Scattered white spots (SWSs) in the descending duodenum are an uncommon finding of upper gastrointestinal system endoscopy (UGSE). Intestinal lymphangiectasia, chronic nonspecific duodenitis and giardiasis are associated with a SWS appearance. The aim of this study was to determine the frequency of SWS during routine endoscopy, as well as to evaluate the effect of treatment on this finding.
Materials and methodsPatients undergoing UGSE with a SWS appearance in the descending duodenum were included prospectively. Appearance of SWSs was graded endoscopically based on density, after which patients were divided into two groups; group 1 (treated group) and group 2 (untreated group). Patients with Helicobacter pylori infection were given eradication therapy, whereas a diet was recommended to patients with intestinal lymphangiectasia. Proton pump inhibitors were initiated for patients with H. pylori negative gastritis. All patients were re-evaluated three months after therapy for the presence of any changes in the SWS appearance.
ResultsSWSs were observed in 97 (3.2%) out of 3010 patients. This appearance was most commonly associated with chronic non-specific duodenitis followed by intestinal lymphangiectasia. While in the untreated group no statistically significant change in SWS appearance was observed, the decrease in endoscopic grade seen in the treated group was statistically significant (p<0.001).
ConclusionThe prevalence of SWSs during routine UGSE was 3.2%, with this finding being more commonly associated with chronic non-specific duodenitis and intestinal lymphangiectasia. Treatment of the underlying causes, including H. pylori eradication, proton pump inhibitors and diet decreased the density of the SWSs.
Las manchas blancas dispersas (MBD) en la porción descendente del duodeno son un hallazgo poco frecuente en la endoscopia del sistema digestivo superior (ESDS). Las MBD se han asociado con linfangiectasia intestinal, duodenitis crónica inespecífica y giardiasis. El objetivo de este estudio fue determinar la frecuencia de hallazgos de MBD en endoscopias rutinarias, así como evaluar el efecto del tratamiento de dicho hallazgo.
Material y métodosSe incluyeron de forma prospectiva a pacientes sometidos a ESDS con MBD en el duodeno. La apariencia de las MBD se clasificó mediante endoscopia, de acuerdo con la densidad, después de dividir a los pacientes en dos grupos: grupo 1 (grupo tratado) y grupo 2 (grupo sin tratar). Los pacientes con infección por Helicobacter pylori recibieron un tratamiento de erradicación, mientras que a los pacientes con linfangiectasia intestinal se les recomendó una dieta. Los pacientes con gastritis y negativos en H. pylori iniciaron un tratamiento con inhibidores de la bomba de protones. Todos los pacientes volvieron a ser evaluados 3 meses después del inicio del tratamiento para detectar cambios en la presencia de MBD.
Resultadosse observaron MBD en 97 pacientes (3,2%) de los 3.010 incluidos en el estudio. Las MBD se asociaron más comúnmente con duodenitis crónica inespecíficas, seguida de linfangiectasia intestinal. En el grupo sin tratamiento, no se observaron cambios estadísticamente significativos en el aspecto de la MDB, pero se observó una disminución estadísticamente significativa de la densidad en el grupo tratado (p<0,001).
ConclusiónLa prevalencia de MBD durante ESDS rutinaria fue del 3,2%. Este hallazgo se asoció más frecuentemente con duodenitis crónica inespecífica y linfangiectasia intestinal. El tratamiento de las causas subyacentes, entre ellas la erradicación de H. pylori, los inhibidores de la bomba de protones y la dieta, disminuyeron la densidad de ESDS.
The duodenum is affected by numerous local and systemic factors, such as acidic gastric content, Helicobacter pylori (HP), intestinal lymphangiectasia (IL), medications, infections (viral, bacterial and parasitic), celiac disease, eosinophilic gastroenteritis, autoimmune enteritis, Crohn's disease, tropical sprue and malignancies. Each of these disorders is associated with endoscopic and histopathological changes in the duodenum.1,2
Diseases involving the duodenum usually present with varying signs and symptoms, including diarrhea, weight loss, anemia, and malabsorption.1,2 Histopathological examination of biopsy specimens is essential for the differential diagnosis of duodenal pathologies. Recent studies have shown that gastric pathologies may also affect the duodenum. In a study on pediatric patients, a duodenal pathology was detected in 17.4% of routine duodenal biopsy specimens obtained.1 Reported findings included giardia, moderate inflammation of the lamina propria with an increase in plasma cell infiltration associated with HP colonization of gastric tissue, and an increase in intraepithelial lymphocyte count.1 Based on these findings, it is recommended to also obtain specimens from the stomach of patients with duodenal pathology.1,2
Endoscopic finding of SWS in the descending duodenum is not specific and etiologies comprise a wide range of disorders. To date there has been only a single study published on scattered white spot (SWS) appearance in the duodenum.3 Incidence, endoscopic course and response to treatment of SWS have not been previously investigated. The aim of this study was to evaluate the incidence, etiological factors, and response to etiology-specific treatment of SWS appearance in the descending duodenum.
Materials and methodsStudy populationThis prospective study was undertaken at the Ankara Education and Research hospital in accordance with the guidelines of the Helsinki declaration and with the approval of the local ethics committee. All patients who were referred for upper gastrointestinal system endoscopy (UGSE) from several departments/outpatient clinics between January 2010 and May 2010 were screened for inclusion in this study.
Consenting patients in whom UGSE revealed the presence of SWS were included in the final analysis. Participants were then stratified into two groups based on treatment status. Group 1 (treatment group) consisted of subjects for whom treatment was initiated based on specific findings of gastric and duodenal biopsy specimens, whereas patients who did not receive any treatment were placed in Group 2 (control group).
Patients were excluded from the study in the event of active upper gastrointestinal bleeding, pregnancy, previous gastric surgery, presence of a duodenal ulcer of mass, a decompensated cardiac or pulmonary condition for which endoscopic evaluation was deemed too risky, or withheld consent.
The symptoms of enrolled subjects were recorded in detail (bloating, epigastric pain, heartburn, nausea, vomiting, bad breath, weight loss, etc.) along with a history of previous and current medications and laboratory findings.
Endoscopic procedureFollowing a 12-hour fast UGISE was performed on each patient. Written informed consent was obtained prior to each procedure from all patients. All procedures were performed by three designated senior endoscopists using a front-view videogastroscope (Fujinon EVE S400, Saitama City, Japan). All endoscopists had performed upper gastrointestinal endoscopy for at least three years and were aware of SWS in the duodenum, since there have been ongoing studies in our clinic since 2008.3 After premedication with topical anesthesia of the oropharynx, patients assumed the left lateral decubitus position. When necessary, administration of 2.5–5mg of a combination with midazolam was performed until sufficient conscious sedation was achieved.
Endoscopic findings for each procedure were recorded based on minimal standard terminology, including the presence of esophagitis, Barrett's esophagus, hiatal hernia, open cardia, gastritis, bulbitis and gastric and duodenal ulcers.
In the event of the discovery of SWSs in the descending duodenum, the appearance was graded based on extent of involvement into three grades; Grade 1 – detection of SWS requires careful examination (Fig. 1), Grade 2 – immediately apparent but scarce SWS (Fig. 2), Grade 3 – dense appearance of SWS in duodenal mucosal (Fig. 3). The questionnaire was non-validated but was based on personal experience. At least four duodenal biopsies were obtained from the second and/or third portion of the duodenum for histological investigation. Gastric antrum and corpus biopsy specimens (at least two biopsies from each) were also obtained from all participants.
Biopsy specimens were fixed with 10% formalin solution before being embedded in paraffin. Following staining with HE, stained sections of all four duodenal biopsies were examined for the presence of pathogens (Giardia lamblia and Cryptosporidium spp.), inflammation in the lamina propria and intraepithelial lymphocytes. Villous architecture was also evaluated and documented. Additional staining with periodic acid-Schiff (PAS) stain was performed to rule out Whipple's disease.
By careful examination of duodenal biopsy specimens and measurement of antibodies for Celiac disease (anti-tissue transglutaminase IgA and IgG), the presence of gluten-sensitive enteropathy was ruled out in all participants of our study.
Pre- and post-treatment grading of SWS appearance was performed by a designated senior endoscopist, who for post-treatment evaluation was blinded to pre-treatment results of grading. Changes in SWS appearance with treatment were evaluated for statistical significance.
Post-procedural follow-upAll patients in group 1 were treated based on biopsy results. Those in whom the presence of HP was confirmed were given stand eradication therapy with lansoprazole 30mg bid, amoxicillin 1g bid, and clarithromycin 500mg bid for 14 days, followed by 1 month maintenance treatment with lansoprazole 30mg od. Patients with HP-negative chronic gastritis or nonspecific duodenitis were treated with lansoprazole 30 bid for three months. In patients with lymphangiectasia confirmed by duodenal biopsy, dietary modification (rich in short- and medium-chain fatty acids) and treatment of the underlying disorder (heart failure, hyperlipidemia) were recommended for three months. Success of eradication of HP was evaluated by a urea breath test 4 weeks after completion of treatment. All patients in group 1 subsequently underwent repeat endoscopy 3 months after initial evaluation to determine the presence of any changes in SWS appearance.
Patients in group 2 were not given any treatment but were still subject to a repeat endoscopy three months after the first evaluation to document any changes in SWS appearance.
Statistical analysisAnalysis was performed using the Statistical Package for Social Sciences program for Windows™, version 15.0. Comparison of categorical variables between groups was performed using the Chi-square test, and in case of a sample size of ≤ 5, Fisher's exact test was used. The t-test was used for comparison of continuous variables, and in the event of a skewed distribution, the Mann–Whitney U test was used. A p-value less than 0.05 was considered indicative of statistical significance.
ResultsA total 3450 patients were approached for inclusion in this study, out of which 3010 consented for participation. Overall, SWS were observed in 97 patients (3.2%), with which the final analysis was performed. Eight patients in group 1 did not complete the study for several reasons (2 became pregnant, 3 were diagnosed with gastric adenocarcinoma, 1 patient was diagnosed with ovarian cancer, and for 2 patients who were on warfarine, biopsy specimens could not be obtained). The study design is schematized in Fig. 4.
Fifty-eight (59.8%) of the 97 patients with SWS were women, and 39 (40.2%) were men, with a mean age of 51.1±15.8 (19–83) years. There was no difference between groups in terms of presenting complaints (Table 1).
Summary of presenting complaints of study population.
| Complaint | Overall (n=97) | Paitents (n=82) | Controls (n=15) | p-value |
| Epigastric pain | 76 (78.3%) | 64 (78%) | 12 (80%) | 0.865 |
| Epigastric discomfort | 34 (35.1%) | 29 (35.4%) | 5 (33.3%) | 0.879 |
| Nausea | 19 (19.6%) | 16 (19.5%) | 3 (20%) | 0.965 |
| Vomitting | 3 (3.1%) | 3 (3.7%) | 0 | 0.311 |
| Lower extremity edema | 1 (1.0%) | 1 (1.2%) | 0 | 0.561 |
| Bloating | 63 (64.9%) | 54 (65.9%) | 9 (60%) | 0.665 |
| Periumbilical pain | 17 (17.5%) | 13 (15.9%) | 4 (26.7%) | 0.333 |
| History of GISBa | 4 (4.1%) | 2 (2.4%) | 2 (13.3%) | 0.097 |
| Weight loss | 4 (4.1%) | 4 (4.9%) | 0 | 0.241 |
| Diarrhea | 6 (6.1%) | 4 (4.9%) | 2 (13.3%) | 0.260 |
aGISB; gastrointestinal system bleeding.
The endoscopic findings of all participants were documented. There was no significant difference between groups with regard to endoscopic and histopathological findings of gastric and duodenal specimens (Table 2).
Endoscopic and histopathological findings of study population.
| Finding | Overall (n=97) | Patients (n=82) | Controls (n=15) | p-value |
| Esophagitis | 13 (13.4%) | 11 (13.4%) | 2 (13.3%) | 0.840 |
| Loose LES | 7 (7.2%) | 6 (7.3%) | 1 (6.7%) | 0.928 |
| Hiatal hernia | 2 (2.1%) | 2 (2.4%) | 0 | 0.410 |
| Pangastritis | 11 (11.3%) | 10 (12%.2) | 1 (6.7%) | 0.530 |
| Antral gastritis | 85 (87.6%) | 72 (84.7%) | 13 (86.7%) | 0.614 |
| Bulbitis | 4 (4.1%) | 4 (4.9%) | 0 | 0.241 |
| Gastric mass | 3 (3.1%) | 3 (3.7%) | 0 | 0.311 |
| Gastric biopsy | ||||
| Chronic gastritis | 77 (81.1%) | 66 (80.4%) | 11 (73.3%) | 0.422 |
| Helicobacter pylori (+) | 45 (46.4%) | 37 (45.1%) | 8 (53.3%) | 0.558 |
| Intestinal metaplasia | 7 (7.2%) | 5 (6.1%) | 2 (13.3%) | 0.372 |
| Gastric adenocancer | 3 (3.1%) | 3 (3.7%) | 0 | 0.306 |
| Duodenal biopsy | ||||
| CND | 75 (77.3%) | 64 (78%) | 11 (73.3%) | 0.772 |
| IL | 43 (44.3%) | 37 (45.1%) | 6 (40%) | 0.350 |
| CND+IL | 21 (21.6%) | 19 (23.1%) | 2 (13.3%) | 0.420 |
CND, chronic nonspecific duodenitis; IL, intestinal lymphangiectasia; LES, lower esophageal sphincter.
Blood counts and biochemical parameters of both groups on first presentation did not reveal a statistically significant difference.
Patients were evaluated according to the given treatments. Fifteen patients (15.5%) in the control group did not receive any medication. Forty-four patients in group 1 with chronic gastritis and nonspecific duodenitis were given lansoprazol (30mg per day, for 2 months). HP eradication therapy was given to 36 patients and dietary modification and/or antihyperlipidemic treatment were given to 37 patients with intestinal lymphangiectasia.
Table 3 summarizes the results of pre- and post-treatment grading of SWS appearance on endoscopy. No change in SWS grade was observed in the control group, a statistically significant change was demonstrated in the treatment group before and after treatment (p<0.001) (Table 3).
Pre- and post-treatment endoscopic grading of scattered white spot appearance in study population.
| Endoscopic grading | Group 1 | Group 2 | ||||
| Pretreatment | Posttreament | p-value | Pretreatment | Posttreament | p-value | |
| Normal | 0 | 42 (51.2%) | <0.001 | 0 | 0 | 0.146 |
| Grade 1 | 15 (18.3%) | 25 (30.5%) | 0 | 2 (13.3%) | ||
| Grade 2 | 51 (62.2%) | 5 (6.1%) | 9 (60%) | 10 (66.7%) | ||
| Grade 3 | 16 (19.5%) | 2 (2.4%) | 6 (40%) | 3 (20%) | ||
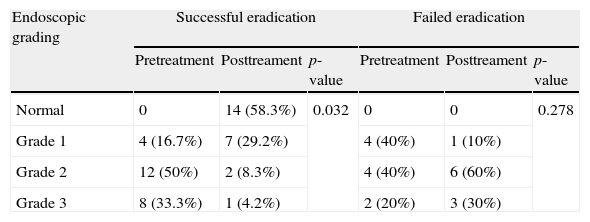
HP eradication was successful in 24 of the 34 patients who received treatment, whereas in 10 patients HP infection persisted. With regard to pre-treatment distribution of endoscopic appearance of SWS in the HP eradicated group, the treatment response was statistically significant (p=0.032). In the 10 patients for whom HP eradication was not successful, no statistically significant change was observed with treatment (Table 4).
Pre- and post-treatment endoscopic grading of scattered white spot appearance in patients with successful and failed HP eradication.
| Endoscopic grading | Successful eradication | Failed eradication | ||||
| Pretreatment | Posttreament | p-value | Pretreatment | Posttreament | p-value | |
| Normal | 0 | 14 (58.3%) | 0.032 | 0 | 0 | 0.278 |
| Grade 1 | 4 (16.7%) | 7 (29.2%) | 4 (40%) | 1 (10%) | ||
| Grade 2 | 12 (50%) | 2 (8.3%) | 4 (40%) | 6 (60%) | ||
| Grade 3 | 8 (33.3%) | 1 (4.2%) | 2 (20%) | 3 (30%) | ||
HP, Helicobacter pylori.
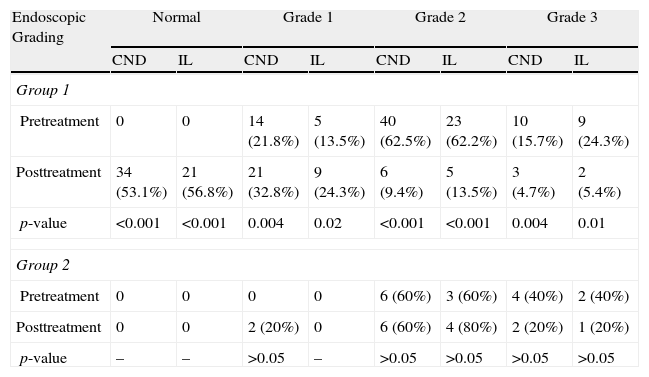
Comparison of pre- and post-treatment SWS appearance was also performed for 74 patients who had CND on duodenal biopsy and 42 patients with endoscopic findings consistent with IL (Table 5). Treatment resulted in a statistically significant change in grade of SWS appearance in both groups (p<0.001). In group 2 (untreated group) there was no difference in endoscopic grading of SWS appearance in patients with CND and IL by the end of the study period (Table 5).
Pre- and post-treatment endoscopic grading of scattered white spot appearance in patients with chronic non-specific duodenitis and intestinal lymphangiectasia.
| Endoscopic Grading | Normal | Grade 1 | Grade 2 | Grade 3 | ||||
| CND | IL | CND | IL | CND | IL | CND | IL | |
| Group 1 | ||||||||
| Pretreatment | 0 | 0 | 14 (21.8%) | 5 (13.5%) | 40 (62.5%) | 23 (62.2%) | 10 (15.7%) | 9 (24.3%) |
| Posttreatment | 34 (53.1%) | 21 (56.8%) | 21 (32.8%) | 9 (24.3%) | 6 (9.4%) | 5 (13.5%) | 3 (4.7%) | 2 (5.4%) |
| p-value | <0.001 | <0.001 | 0.004 | 0.02 | <0.001 | <0.001 | 0.004 | 0.01 |
| Group 2 | ||||||||
| Pretreatment | 0 | 0 | 0 | 0 | 6 (60%) | 3 (60%) | 4 (40%) | 2 (40%) |
| Posttreatment | 0 | 0 | 2 (20%) | 0 | 6 (60%) | 4 (80%) | 2 (20%) | 1 (20%) |
| p-value | – | – | >0.05 | – | >0.05 | >0.05 | >0.05 | >0.05 |
CND, chronic non-specific duodenitis; IL, intestinal lymphangiectasia.
In this study population, SWS appearance on endoscopy was detected in 3.2% of patients, most of whom had findings consistent with either HP-positive or HP-negative chronic gastritis. Similarly, most of the duodenal specimens obtained confirmed the presence of CND and IL. When patients with HP-positive gastritis, CND and IL were considered separately, it was observed that in all three sub-groups treatment resulted in statistically significant decreases in endoscopic grade of SWS appearance.
SWS appearance in the duodenum may occur as a result of numerous conditions. The only study evaluating the causes of SWS appearance was undertaken by Biyikoglu et al. Out of 107 patients with duodenal SWS, 36.4% had IL, 29.1% had CND, and 14% had giardiasis. Among the patients with more than one finding, 18.7% had both IL and CND, 1.9% had IL and giardiasis, and 0.9% had CND and giardiasis.3 However, they did not evaluate the status of H. pylori in those patients. Moreover, they did not evaluate the endoscopic findings after the treatment of underlying causes. In our study population, SWS appearance was most commonly associated with the presence of CND, followed by IL, either alone or in combination with CND. Giardia was not detected in any of our subjects, which may be attributed to the fact that very few of the participants complained of diarrhea, or duodenal samples did not coincide with sites colonized by Giardia.
SWS appearance is not a very commonly encountered feature of UGSE. It is usually detected in the proximal duodenum and is generally not associated with mucosal erosions.4–6 In the pertinent literature, we did not come upon a study on the incidence of SWS, which perhaps highlights the significance of our study in this sense.
In our study, the presence of CND seemed to be the primary cause responsible for SWS appearance. CND is defined by the presence of inflammatory cell infiltration on the surface or within the crypts, resulting in crypt atrophy.7 Known etiologies for CND are peptic duodenitis, duodenitis secondary to HP, gluten-sensitive enteropathy, Crohn's disease, ulcerative colitis, Whipple's disease, parasitic infections and drugs.8 CND or peptic duodenitis develop as a result of HP-related acid injury.9 HP frequently colonizes the proximal duodenum leading to chronic active duodenitis.10,11
In a study by Madsen et al., the authors reported an association of HP with microscopic chronic active duodenitis in 94.1% of subjects.11 Inflammation of the duodenal bulbus has been linked to the presence of villous atrophy in the distal duodenum.7 A high prevalence of duodenitis has been reported in children infected with HP.12 It has been postulated that the chronic inflammatory process in CND causes disruptions in epithelial lymphatic flow.3 In our study, the presence of HP was confirmed by biopsy in 34 patients. While eradication therapy was successful in 24 participants, eradication was not achieved in 10. We managed to demonstrate statistically significant decreases in endoscopic grade of SWS appearance, particularly in patients for whom eradication therapy was successful. This improvement may be attributed to the elimination of the duodenal inflammatory response triggered by HP.1 Significant improvements in SWS appearance were observed after treatment in all 64 patients with CND confirmed by duodenal biopsy, regardless of HP status.
IL is characterized by the presence of focal or generalized changes in the intestinal lymphatic channels, which may result in a protein-losing enteropathy, steatorrhea or lymphopenia. Primary intestinal lymphangiectasia is a generalized congenital malformation of the lymphatic system affecting children and young adults.13 Secondary causes include obstruction of the lymphatic channels and increased central pressure.14,15 Several studies have reported on endoscopic evidence of duodenal lymphangiectasia without the presence of malabsorption.16,17 Consistent with these results, none of our patients with IL had hypoalbumenia, lymphopenia or chronic diarrhea.
Endoscopically, IL has three distinct appearances; multiple small white spots (50.8%), diffuse whitening of villi (27.1%) and large macular or nodular focal white lesions (22.1%). In a retrospective analysis by Kim et al., the prevalence of IL after endoscopic evaluation was 3.2%, while with histopathological confirmation this value was only 1.9%.18 The same authors followed this up with a prospective study on asymptomatic individuals who underwent UGISE where they reported an endoscopic prevalence of 11.2% and a microscopic prevalence of 8.9% for IL.18
In a study by Biyikoglu et al.3, the prevalence of IL in patients with SWS appearance on endoscopy was reported to be 57%. In our study, we detected IL in 44.2% of patients with duodenal SWS. Histologically, IL is characterized by the presence of marked dilatation of lymphatic channels, whereas SWSs are believed to occur as a result of disruptions in intestinal lymphatic flow.19 Patients with duodenal SWS and IL were asked to adhere to a diet rich in short- and medium-chain fatty acids20 for a period of 2 months, after which control endoscopy revealed significant decreases in the grade of SWS appearance. Short and medium-chain fatty acids are absorbed directly into the portal system without contributing to the formation chylomicrons, which in turn relieves the load on lymphatic flow and decreases lymphatic pressure. Consequently, this unhindered lymphatic flow leads to a reversal in duodenal SWS appearance.
One of the limitations of our study is the small size of the control group. Moreover, we could have performed quantitative evaluation of alpha-1 antitrypsin in dry stool to rule out the presence of protein-losing enteropathy in patients with IL. Undertaking such a feat would have added to the cost of the study without providing patients with any clinical benefit. Another limitation of the study is the absence of a validated classification for the endoscopic appearance of SWS. We described a grading system based on personal experience. Endoscopic grading was performed, both before and after treatment, by a designated endoscopist with 15 years of experience, while ensuring that the endoscopist was blinded to the results of pre-treatment endoscopic evaluation.
In conclusion, the presence of SWS in the descending duodenum is not specific to any particular disorder, although CND and IL are the most commonly encountered etiologies. Patients with an endoscopic appearance of SWS should undergo gastric and duodenal biopsy to help guide treatment. But there are still further questions remaining regarding the real need of treating SWS because of lack of long-term follow-up. Moreover, regardless of H. pylori status, a majority of patients in this study can be labeled as functional dyspeptics, which are usually difficult-to-treat patients and poor responders to drugs. For that reason, further prospective randomized case–control studies are required to establish the benefits of long-term treatment of SWS patients.
Conflict of interest statementThe authors declare no conflict of interest.