Ustekinumab is an effective treatment for inflammatory bowel diseases. However, some patients do not respond to conventional doses. The aim of the study was to evaluate the effectiveness of intravenous maintenance ustekinumab in patients with secondary failure.
MethodsSingle-center, retrospective study in adult patients with intravenous maintenance ustekinumab. The reduction of biochemical activity markers, ustekinumab trough levels and clinical indices of activity were evaluated. Biological remission was defined as the percentage decrease fecal calprotectin ≥80% and/or final fecal calprotectin ≤250 and C reactive protein <5mg/L.
ResultsThirty-one patients were included: Crohn's disease 77.4%. All included patients were bio-exposed and 61.3% had carried ≥2 biologics.
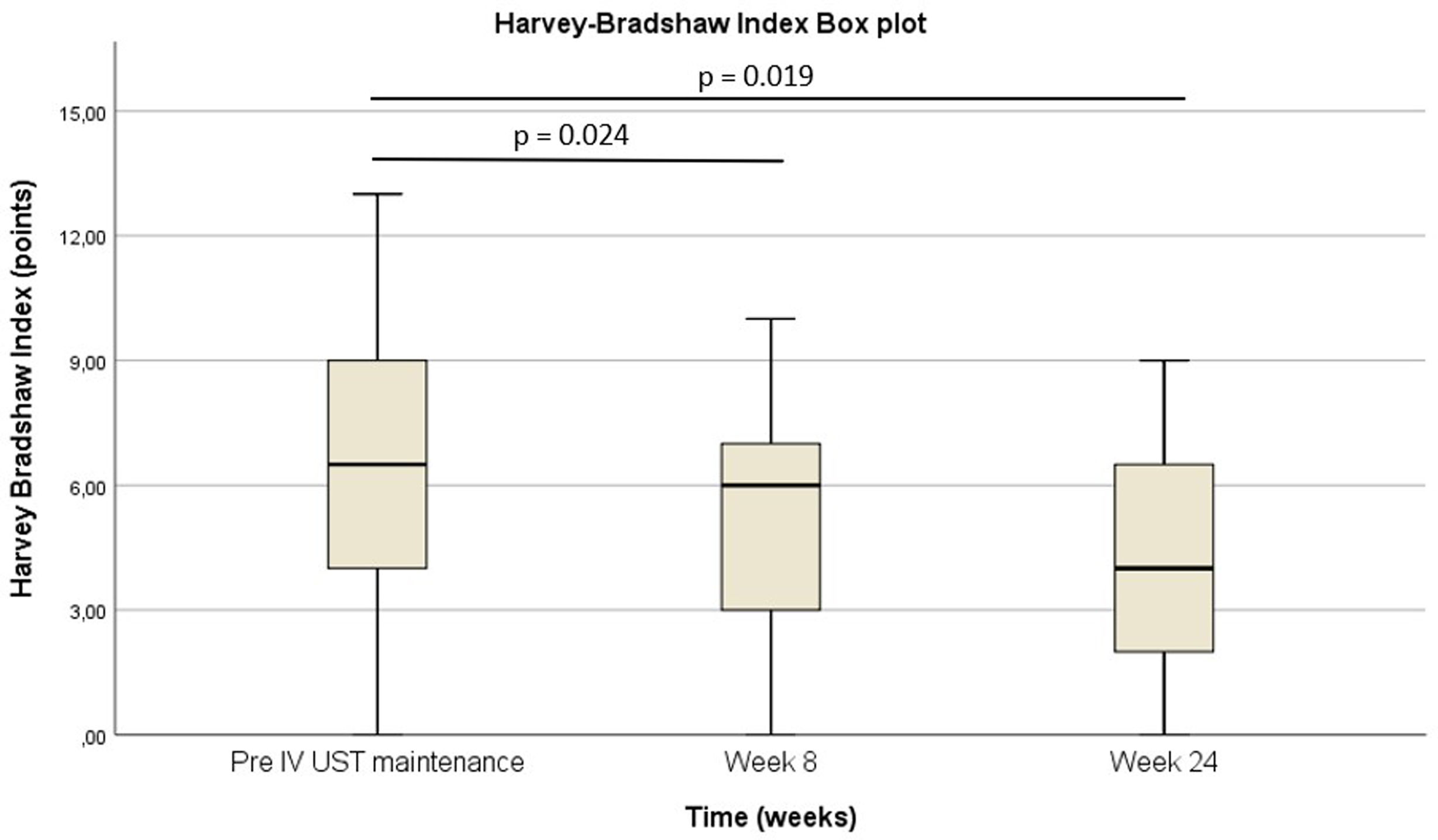
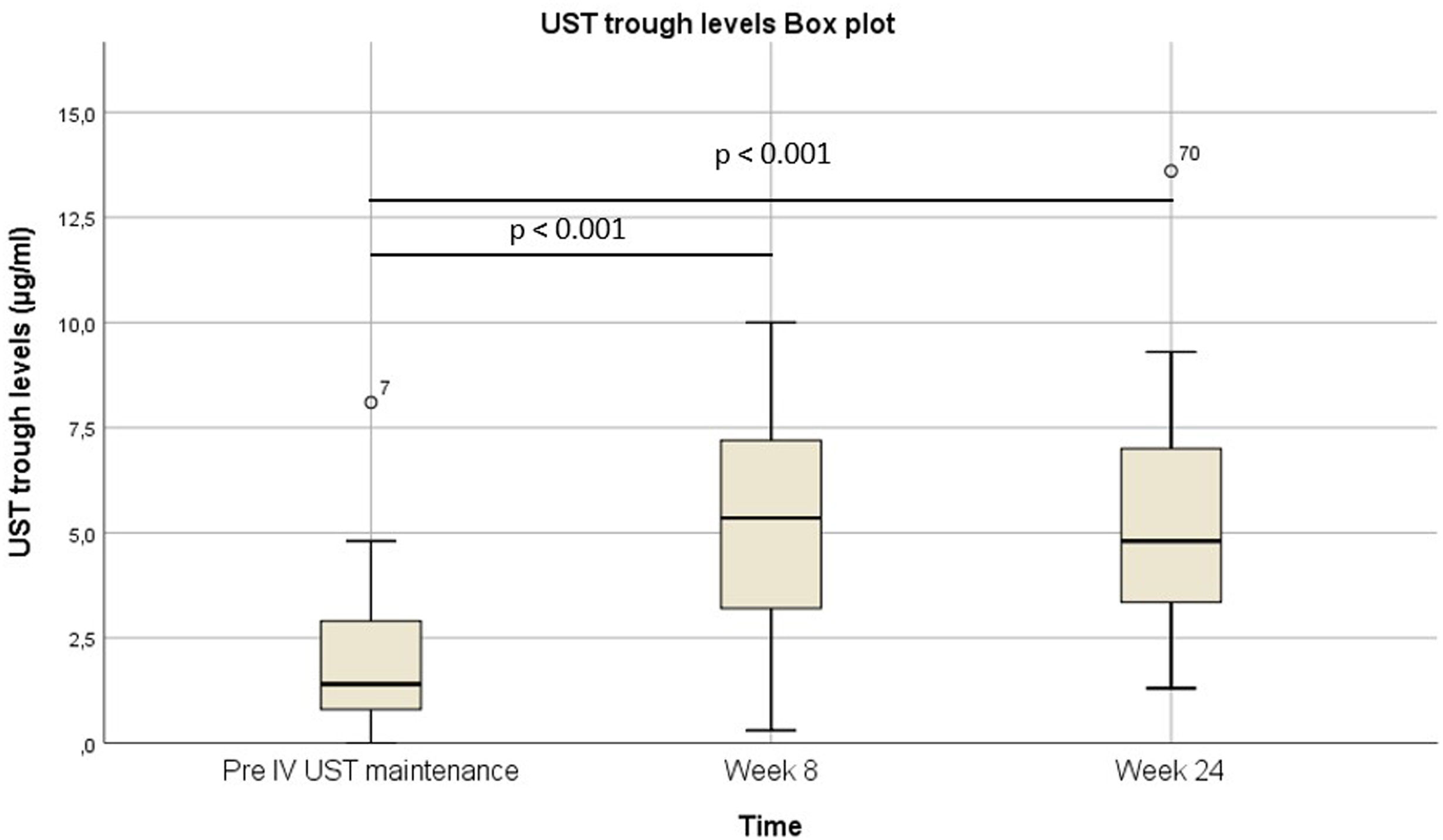
Pre-intravenous maintenance mean Harvey–Bradshaw Index was 6.5±4.38 vs 5±3.1 at week 8 (p=0.024) vs 4.1±3.1 at week 24 (p=0.019). The median ustekinumab trough level pre-intravenous maintenance was 1.40μg/ml [IQR 2.3] vs 5.35μg/ml [IQR 4.1] at week 8 (p<0.001) vs 4.8μg/ml [IQR 3.9] at week 24 (p<0.001). The pre-intravenous maintenance median fecal calprotectin was 809μg/g [IQR: 2256] vs 423μg/g [IQR: 999] at week 8 (p=0.025) vs 333μg/g [508] (p=0.001) at week 24.
At the end of follow-up 48% went into biological remission. The presence of perianal disease was associated with lower biological remission (70.6% vs 27.3%, p=0.025). Median intravenous ustekinumab maintenance time was 8.55 [IQR 23.9] months. In 83.9% of patients no serious infections or malignancy were documented.
ConclusionsThe use of maintenance intravenous ustekinumab appears to be an effective and safe strategy that can be evaluated as a salvage treatment especially in highly bio-exposed patients.
Ustekinumab es un tratamiento eficaz para la enfermedad inflamatoria intestinal. Sin embargo, algunos pacientes no responden a las dosis convencionales. El objetivo del estudio fue evaluar la eficacia de ustekinumab intravenoso de mantenimiento.
MétodosEstudio retrospectivo unicéntrico en pacientes adultos con ustekinumab intravenoso de mantenimiento. Se evaluó el descenso de los marcadores bioquímicos de actividad, los niveles valle de ustekinumab y los índices clínicos de actividad. La remisión biológica se definió como la disminución porcentual de calprotectina fecal ≥80% y/o calprotectina fecal final ≤250 y proteína C reactiva <5mg/l.
ResultadosSe incluyeron 31 pacientes: enfermedad de Crohn 77,4%. Todos los pacientes incluidos estaban bioexpuestos, y el 61,3% habían llevado ≥2 biológicos.
La mediana del índice Harvey-Bradshaw antes del mantenimiento intravenoso fue de 6,5±4,38 vs. 5±3,1 en la semana 8 (p=0,024) vs. 4,1±3,1 en la semana 24 (p=0,019). La mediana de los niveles valle de ustekinumab antes del mantenimiento intravenoso fue de 1,40μg/ml (IQR: 2,3) vs. 5,35μg/ml (IQR: 4,1) en la semana 8 (p<0,001) vs. 4,8μg/ml (IQR: 3,9) en la semana 24 (p<0,001). La mediana de calprotectina fecal antes del mantenimiento intravenoso fue de 809μg/g (IQR: 2.256) vs. 423μg/g (IQR: 999) en la semana 8 (p=0,025) vs. 333μg/g (508) (p=0,001) en la semana 24.
Al final del seguimiento, el 48% entró en remisión biológica. La presencia de enfermedad perianal se asoció a una menor remisión biológica (70,6 vs. 27,3%; p=0,025). La mediana del tiempo de mantenimiento con ustekinumab intravenoso fue de 8,55 (IQR: 23,9) meses. En el 83,9% de los pacientes no se documentaron infecciones graves ni neoplasias.
ConclusionesEl uso de ustekinumab intravenoso de mantenimiento parece ser una estrategia eficaz y segura que podría servir como tratamiento de rescate especialmente en los pacientes altamente bioexpuestos.
Ustekinumab (UST) is a monoclonal antibody that binds with high affinity to the p40 subunit of human interleukin 12 and 23 and has been approved for treatment of patients with moderate to severe Crohn's disease (CD) and ulcerative colitis (UC). It is indicated in adult patients who have failed to conventional therapy or anti-tumor necrosis factor (TNF) therapy, and for whom conventional therapy or anti-TNF therapy is contraindicated.1,2 UST has been shown to be effective in both CD and UC in real-world studies.3,4
Although the effectiveness of biologic drugs is high, some patients still do not respond to treatment. With anti-TNFs we learned to recognize different types of therapeutic failure. While immunogenic failure does seem to be more closely related to anti-TNF drugs than to the more recently approved biologics for IBD – ustekinumab, vedolizumab – pharmacodynamic and pharmacokinetic failure is presented with all biologics. The first type of failure is difficult to predict and to address at present despite a multitude of ongoing efforts to achieve precision medicine in this disease. However, pharmacokinetic failure, due to insufficient drug exposure, secondary for example to a significant inflammatory burden, can be detected and reversed with therapeutic drug monitoring (TDM). This therapeutic strategy based on drug levels allows to adjust the drug dose in the event of a decrease in drug levels.
Some pharmacokinetic studies, commonly carried out in patients with CD, in part because of its prior approval to UC as a treatment, have already shown how plasma concentrations of UST are proportional to the administered dose and that these concentrations also influence the effectiveness of the treatment.5
It has been shown that standard doses of UST are probably insufficient in a significant number of patients, and that by increasing the dose we can rescue patients who do not respond to the usual dose.6–8 As a strategy to achieve higher UST trough levels, intensification of subcutaneous (SC) treatment to every 4 weeks is effective and safe.9,10 In patients with CD in whom SC escalation of treatment has not been effective, the option of weight-adjusted intravenous reinduction helps rescue approximately half of patients and increases the durability of response.11,12 In addition, there is already evidence that an induction with two initial IV doses in patients with severe CD is associated with a higher clinical, endoscopic response and mucosal healing at weeks 16 and 48.13
The use of maintenance IV UST is another, and more recently described, dosage intensification strategy that could help rescue partial responders or patients with secondary loss of response with standard doses of UST.14–16
The aim of the study was to evaluate the effectiveness, security, and persistence of maintenance IV UST in patients with secondary failure to SC UST.
MethodsStudy design and patient populationUnicentric observational and retrospective study based on a database with prospective data collection in adult patients (≥18 years old) with IBD under maintenance with IV UST treatment. Patient data were obtained from the ENEIDA (Estudio Nacional en Enfermedad Inflamatoria Intestinal sobre Determinantes Genéticos y Ambientales [Spanish National Study on Inflammatory Bowel Disease: Genetic and Environmental Determinants]) database, a registry of the GETECCU, which includes patients with inflammatory bowel disease (IBD) and records prospectively clinical characteristics, outcomes, and treatments. It was approved by the Hospital Clínic de Barcelona Ethics Committee in 2006 – ref. 2006/3155, and its use by the participating centers complies with all local and national regulations.
All patients were treated with an initial IV induction therapy of UST according to the patient's body weight (260mg <55kg, 390mg 55–85kg, and 520mg >85kg), followed by SC maintenance therapy of 90mg SC maintenance Q8 or Q4 before starting IV maintenance. All patients included had received at least three maintenance doses of UST IV.
Activity markers such as C reactive protein (CRP) and fecal calprotectin (FC), UST trough levels pre- and post IV maintenance as well as clinical indices of activity (Harvey–Bradshaw Index [HBI] or Partial Mayo Score [pMS]) were extracted from electronic medical records (EMR) and were retrospectively analyzed. IV dose adjustments and their effect on levels were also collected, as well as persistence and adverse effects. UST trough levels were measured using a commercially available validated chemiluminescent immunoassay (CLIA) – i-Tracker Ustekinumab, Theradiag. Anti-drug antibodies to UST were automatically measured if UST trough levels were very low (<0.5μg/ml) or undetectable.
The choice of maintenance IV regimen, as well as for the initial loading dose, was based on the subjective assessment of the physician considering patient's previous UST SC levels, current inflammatory burden, previous history of inflammatory bowel disease (e.g. refractoriness to multiple lines of treatment) and the intended therapeutic goal (clinical remission, endoscopic remission, etc.).
Outcomes and definitionsMain outcome was biological remission at week 24 defined as percentage decrease of FC ≥80% and/or final FC ≤250μg/g and CRP <5mg/L.17
Secondary outcomes were clinical remission and clinical response. Clinical remission was defined as a HBI <5 without steroids for CD and pMS <3 for UC and clinical response was defined by HBI reduction ≥3 without steroids for CD or a reduction of in pMS ≥3. Other secondary outcomes were the improvement of biochemical tests (CRP and FC) between baseline and weeks 8 and 24.
Immunosuppressive therapy was defined as any dose of either methotrexate, azathioprine or 6-mercaptopurine at study enrolment. UST therapeutic range in our laboratory is 1–4.5μg/ml.
Statistical analysisData are expressed as the mean (standard deviation) or median (interquartile range) for numerical variables, depending on whether they had a normal or non-parametric distribution, respectively, based on the Shapiro–Wilks test. Qualitative variables are expressed as the absolute frequency (%). We used T-student or Mann–Whitney for continuous variables (normal and non-parametric, respectively) and Chi-square (or Fisher's exact test if needed) for categorical variables. A p-value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 25.0 (IBM, Armonk, USA).
EthicsThis project was approved by the Ethics Committee of La Fe Institute of Health Research and by the Spanish Agency for Medicines and Medical Devices (AEMPs). All patients included in the registry signed an informed consent document authorizing the use of their clinical data for research purposes. The stipulations of Good Clinical Practice and the Declaration of Helsinki were adhered to at all times.
ResultsBaseline characteristicsOf 335 patients in our centre under treatment with UST between the years 2017 and 2023, 31 patients (9.25%) were included. Baseline characteristics of patients are shown in Table 1. A total of 77.4% (24/31) of patients had a diagnosis of CD. Up to 2/3 of patients with CD had a complicated phenotype (B2/B3) and 38.7% had associated perianal disease (PD). All patients with UC included had pancolitis. All included patients were bio-exposed and 61.3% had carried ≥2 biologics. Concomitant treatment was used at the start of ustekinumab in 54.8% of patients (immunosuppressants 37.9%, prednisone 34.5% and mesalazine 20.7%).
Baseline characteristics.
| Total (n)=31 | N (%) |
|---|---|
| Mean age (years)a | 42.49±13.23 |
| Males,n(%) | 17 (55) |
| BMI ≥25,n(%) | 11 (36.6) |
| Smoking | |
| - Active smoking | 10 (33.3) |
| - Ex-smokers | 8 (26.7) |
| Previous surgical interventions | 18 (58.1) |
| Disease duration (years)b | 14.3 (14.7) |
| CD,n(%) | 24 (77.4) |
| Montreal CD classificationc | 24/31 |
| - Age | |
| ∘ A1, n (%) | 6 (25) |
| ∘ A2, n (%) | 17 (70.8) |
| ∘ A3, n (%) | 1 (4.2) |
| - Location | |
| ∘ L1, n (%) | 12 (50) |
| ∘ L2, n (%) | 2 (8.3) |
| ∘ L3, n (%) | 10 (41.7) |
| ∘ L4, n (%) | 6 (26.1) |
| - Behavior | |
| ∘ B1, n (%) | 8 (33.3) |
| ∘ B2, n (%) | 11 (45.8) |
| ∘ B3, n (%) | 5 (20.8) |
| ∘ Perianal disease, n (%) | 12 (50) |
| Montreal UC classification | 7/31 |
| - Extent | |
| ∘ E1 (proctitis), n (%) | 0 (0) |
| ∘ E2 (left colitis), n (%) | 0 (0) |
| ∘ E3 (pancolitis), n (%) | 7 (100) |
| Prior medication exposure | 31 (100) |
| - 1 biologic drug, n (%) | 10 (32.3) |
| - 2 biologic drugs, n (%) | 13 (41.9) |
| - 3 or more biologic drugs, n (%) | 7 (22.6) |
| Type of prior biologic drug | |
| - Infliximab, n (%) | 23 (74.2) |
| - Adalimumab, n (%) | 20 (64.5) |
| - Vedolizumab, n (%) | 10 (32.3) |
| - Golimumab, n (%) | 1 (3.2) |
| - Certolizumab, n (%) | 1 (3.2) |
| Previous Jak-inhibitor,n(%) | 3 (9.7) |
| Baseline fecal calprotectineb | 809 (IQR: 2256) |
| Baseline Harvey–Bradshaw indexa | 6.5±4.38 |
| UST trough levels prior to IV maintenanceb | 1.4 [IQR 2.3] |
| Modification of IV UST maintenance regimen,n(%) | 11 (35.5) |
| Duration of IV UST treatment (months)b | 8.55 [IQR 23.9] |
(%): numbers in parentheses indicate the prevalence of the variable.
Abbreviations: BMI, body mass index; CD, Crohn's disease; UC, ulcerative colitis; UST, ustekinumab, IV, intravenous.
The main indication for initiation of UST was active disease due to secondary failure to previous biologic therapy (80%). The induction dose was adjusted by weight (260mg in 6 patients, 390mg in 20 patients and 520mg in 5 patients) and subsequent SC maintenance was every 8 weeks in 76% (n=23) and every 4 weeks in 23.3% (n=7). Of the patients with the initial standard regimen, about 60% were later intensification cases after a median time from initiation of UST of 7.53 [IQR 7.66] months. Reinduction followed of SC administration was performed in 36.7% of patients.
Ustekinumab IV maintenanceThe reason to start IV maintenance was in 10 (32.2%) patients due to low trough levels with inflammatory activity; 13 (41.9%) due to persistent inflammatory activity with UST trough levels in therapeutic range; 7 (22.6%) with loss of response and improvement after reinduction; and 1 (3.2%) due to the route of drug administration preference. The median time from drug initiation to IV maintenance was 10.23 [IQR 30.7] months. In 54.8% a higher dose was administered in the first IV maintenance infusion at discretion of the physician (the most common 390mg). The most common maintenance regimen was 130mg (64.5%) every 4 weeks (54.8%). A modification of the maintenance IV regimen was made during follow-up in 35.5% (the new regimen was 260mg in 54.5% – every 6 weeks in 45.5%). Fig. 1 shows flow chart of the route and doses administered and dosing schedule adjustments. No anti-drug antibodies were detected.
Primary outcome and predictive factors of responseAt the end of follow-up 48% of patients (n=15) went into biological remission at week 24. The presence of PD was associated with lower biological remission (70.6% remission without perianal disease vs 27.3% with perianal disease, p=0.025). Pre-maintenance IV loading dose, concomitant immunosuppressant therapy and the type of IBD (CD vs UC) did not influence biological remission. In patients with CD, response was achieved in 66.6% (10/15) and clinical remission was achieved in 60% (9/15) of patients.
Outcomes at weeks 8 and 24While UST trough levels significantly increased following the switch from IV to SC UST, HBI, CRP and FC decreased significantly during follow-up.
In patients with CD, the pre-IV maintenance mean HBI was 6.5±4.38 vs 5±3.1 at week 8 (p=0.024) vs 4.1±3.1 at week 24 (p=0.019) (Fig. 2). The median UST trough level pre-IV maintenance was 1.40μg/ml [IQR 2.3] vs 5.35μg/ml [IQR 4.1] at week 8 (p<0.001) vs 4.8μg/ml [IQR 3.9] at week 24 (p<0.001) (Fig. 3). The median UST trough level at week 24 in the responder group was 6.2 (IQR 4) vs 4.85 (IQR 3.7) in the non-responder group, p=0.367. In patients with UC, the median pMS was 2.5±6 vs 1±2 at week 24 (p=0.066).
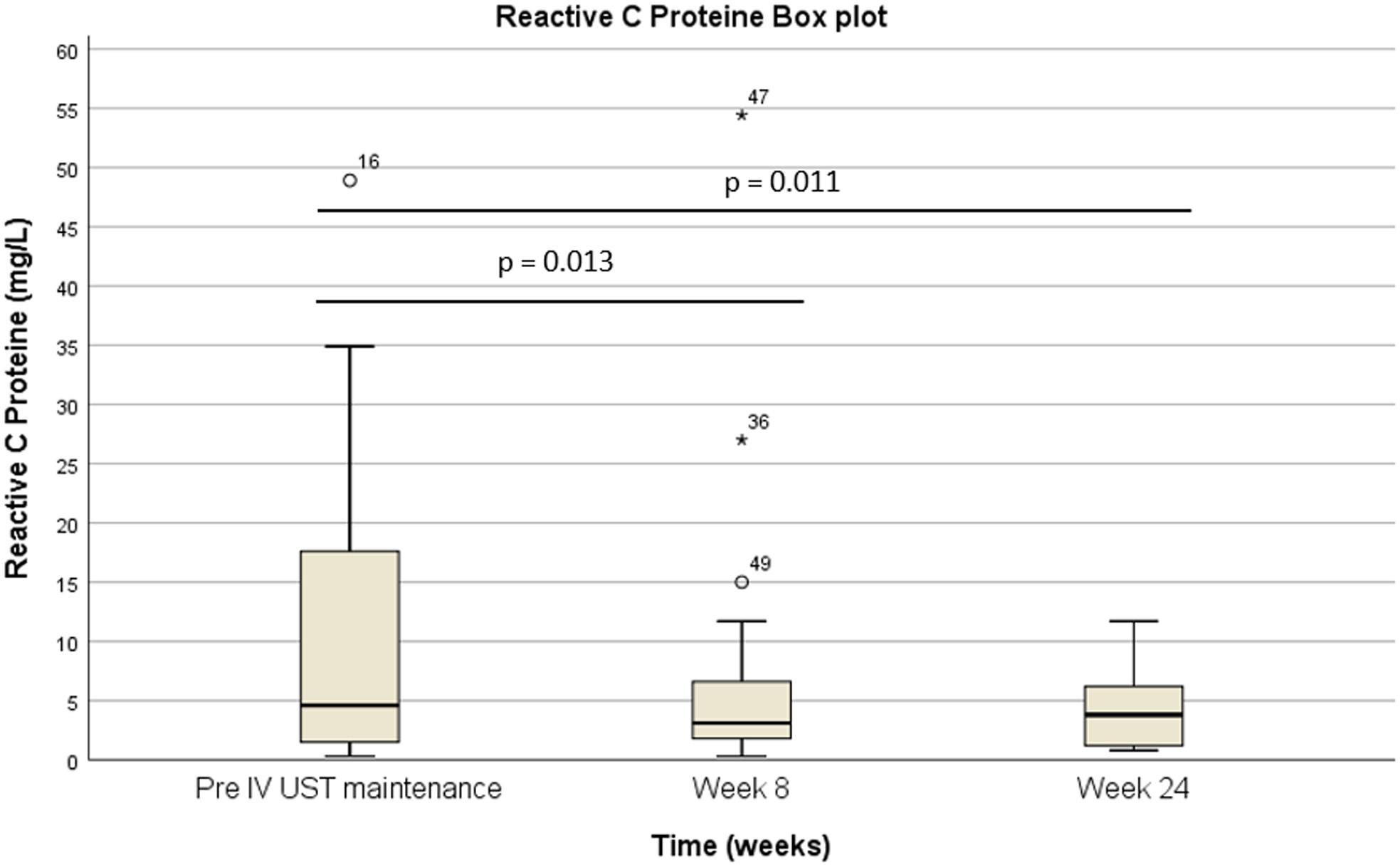
The pre-IV maintenance median FC was 809μg/g [IQR: 2256] vs 423μg/g [IQR: 999] at week 8 (p=0.025) vs 333μg/g [508] (p=0.001) at week 24 (Fig. 4). Basal RCP was 4.6 [17.4] vs 3.1 [IQR 5.6] at week 8 (p=0.013) vs 3.8mg/L [IQR 10.9] at week 24 (p=0.011) (Fig. 5).
SafetyIn 83.9% of patients no serious infections or malignancy were documented. A mild adverse effect, that did not require withdrawal/modification nor the addition of any new treatment, was documented in 3 patients (9.7%). In 2 patients, an adverse effect classified as serious was documented due to the need to associate a new treatment for that adverse effect: antiviral in a reactivation of ocular herpes zoster and antibiotic and drainage in a tube-ovarian abscess. However, UST was not discontinued in any of the 2 patients with good outcomes from both infections.
PersistenceThe median IV UST maintenance time was 8.55 [IQR 23.9] months and 96.6% of patients (n=30) are continuing treatment. Treatment was only suspended in 1 patient due to loss of follow-up due to return to their country of origin.
DiscussionThis single-center, observational, retrospective study demonstrates the effectiveness and safety of IV UST administration, a drug whose maintenance treatment is SC in bio-exposed patients, with reinduction and intensification. However, in certain situations this route of administration and dosage may not be sufficient. Approximately half of the patients in the cohort achieved biological remission and more than half clinical remission with UST IV maintenance at 24 weeks. It is important to highlight the multi-refractoriness of the cohort, with two thirds of patients bio-exposed to 2 or more biologics or with complex CD phenotype. To our knowledge, this is the study with the largest sample size published to date of patients on maintenance IV UST.
Subsequent analyses of pivotal studies of UST have shown that the pharmacokinetics behave similarly to previous biologics (anti-TNF), and that administration of UST treatment in bio-naïve patients results in a higher treatment response rate than in bio-exposed patients.18 IV drug maintenance and higher drug levels could be an alternative to failure with standard doses in this situation of refractoriness to previous lines, which is increasingly common in clinical practice. Meanwhile, therapeutic goals we currently set for IBD have changed and have become more ambitious as more effective treatments are available. In the recently published GETECCU position paper on the TDM with biologic drugs, recommendations are given on the trough levels of UST both post-induction and in the maintenance phase, according to the targets set. Thus, for an endoscopic response with UST we would need maintenance levels >4.5μg/ml.19,20 In clinical practice, especially in patients with a higher body mass index (BMI) or high initial inflammatory load, it is very difficult to achieve these target concentrations with the standard SC dose.
Pharmacokinetic studies have also been conducted with model development to guide UST dosing in CD patients. UST clearance is increased with decreasing albumin and weight gain.21 In our study one third of the patients had a BMI ≥25. Regarding albumin, in our series the median was 4.4 before initiation of maintenance IV UST and 4.3 at the end of follow-up. In our patient cohort we did not use a pharmacokinetic model to calculate the selected dose. The choice of initial dose was based on previous UST SC levels, current inflammatory burden, previous history of IBD and the intended therapeutic goal. The subsequent modification of the IV maintenance dose was marked by not achieving the proposed objectives or by a non-significant increase in drug levels. Although the initial dose of 5 patients was 130mg Q8w, at the end of follow-up none of the patients continued with that dosage regimen, probably due to a very slight increase in levels compared to the intensified SC doses. Although the choice of dosage in our patients was not greatly limited economically, we believe it is worth noting that some used doses were inefficient, and this should be taken into account when planning treatment.
Regarding the UST levels reached with the IV regimen, they are lower than those of other published studies15 or published abstracts of congresses.22,23 This could be explained by the difference in measurement methods between laboratories (trough levels are also lower in our study with the subcutaneous regimen) or by the refractoriness of IBD in these patients and their difficulty in reaching optimal levels.
There is limited evidence in the literature to predict which patients are at increased risk of failure of UST intensification. Having PD, an elevated HBI or concomitant corticosteroid use has been shown to be associated with failure after UST intensification.24 In our study PD was associated with a lower biochemical response at 24 weeks after intravenous treatment. Perhaps, as with anti-TNF drugs, in the clinical setting of perianal disease, higher levels of UST are required, and maintenance could be considered directly with IV doses.
With SC UST it has been demonstrated long-term maintenance of clinical, endoscopic and radiological response in patients with CD.25 Our series evaluates both the improvement in biochemical and clinical parameters at week 8 and week 24 after the start of IV treatment. We evaluated biological response at week 24 given that in many cases these are patients with a long evolution of their disease, severe lesions in morphological tests and with difficulty controlling the disease with previous treatments. Regarding the persistence of the drug in intravenous administration, in our patients it was very high, greater than 95% 8 months after switching to the intravenous route, as reported in previously published studies.26
About the safety of maintenance UST treatment, a large number of previous publications have already demonstrated the safety of different UST intensification strategies.8,14 In our study, treatment suspension was not necessarily due to adverse effects. However, additional treatment was required in 2 of the patients for viral and bacterial infections. Despite a higher drug concentration and therefore a theoretical greater immunosuppression with the intravenous dose, we cannot assure that these adverse effects would not have occurred without IV maintenance or without receiving the drug.
To our knowledge, this is the study with the largest sample size published to date, without considering abstracts in congress.22,23 Previously, the study by Argüelles-Arias et al. had presented the largest series of patients.15 Another strength of our research is that our series provides information on both short-term response (8 weeks) and medium to long-term response (24 weeks) with UST IV maintenance in real world.
The limitations of the study are its retrospective nature with associated biases: in the case of safety, the possible collection bias and with respect to drug persistence, the fact that in many patients there were no other treatment options, which could lead to a false persistence of patients still with inflammatory activity. Also, the small sample size, the absence of baseline and post-maintenance IV endoscopy or imaging in most patients, that would provide demonstration of an endoscopic response, and the subjective choice of IV maintenance regimen due to the lack of a pharmacokinetic model.
In conclusion, this research demonstrates that UST as maintenance IV appears to be an effective and safe strategy that can be evaluated as a salvage treatment especially in highly bio-exposed patients or in patients with complex CD phenotype. It has been clearly studied how the effectiveness of a second or subsequent therapeutic line is always lower than that of the drug in a biologic-naïve patient. Through the results of this study, we can avoid a second, third or fourth line of therapy due to UST failure and we can increase the persistence of the drug, avoiding possible risks of worsening or adverse effects of the new treatment. Longer-term studies and larger sample sizes are necessary to confirm our results.
Authors’ contributionsA.M and G.B equally participated in the design of the study, the analysis and drafted the manuscript; A.M, G.B, E.C, A.G, P.R, M.G, M.I, M.A, E.C and P.N participated in the interpretation of the data and critically reviewed the manuscript; A.M, G.B, E.C, A.G, P.R, M.G, M.I, M.A, E.C, L.T, V.B, N.B, M.J.F, R.M and P.N recruited patients for the study and contributed to the critical review of the manuscript.
FundingThis research has not received specific aid from public sector agencies, the commercial sector, or non-profit entities.
Conflict of interestsThe authors declare no conflict of interest.