, was overexpressed, indicating P53 gene mutation, based on the immunohistochemical study (see sup Fig. 4F, G); therefore, the malignant nature of the lesion was confirmed. The diagnosis of signet ring cell hilar cholangiocarcinoma was finally established. The biopsy procedure was uneventful, and chemotherapy was continued with oral capecitabine. The interesting points of the present study were the sampling technique and the pathological aspect. From the technical point of view; sampling procedures for hilar CCA may include brush cytology which has low sensitivity, EUS guided fine needle aspiration (FNA) which has limited role in the diagnosis of hilar (proximal) strictures from CCA, tansperitoneal FNA biopsy which possesses risk of peritoneal metastasis.2 Cholangioscopy-guided targeted biopsy has a better sensitivity for the diagnosis of malignant biliary strictures; nevertheless, it is an expensive procedure. As an alternative to cholangioscopy-guided biopsy, endoscopic transpapillary forceps biopsy, which was also performed in our case, can be considered an effective and inexpensive sampling technique that needs to be operated by an experienced endoscopist.3
From pathological aspect, signet ring cell carcinoma of biliary tract, especially in hilar region, is extremely rare and the first case of signet ring cell hilar CCA was reported in 2015 which had been diagnosed via surgical resection of biliary tree. To the best of our knowledge, our case is the second case of signet ring cell hilar CCA and the first case diagnosed through transpapillary direct cold forceps biopsy under fluoroscopy. Hilar CCA has a poorer prognosis than distal bile duct cancers, and the presence of signet ring cells may confer further aggressiveness to this tumor, as observed in our case with poor response to chemotherapy.4
As mentioned before, signet ring cell carcinoma of primary biliary tract origin is very rare and this type of adenocarcinoma usually affects the stomach and the colon, thus confirming biliary origin and exclusion of a secondary tumor is necessary. In this patient no tumoral lesion was identified on esophagogastroscoy and colonoscopy. Moreover, strong and diffuse expression of CK7, CK19, and CA19-9 by tumor cells in the immunohistochemical study is compatible with the biliary tract origin of signet ring cell carcinoma. Therefore, providing sufficient diagnostic materials for a complete pathological evaluation is of great importance. In our case, the two initial sampling procedures failed to confirm the diagnosis of malignancy. In our third ERCP and sampling attempt, brush cytology was only suggestive of malignancy with no further diagnostic information. Although the prepared cell block from the brush container confirmed the malignant nature of the lesion, the yielded materials were not sufficient for further immunohistochemical studies. However, the endoscopic transpapillary forceps biopsy of this patient provided fair and adequate materials for a proper and complete histopathological evaluation. Xu Ming-Ming et al. also recommend combining sampling techniques, such as cytology, directed biopsy, and molecular analysis, to improve the yield of tissue diagnosis for malignant biliary strictures.5
In this study, we found that there are different sampling procedures for CCA, including ERCP using forceps with a combination of fluoroscopy, cholangioscopy-guided targeted biopsy and EUS-guided tissue acquisition, among others.6 A recent meta-analysis of nine studies demonstrated that the yield of intra-ductal biopsies obtained during ERCP was low, with a sensitivity less than 50%, but a specificity also around ∼99%.7 With regard to cholangioscopy and targeted tissues biopsies a meta-analysis of 10 studies involving 456 patients showed that the sensitivity of cholangioscopy was 60% with a specificity of 98%.2 Overall, none of the techniques alone has shown to be sufficient in the diagnosis of CCA, the best approach being a combination of two or more methods depending on the expertise of the physician, local availability and the patient's tumor characteristics. Finally, we suggest endoscopic forceps biopsy in conjunction with brushing for suspected cases of hilar CCA if the procedure can be performed by experienced experts.
Authors’ contributions[Mahdiieh Ghoddoosi], writing original draft & conceptualization. [Ahmad Hormati], correspondence. [Davoud Ouladdameshghi], data analyzing. [Sajjad Ahmadpour], writing review & editing.
Ethical approvalThis study was approved by the Ethics Committee of the First Affiliated School of Qom University of Medicine (No. 2019-0189). Written informed consent was obtained from all participants.
FundingThis study was supported by a grant from the Qom University of Medical Sciences, Qom, Iran (No. 539001).
Conflict of interestNo benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
The authors would like to thank Sanam Ahmadpour for her contribution to the edit of the present manuscript.