Inflammatory bowel disease is a premalignant condition for developing colorectal cancer. Since a correlation has been suggested between telomere length, chromosomal instability and neoplastic transformation in this setting, we sought to investigate whether telomerase expression in colorectal mucosa may constitute a biomarker for malignant transformation in patients with inflammatory bowel disease.
Patients and methodsForty-seven patients with inflammatory bowel disease with and without cancer or dysplasia were evaluated for human telomerase reverse transcriptase hTERT immunostaining in paraffin-embedded, formalin-fixed colorectal tissues. In addition, hTERT mRNA expression was assessed in fresh frozen specimens from a second set of 35 patients with inflammatory bowel disease at high or low risk for neoplastic transformation.
ResultsFive out of 10 patients (50%) with colorectal cancer or high-grade dysplasia exhibited hTERT immunochemical detection in adjacent, non-transformed colonic mucosa. However, this phenomenon was also observed in non-affected mucosa of patients with either long-standing (13 out of 19 patients; 68%) or short duration (13 out of 18 patients; 72%) disease without cancer or dysplasia. On the other hand, hTERT mRNA expression in non-affected colorectal mucosa from patients at high risk for neoplastic transformation due to long-standing disease was higher than in those at low risk (7.42±6.43 vs. 2.87±1.47, respectively; p=0.006).
ConclusionsWhereas hTERT immunostaining provides equivocal results, the observation that patients at high risk for colorectal cancer because of long-standing inflammatory bowel disease overexpress hTERT mRNA in non-affected colorectal mucosa suggests its potential usefulness as a biomarker of the risk of malignant transformation.
La enfermedad intestinal inflamatoria es una condición premaligna para el desarrollo de cáncer colorrectal. Puesto que se ha propuesto la existencia de una correlación entre la longitud de telomero, la inestabilidad cromosómica y la transformación neoplásica en este contexto, nos propusimos investigar si la expresión de la telomerasa en la mucosa colorrectal puede constituir un marcador biológico de transformación neoplásica en pacientes con enfermedad intestinal inflamatoria.
Pacientes y métodosSe evaluaron 47 pacientes con enfermedad intestinal inflamatoria, con y sin cáncer o displasia, para inmunotinción de hTERT (transcriptasa inversa de telomerasa humana) en tejidos colorrectales preservados en parafina y fijados con formol. Además, se evaluó la expresión de la ARNm de la hTERT en muestras congeladas procedentes de un segundo grupo de 35 pacientes con enfermedad intestinal inflamatoria clasificados de alto o bajo riesgo de transformación neoplásica.
ResultadosCinco de 10 pacientes (50%) con cáncer colorrectal o un grado elevado de displasia presentaban detección inmunoquímica de la hTERT en la mucosa colónica adyacente sin transformación maligna. Sin embargo, este fenómeno también se observó en mucosa no afecta de pacientes que presentaban enfermedad de larga duración (13 de 19 pacientes; 68%) y de corta duración (13 de 18 pacientes; 72%), sin presencia de cáncer ni displasia. Por otro lado, la expresión del ARN de la hTERT en mucosa colorrectal no afecta de pacientes con alto riesgo de transformación neoplásica, debido a enfermedad prolongada, fue mayor que en aquellos con bajo riesgo (7,42±6,43 frente a 2,87±1,47, respectivamente; p=0,006).
ConclusionesSi bien la inmunotinción para hTERT aporta resultados equívocos, la observación de que los pacientes con alto riesgo de cáncer colorrectal, con sobreexpresión del ARNm de la hTERT en la mucosa colorrectal no afecta, en enfermedades intestinales inflamatorias de larga duración, sugiere que podría ser útil como marcador biológico del riesgo de transformación maligna.
Inflammatory bowel disease (IBD) as a premalignant condition for developing colorectal cancer was first described in 1925 for ulcerative colitis (UC)1 and in 1948 for Crohn's disease (CD).2 Subsequently, several epidemiologic studies have confirmed an increased cumulative risk for developing cancer in UC3 and CD4–6 patients, which raises with early age of onset,7 disease duration and extent and severity of inflammation.8 The precise mechanism by which chronic colonic mucosal inflammation causes malignancy in IBD is poorly understood, although it is supposed to be related to a failure in regulatory mechanisms during cell division.
Telomeres are non-codifying sequences constituted by short tandem DNA repeats and associated proteins, placed at the end of lineal chromosomes, necessary for maintaining chromosomal integrity through cell division. They help to distinguish natural end of chromosomes from random DNA breaks, thus preventing end-to-end fusion between them. Besides this function, they are implicated in other cellular processes such as chromatin organization and control of cell proliferation.9 Telomerase is a ribonucleoprotein that has been identified as one of the most important regulatory mechanisms during chromosomal replication in cell division, and it has been directly implicated in developing cancer.10 Telomerase is a specialized inverse transcriptase that rebuilds telomeres by synthesizing telomeric simple sequences on the 3′ region within its integral RNA component. Telomerase activity is present in all germline cells but not in most somatic cells late from embryonic phases, except for those tissues in rapid proliferation.11 Expression of hTERT, the catalytic subunit of human telomerase reverse transcriptase, detected by RT-PCR, correlates with telomerase activity both in vitro and in vivo.12
During cell division and chromosomal replication DNA-dependent DNA polymerases fail to replicate telomeres, so in mature tissues lacking telomerase, up to two hundred base pairs can be lost. This phenomenon is cumulative, thus shortening telomeres with age.13 Eventually these shortened telomeres reach a critical point at which the chromosome integrity is lost and proliferation ceases. Rarely, somatic cells escape from this apoptotic mechanism by activating telomerase that restores telomere length, increasing cell survival and providing them the opportunity to acquire other genomic insults that lead to neoplastic transformation.
It has been recently demonstrated that colonocyte telomeres in patients with UC shorten with age almost twice as rapidly as in normal control subjects.14 This extensive shortening occurs within approximately 8 years of disease duration,14 which parallels the risk of colorectal cancer development.15 Interestingly, it has also been shown that telomeres are shorter in non-dysplastic colorectal mucosa from patients with UC who progress to dysplasia or cancer than in those from non-progressing patients or control individuals without UC, and that this reduced telomere length is correlated with increased chromosomal instability.16
Taking into account the above-mentioned correlation between telomere length, chromosomal instability and neoplastic transformation in UC, we sought to investigate if telomerase expression in colorectal mucosa may constitute a biomarker for malignant transformation in patients with IBD. For this purpose, we compared hTERT immunohistochemical detection and/or hTERT mRNA expression in colorectal mucosa from patients affected with UC or colonic CD with and without cancer or dysplasia, as well as from those at high or low risk for neoplastic transformation because of the extension and duration of their disease.
Patients and methodsPatients and samplesTwo sets of patients with IBD were evaluated. The first set included 47 patients with UC or colonic CD (with more than 30% of the colonic length involved by the disease) in whom paraffin-embedded, formalin-fixed colorectal specimens were available for hTERT immunostaining (Table 1). The vast majority of them were recruited in the Hospital Clínic, Barcelona, whereas some additional cases with cancer or dysplasia were obtained from Hospital Meixoeiro, Vigo, and Hospital de Bellvitge, L’Hospitalet de Llobregat. This set included 10 patients affected by cancer or high-grade dysplasia, in whom samples were obtained from surgical specimens, including normal appearing colorectal mucosa, acute and chronic inflammatory mucosa, and neoplastic tissue. However, for the purpose of this study, only those obtained from cancer/dysplasia and morphologically normal colorectal mucosa were evaluated. In this subset of patients, an effort was made to collect any tissue sample from previous examinations. On the other hand, 19 patients with long-standing IBD without dysplasia or cancer, matched by age and duration of the disease (ratio 1:2) with those patients with neoplastic transformation, were included as control group. In them, tissue samples were obtained during endoscopic surveillance. Finally, a third group of 18 patients with a short duration disease (duration shorter than 8 years), in whom tissue samples were obtained during examinations performed to confirm disease remission, was also included. In these two control groups, samples comprised normal appearing mucosa, and acute and chronic inflammatory tissues, but only those obtained from morphologically normal colorectal mucosa were evaluated. Standard criteria for histological grading were used.
Characteristics of patients with inflammatory bowel disease evaluated for hTERT immunostaining.
| Cancer or dysplasia | Long-standing disease | p valuea | Short duration disease | p valueb | |
| (n=10) | (n=19) | (n=18) | |||
| Age (years)c | 68.8±12.0 | 60.0±16.7 | 0.16 | 42.8±18.7 | 0.005 |
| Gender—no. (%) | |||||
| Male | 7 (70%) | 9 (47%) | 0.43 | 8 (44%) | 0.89 |
| Female | 3 (30%) | 10 (53%) | 10 (56%) | ||
| Group of disease—no. (%) | |||||
| Ulcerative colitis | 8 (80%) | 13 (68%) | 0.82 | 13 (72%) | 0.92 |
| Crohn's disease | 2 (20%) | 6 (32%) | 5 (28%) | ||
| Extension of disease—no. (%) | |||||
| Pancolitis | 8 (80%) | 5 (26%) | 0.01 | 9 (50%) | 0.28 |
| Proximal colon (CD) | 2 (20%) | 6 (32%) | 5 (28%) | ||
| Left-sided | – (–) | 8 (42%) | 4 (22%) | ||
| Duration of disease (years)c | 17.2±9.7 | 14.6±9.7 | 0.34 | 5.7±1.1 | 0.005 |
CD, Crohn's disease.
The second set included 35 patients with UC or colonic CD in whom fresh frozen biopsy specimens were available for hTERT mRNA expression analysis (Table 2). All of them were recruited in the Hospital Clínic, Barcelona. In 20 high-risk patients because of a long-standing disease (duration longer than 10 years) but without dysplasia or cancer, endoscopic biopsies were obtained at four colonic segments (right colon, transverse colon, left colon and rectum–sigmoid colon) during surveillance colonoscopies. On the other hand, in 15 low-risk patients because of a short duration disease (duration shorter than 8 years), samples were obtained at rectum–sigmoid colon during colonoscopies performed to confirm remission of the disease. Samples obtained from morphologically normal colonic mucosa were evaluated in these non-progressing patients. These biopsies were introduced in RNA-later solution (Ambion, Austin, TX), snap-frozen and preserved at −80°C until evaluation.
Characteristics of patients with inflammatory bowel disease evaluated for hTERT mRNA expression analysis.
| High risk of neoplastic transformation | Low risk of neoplastic transformation | p value | |
| (n=20) | (n=15) | ||
| Age (years)a | 53.7±15.2 | 44.3±15.3 | 0.05 |
| Gender—no. (%) | |||
| Male | 6 (30%) | 9 (60%) | 0.15 |
| Female | 14 (70%) | 6 (40%) | |
| Group of disease—no. (%) | |||
| Ulcerative colitis | 18 (90%) | 15 (100%) | 0.59 |
| Crohn's disease | 2 (10%) | – (–) | |
| Extension of disease—no. (%) | |||
| Pancolitis | 13 (65%)a | 7 (47%) | 0.46 |
| Left-sided | 7 (35%) | 8 (53%) | |
| Duration of disease (years)b | 15.4±6.3 | 4.4±2.1 | 0.005 |
DNA and total RNA were isolated from colonic tissue using the AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. After RNA was extracted, cDNA was prepared for each sample from 1μg of RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol with a minor modification—the addition of RNAse Inhibitor (Applied Biosystems, Foster City, CA) at a final concentration of 0.4U/μl. Samples were incubated at 25°C for 10min and at 37°C for 120min.
The study was approved by the Institutional Ethics Committee of each participating hospital and written informed consent was obtained from all patients.
hTERT immunostainingOn the basis of the previous studies,17 the monoclonal antibody against hTERT (NCL-L-hTERT, Clone 44F12, Novocastra, Newcastle, United Kingdom) was selected for hTERT immunostaining. After paraffin removal and hydration, antigen retrieval was performed using pressure cooker in citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked in 3% hydrogen peroxide for 10min. The slides were incubated with the primary antibody (dilution 1/25) for 80min at room temperature. EnVision Detection System (Dako, Glostrup, Denmark) was used to detect the antigen–antibody reaction. Sections were visualized with 3,3′-diaminobenzidine as a chromogen and counterstained with Mayer's hematoxylin. Nuclear staining of mucosal lymphocytes was taken as positive internal control. Negative controls were carried out by substituting the primary antibody with an equivalent concentration of non-immune immunoglobulin.
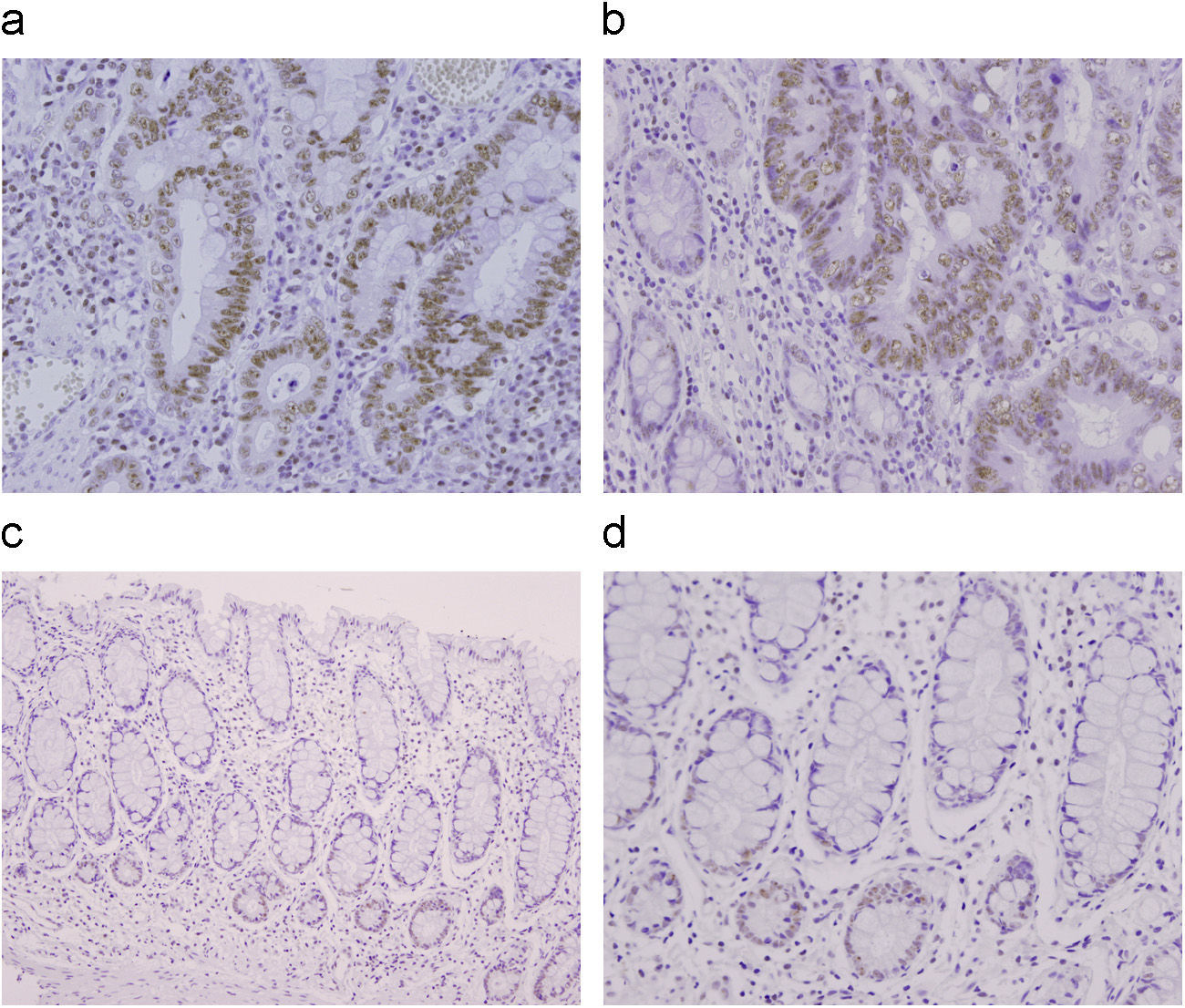
hTERT staining in colonic cells was scored for intensity (0, lower; 1, similar; 2, higher; with respect to the corresponding staining in tissue lymphocytes) and extension (1, bottom part of colonic crypt; 2, bottom and middle part of the crypt; 3, whole crypt) (Figure 1). Arbitrarily, the value resulting from the multiplication of intensity and extension counts was used as the final immunostaining score. The pathologist (AP) was blinded with respect to the clinical characteristics of patients.
Immunohistochemical expression of hTERT in high grade dysplasia (a) and colorectal adenocarcinoma (b). Note strong and diffuse nuclear staining of hTERT in neoplastic glands in contrast with absence of expression in non-neoplastic glands and less intense staining in lymphocytes. Paired non-affected colonic mucosa from one patient who developed high grade dysplasia showing hTERT expression confined to the bottom of crypts and with intensity similar to the one observed in lymphocytes (c,d). Original magnification of photomicrographs ×200.
The expression level of hTERT was measured by real-time quantitative PCR using the TaqMan technology on a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers and FAM dye-MGB probe were developed by the manufacturer as a TaqMan Gene Expression Assay bridging exons 11 and 12 of the hTERT mRNA sequence (NM_198253.2). TBP expression was monitored as the endogenous control gene in a duplicate of the same samples, being the assay available from the manufacturer as a VIC dye-MGB probe.
PCR reactions were prepared using 5μl of cDNA in a final volume of 25μl with final concentrations of 1× TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA) with uracil DNA-glycosylase (AmpErase UNG), and the mentioned specific hTERT assay. Amplification conditions comprised an initial UNG incubation at 50°C for 2min, AmpliTaq Gold DNA Polymerase activation at 95°C for 10min, 50 cycles of denaturation at 95°C for 15s and annealing/extension at 60°C for 1min.
Each measurement in a given sample was performed in triplicate for both hTERT and TBP, and the threshold cycle (Ct), the fractional number at which the amount of amplified target reached a fixed threshold, was determined. The standard deviation in sample triplicates was always below 0.2. Relative amounts of both genes were also normalized to a pool of control colonic tissue RNAs acting as calibrator to allow comparison across all tested samples. The comparative Ct method,18 also known as the 2−ΔΔCt method, was calculated from
where ΔCt,sample was the hTERT Ct value for any sample normalized to TBP, and ΔCt,calibrator was the hTERT Ct value for the calibrator also normalized to TBP. For the ΔΔCt calculation to be valid, the amplification efficiencies of the target and the endogenous reference must be approximately equal, which was previously confirmed by checking how ΔCt varied with template dilution for each tested gene.The investigator performing the real-time quantitative PCR experiments (SC-B) was blinded to the clinical characteristics of patients.
Statistical methodshTERT expression by immunohistochemistry was analyzed both qualitatively and quantitatively. For qualitative analyses, patients were classified as overexpressing hTERT (immunostaining score equal or greater than 1) or non-overexpressing hTERT (immunostaining score equal to 0). hTERT expressions by immunohistochemistry in non-affected colorectal mucosa and cancer/dysplastic tissue from patients with neoplastic transformation were compared by the McNemar's test (qualitative analysis) and the Wilcoxon test (quantitative analysis) for paired samples. A similar approach was done to analyze historical samples obtained in previous colonic examinations from this subset of transforming patients. Differences in hTERT expression in non-affected colonic mucosa between patients showing dysplasia or cancer and matched patients without neoplastic transformation, as well as between non-progressing patients with long- and short-duration IBD, were evaluated by the chi-square test (qualitative analysis) and the Mann–Whitney's U test (quantitative analysis).
hTERT expression by RT-PCR in each colonic segment from patients at high risk for neoplastic transformation because of a long-standing IBD was compared by the Friedman's test for paired samples. Differences in hTERT expression in non-affected colorectal mucosa from patients at high and low risk for malignant transformation were evaluated by the Mann–Whitney's U test.
Quantitative variables were expressed as mean±standard deviation. All p values were two sided. A p value less than 0.05 was considered to indicate a statistically significant difference. All calculations were performed using the 11.0 SPSS software package (SPSS Inc., Chicago, IL).
ResultshTERT immunostaininghTERT immunostaining in colonic cells from neoplastic tissues and non-affected mucosa of patients with long-standing extensive colitis who had developed cancer (n=6) or dysplasia (n=4) was scored. Nine out of these 10 patients (90%) exhibited hTERT overexpression in the neoplastic tissue, whereas this phenomenon was present in 5 out of 10 (50%) corresponding non-transformed colorectal mucosa (p=0.13). Indeed, the ìmmunostaining score for hTERT protein expression was higher in neoplastic tissue than in paired-normal mucosa (5.40±1.89 vs. 1.11±1.17, respectively; p<0.05). No differences were found when patients with cancer were compared with those with dysplasia regarding hTERT expression in either neoplastic tissue (6.00±0.00 vs. 4.50±3.00, respectively; p=0.22) or non-affected colorectal mucosa (0.80±1.09 vs. 1.50±1.29, respectively; p=0.36). On the other hand, in the subset of patients in whom tissue samples from previous examinations were available for analysis, hTERT expression in normal appearing colorectal mucosa from historical samples was similar to that in the corresponding tissue when patients presented with neoplastic transformation (1.17±0.98 vs. 1.10±1.00, respectively; p=1.0).
Patients with long-standing IBD without malignant transformation were similar to those with cancer or dysplasia regarding clinical characteristics (Table 1). hTERT overexpression in non-affected colorectal mucosa was observed in 13 out of 19 patients (68%) who did not develop cancer/dysplasia in comparison with 5 out of 10 patients (50%) with malignant transformation (p=0.43). Similarly, there was no significant difference between both groups in terms of quantitative hTERT protein expression in non-affected colorectal mucosa (1.79±1.65 vs. 1.11±1.17, respectively; p=0.34).
hTERT expression was also evaluated by immunostaining in patients with short duration IBD. As shown in Table 1, these patients were younger than those with a long-standing disease (42.8±18.7 years vs. 60.0±16.7 years, respectively; p=0.005). hTERT overexpression in non-affected colorectal mucosa was observed in 13 of 18 (72%) patients with disease of short duration, similar to the proportion observed in those at high risk for malignant transformation due to a long-standing IBD (13 out of 19 patients (68%); p=1.0). Finally, there was no significant differences between both groups in terms of quantitative hTERT expression in non-affected colorectal mucosa (1.78±1.63 vs. 1.79±1.65, respectively; p=0.94).
hTERT mRNA expression analysishTERT mRNA expression in non-affected colorectal mucosa from patients at high risk for neoplastic transformation differed depending on the segment evaluated: right colon, 6.98±5.63; transverse colon, 10.73±13.41; left colon, 6.06±4.72; rectum-sigmoid colon, 4.38±3.12 (p=0.006).
When hTERT mRNA expression in non-affected colorectal mucosa from high-risk patients was calculated as average of all evaluated colonic segments, mRNA expression was higher in these patients than in those at low risk for neoplastic transformation due to a short duration of the disease (7.42±6.43 vs. 2.87±1.47, respectively; p=0.006).
Since differences in hTERT mRNA expression throughout the colon were observed, comparison between high-risk and low-risk patients was repeated considering only the value of rectum–sigmoid colon measurement in order to adjust the analysis for tissue sampling. In such an analysis, hTERT mRNA expression in patients at high risk for malignant transformation remained higher than in those at low risk, but this difference did not achieve statistical significance (4.38±3.12 vs. 2.87±1.47, respectively; p=0.21).
DiscussionThe results of this pilot study indicate that patients with IBD who develop colorectal cancer or dysplasia exhibit hTERT protein expression in adjacent non-affected colonic mucosa. More important, patients at high risk for malignant transformation because of a long-standing disease were shown to overexpress hTERT mRNA in non-affected colorectal mucosa, thus supporting the potential usefulness of this biomarker in the surveillance of such patients.
Results of hTERT immunostaining deserve, however, some clarifications. First, although virtually all tumor samples exhibited hTERT expression and at a higher degree than it was observed in paired non-affected mucosa counterpart, no difference was found in this latter setting when these patients were compared with those with long-standing IBD without malignant transformation. Second, the lack of difference persisted when previous, non-neoplastic samples from patients who eventually develop colorectal cancer or dysplasia were compared in a sequential manner. Finally, and more important, control cases with disease of short duration did also show expression of the catalytic subunit of telomerase in a similar proportion and levels as those at risk of malignant transformation or who actually develop colorectal cancer or dysplasia. Although characteristics of the study—i.e. the reduced sample size—may contribute to explain these results, the putative lack of specificity of the antibody used in this investigation constitutes the most plausible explanation. Initially, the NCL-L-hTERT antibody seemed to recognize specifically the peptide against which it has been claimed to be developed,19,20 with western blotting demonstrating that it was labeled to a unique protein band in the 100kDa rank, consistent with the molecular weight of telomerase.21 Nevertheless, subsequent experiments using hTERT-positive and -negative cells questioned its specificity. The efficacy of this antibody has been recently re-evaluated and compared with other commercially available anti-hTERT antibodies.22 In this study, it was demonstrated that the NCL-hTERT antibody recognizes a protein that shares a number of properties with nucleolin, in both western blot and immunofluorescence analyses, independently of hTERT expression. Indeed, through its RNA binding domain 4 and carboxy-terminal RGG domain, nucleolin interacts with the hTR subunit and, accordingly, this interaction could be critical for hTERT recognition.23 The fact that telomerase and nucleolin shared the same intracellular distribution and both were involved in the same neoplastic processes, thus showing an almost identical pattern of expression, would explain the difficulties in demonstrating this cross-reaction. The lack of specificity showed for the NCL-hTERT antibody was also demonstrated for the remaining commercially available anti-hTERT antibodies,22 thus precluding to perform an accurate and reliable immunohistochemical detection of this protein. Accordingly, when we realized this limitation, no further hTERT immunostaining was performed (i.e. the second set of patients, in whom fresh frozen biopsy specimens were evaluated for hTERT mRNA expression, were not analyzed by immunohistochemistry). However, we decided to maintain this information in the manuscript to point out the restrictions of immunohistochemical analysis in such a setting.
Unlike immunostaining, results regarding hTERT mRNA expression are encouraging. Indeed, patients at high risk for malignant transformation showed hTERT mRNA overexpression in non-affected colorectal mucosa when compared with those individuals with a short duration IBD, thus suggesting its potential usefulness as biomarker of neoplastic transformation. So far, involvement of telomerase in the carcinogenic process associated with IBD was suggested only in two seminal investigations from the same group,14,16 in which telomere length in normal mucosa was carefully evaluated. They initially found that telomeres were shorter in non-dysplastic colorectal mucosa samples taken from patients with UC who progress to dysplasia or cancer than in those from non-progressing patients or control individuals without UC.16 Most recently, they have demonstrated that colonocyte telomeres in patients with UC shorten with age almost twice as rapidly as in normal control subjects.14 In our series, instead of measuring telomere length, widespread quantitative analysis of hTERT mRNA expression following the strict NCI recommendations for telomerase molecular evaluation24 was performed. Evaluation of this surrogate marker of telomerase activity represents a novel approach to investigate the role of telomerase in the neoplastic process associated with IBD. In this sense, it has been extensively demonstrated that hTERT mRNA expression correlated with telomerase activity25–27 and, indeed, hTERT expression constitutes the main determinant regulating telomerase activity in human fibroblasts and cancer cells.12 Unfortunately, mRNA expression analysis had to be limited to the second set of patients, in which frozen tissues were available, thus precluding to confirm its potential predictive value in the group of patients with neoplastic transformation. Likewise, the lack of adequate and sufficient material did not allow us to simultaneously measure telomere length and telomerase activity by TRAP assay in both sets of patients.
Results regarding hTERT as a potential biomarker of malignant transformation deserve some comments. First, it is important to emphasize that the main contribution of this study was to establish a significant correlation between mRNA expression and duration of the inflammatory disease as surrogate indicator of the risk of neoplastic transformation. In this sense, confirmation of the potential utility of hTERT as biomarker does require evaluation of this parameter in a large series of patients with IBD with and without colorectal cancer or dysplasia and, more important, longitudinal studies including large cohorts of patients in whom baseline and sequential hTERT mRNA measurements were taken all over their follow-up. Second, hTERT mRNA expression showed geographical variations throughout the colon. These differences, also observed in other settings (i.e. methylation pattern in patients without IBD,28 may reflect a distinct neoplastic risk depending on the colonic segment, an aspect poorly evaluated in the literature. From a practical point of view, however, this observation could have some clinical implications since it would continue requiring tissue sampling from the whole colon by means of total colonoscopy instead of limiting the evaluation to the rectum or distal sigmoid colon to estimate the risk of malignant transformation. A very attractive, parallel approach to overcome this limitation could be the evaluation of hTERT expression in stool samples. In the last few years, several studies have demonstrated the usefulness of detecting abnormal fecal DNA by means of multitarget analysis,29,30 thus becoming a promising alternative for colorectal cancer screening.31 Although stool RNA detection entails some technical difficulties, fecal hTERT monitoring represents an appealing strategy in patients with IBD. So far, surveillance programs include careful inspection of the entire mucosa and random or cromoendoscopy-guided biopsies in all at-risk patients.3,32,33 Demonstration of the utility of monitoring mRNA hTERT expression in stool samples would help to stratify patients based on their individual risk of developing colorectal cancer or dysplasia and, consequently, to tailor surveillance strategies. This approach would minimize costs and risks associated with unnecessary colonoscopy. Moreover, the use of fecal hTERT measurement could also contribute to better selected patients who may benefit from chemoprevention using aminosalicilates,34 ursodeoxycholic acid,35 6-mercaptopurine36 or folate,37 as well as acting as surrogate marker for monitoring their putative beneficial effect in such a setting.
Considering carcinogenesis as a multifactor and complex process in which many genes and proteins interact to develop an unviable tissue, telomerase should be regarded as a significant step towards cancer in inflammatory tissues.14,16 It operates as a critical factor in predisposing to cancer and, according to the preliminary results of our study, it might constitute a biomarker of the risk of malignant transformation in patients with IBD. Nevertheless, confirmation of this potential field effect of telomerase requires further evaluation in large and long cohort studies in which the primary endpoints were colorectal cancer or dysplasia development.
Conflict of interest statementThis manuscript has been seen and approved by all the authors, who have taken due care to ensure the integrity of the work. All authors had a significant participation in the design of the study, in the recruitment of the patients included in the study, in the analysis and interpretation of the results and in the elaboration of the manuscript. The authors warrant full disclosure of any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
This work was supported by grants from the Ministerio de Educación y Ciencia (SAF 04-07190 and SAF 07-64873), Fondo de Investigación Sanitaria (05/0071 and 08/0024), Asociación Española contra el Cáncer (Fundación Científica and Junta de Barcelona), AGAUR (2009 SGR 849), Fundación Investigación Médica Mutua Madrileña (PI040296), Fundación Olga Torres (PI040212) and Acción en Cáncer (Instituto de Salud Carlos III). Victoria Gonzalo received a research grant from the Hospital Clínic and Sergi Castellví-Bel is supported by a contract from the Fondo de Investigación Sanitaria (CP 03-0070). CIBERehd is funded by the Instituto de Salud Carlos III.