Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2 first started in Wuhan, China, and soon turned to a pandemic and still continue to spread across the world.1,2 With an increasing number of COVID-19 cases outside of China, our clinics in Shahid Beheshti Hospital, Qom city, was faced with a large number of patients who were suspected of being infected with the SARS-CoV-2. Following the outbreak of COVID-19, the prevalence of gastrointestinal (GI) tract symptoms significantly goes up. This incidence in COVID-19 patients is well documented in literatures.3 After February 20, 2020, the day that Iran reported as the start of COVID-19 outbreak, the number of patients referred to our GI clinic was unusually increased. The most important complaint of patients was the incidence of some unusual GI symptoms resistant to medication.4
One of the questions that need to be answered promptly is that whether the incidence of rare GI symptoms is possible in confirmed COVID pneumonia patients. The answer to this question may help physicians take better diagnostic and therapeutic approaches for patients with this symptom. Currently, we have reported the clinical data in details as well as the result of chest CT of a COVID-19 patient with dysentery.
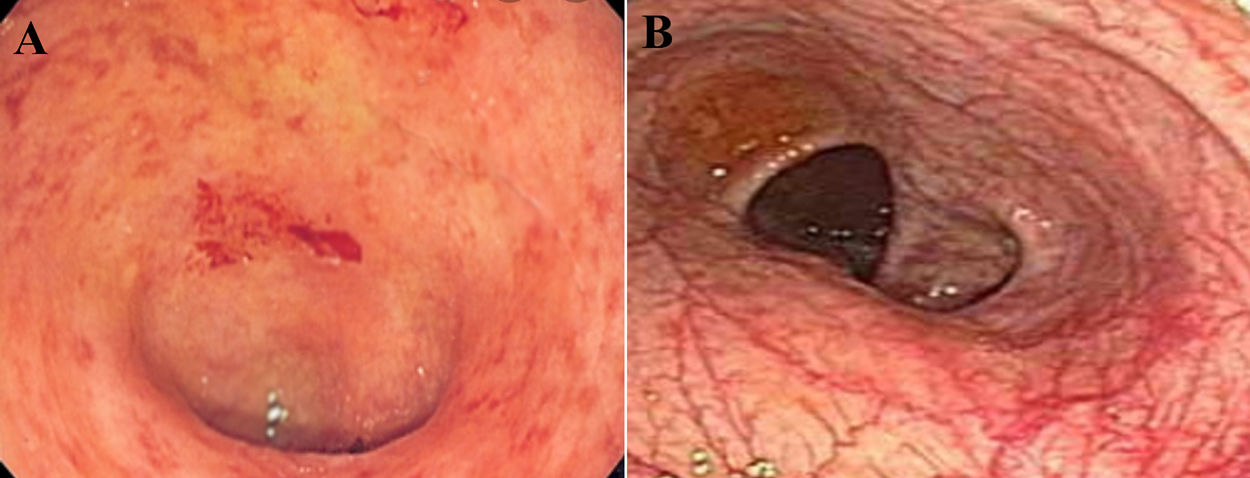
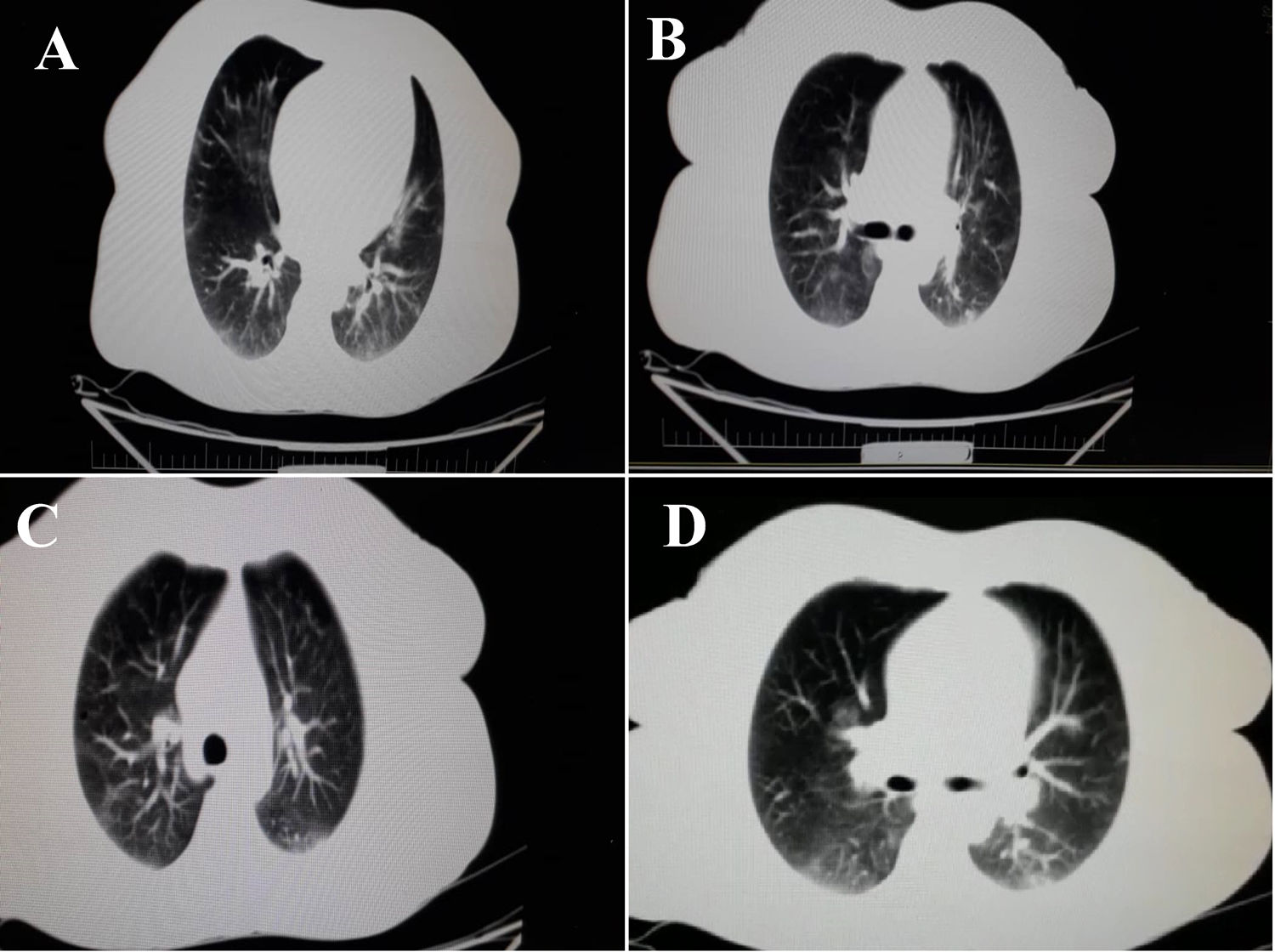
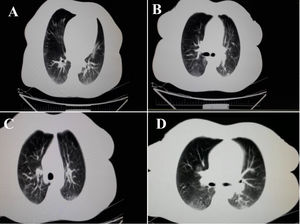
A 43-year-old woman was referred to our gastroenterology clinic, complaining from dysentery for one week. She did not mention any cough, dyspnea or respiratory disorders. Her past medical history was unremarkable and she ignored smoking, drinking or using any drugs and medications. Respiratory rate: 18 per minute and body temperature from oral root: 37.5–38°C. In order to accurately evaluate the patient, laboratory test was performed. The results are summarized in (Table 1). The levels of CRP, LDH and ESR 1h were higher than the normal range and were equal to 8.4, 400 and 21 respectively. Alkaline phosphatase, amylase serum and bilirubin values of the patient were in normal range. Medication with ciprofloxacine, 500mg and metrodinazole, 500mg was started after the first symptoms of dysentery, to reduce the volume of diarrhea and severe dehydration. Due to the persistence of diarrhea and the lack of therapeutic response to medications, as well as evaluation for the probability of inflammatory bowel disease (IBD) in the patient, colonoscopy was performed which was resulted in patchy erythema (Fig. 1). Also, pathological finding was associated with the infiltration of inflammation cells which made us suspect infectious (viral) colitis. Since the incidence of such GI symptoms coincided with the COVID-19 outbreak in Iran, and also more importantly since the patients reported to reside in high risk areas, we suspected patients to be infected by SARS-CoV-2. Therefore, for more validation, laboratory-confirmed COVID pneumonia was performed by using SARS-CoV-2 conventional polymerase chain reaction assay and sequencing of the polymerase chain reaction (PCR) amplicons, which was reported to be positive for the patient. After performing chest CT scan, we noticed the bilateral lung involvement, as revealed bilateral peripheral ground glass, crazy paving and small consolation opacities (Fig. 2). Patient was isolated in a negative pressure room for one week and underwent medications as mentioned above.
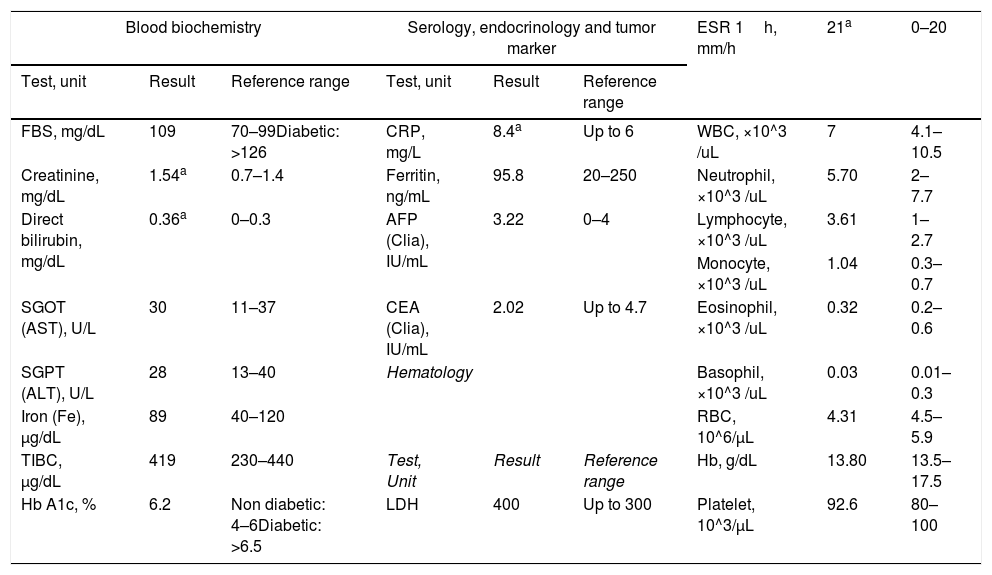
Serial laboratory results of 43-year-old woman with presentation of dysentery due to the infection with SARS-CoV-2.
| Blood biochemistry | Serology, endocrinology and tumor marker | ESR 1h, mm/h | 21a | 0–20 | ||||
|---|---|---|---|---|---|---|---|---|
| Test, unit | Result | Reference range | Test, unit | Result | Reference range | |||
| FBS, mg/dL | 109 | 70–99Diabetic: >126 | CRP, mg/L | 8.4a | Up to 6 | WBC, ×10^3 /uL | 7 | 4.1–10.5 |
| Creatinine, mg/dL | 1.54a | 0.7–1.4 | Ferritin, ng/mL | 95.8 | 20–250 | Neutrophil, ×10^3 /uL | 5.70 | 2–7.7 |
| Direct bilirubin, mg/dL | 0.36a | 0–0.3 | AFP (Clia), IU/mL | 3.22 | 0–4 | Lymphocyte, ×10^3 /uL | 3.61 | 1–2.7 |
| Monocyte, ×10^3 /uL | 1.04 | 0.3–0.7 | ||||||
| SGOT (AST), U/L | 30 | 11–37 | CEA (Clia), IU/mL | 2.02 | Up to 4.7 | Eosinophil, ×10^3 /uL | 0.32 | 0.2–0.6 |
| SGPT (ALT), U/L | 28 | 13–40 | Hematology | Basophil, ×10^3 /uL | 0.03 | 0.01–0.3 | ||
| Iron (Fe), μg/dL | 89 | 40–120 | RBC, 10^6/μL | 4.31 | 4.5–5.9 | |||
| TIBC, μg/dL | 419 | 230–440 | Test, Unit | Result | Reference range | Hb, g/dL | 13.80 | 13.5–17.5 |
| Hb A1c, % | 6.2 | Non diabetic: 4–6Diabetic: >6.5 | LDH | 400 | Up to 300 | Platelet, 10^3/μL | 92.6 | 80–100 |
Abbreviation: FBS: Fasting Blood Sugar; AST: Aspartate Amino Transferase; ALT: Alanine Transferase; TIBC: Total Iron-Binding Capacity; Hb A1c: Glycosylated Hemoglobin; CRP: C-Reactive Protein; AFP: Alpha-Fetoprotein; CEA: Embryonic Carcinoma Antigen; LDH: Lactate Dehydrogenase; WBC: White Blood Cell; RBC: Red Blood Cell; Hb: Hemoglobin.
According to the findings of Zou et al., about the role of ACE-2 as the main host cell receptor for entrance of 2019-nCoV to cell and with notice to the high expression of ACE-2 receptor in the oral cavity, as well as in colon, intestine and gallbladder and enriching in epithelial cells, we supposed that this receptor may mediate some GI symptoms of infected patients.5 Previously, epidemiologic finding of Lai et al., demonstrated that among adult patients, some common respiratory symptoms were followed by diarrhea.3 Previously, we reported that infected patients with COVID-19 can present some unusual GI symptoms.4 These symptoms were resistant to medications and in some cases without any medications, they subsided.
At the age of COVID-19 crisis, in some patients, GI physicians may face rare GI symptoms such as dysentery, hepatic involvement or other ones in patients. Therefore, it is necessary that all GI physicians should be aware of the possible occurrence of these symptoms as an important prognosis of COVID pneumonia. Although, in this letter we only documented one case of this issue, at the age of COVID-19, all rare GI symptoms should be exactly addressed in new referred patients to GI clinic. Recording and investigation of these symptoms may open a new window to help finding valuable information on the characterization of this mysterious disease.
DeclarationsEthics approvalApproval was obtained from the ethics committee of Qom University of Medical Sciences. The procedures used in this study adhere to the tenets of the declaration of Helsinki (Nu: IR.MUQ.REC.1399.044).
Consent to publishPatients signed informed consent regarding publishing their data and photographs.
Availability of supporting dataAll data and materials are available.
Authors’ contributionAhmad Hormati, Writing of the report and therapeutic physician. Mohammad Reza Ghadir, Therapeutic physician. Reza Aminnejad & Fatemeh Khodadust, Review and edit of the manuscript. Mahboubeh Afifian, Collecting of the data. Sajjad Ahmadpour, Writing of the report and correspondence.
FundingNo funding was provided for this manuscript.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to thank Sanam Ahmadpour for her contribution to the edit of the present manuscript.