Capsule endoscopy (CE) has the highest sensitivity in the evaluation of small-bowel mucosa in Crohn's disease (CD). Recent guidelines recommend the use of validated CE scores to assess small-bowel inflammatory activity in CD. Lewis score (LS) and Capsule Endoscopy Crohn's Disease Activity Index (CECDAI) are the currently available validated scores, but comparative studies are scarce. Moreover, correlation of these endoscopic scores with biomarkers and clinical activity is lacking. This study aims to compare LS with CECDAI, to determine cutoff values for CECDAI similar to those of LS (135–790), and to correlate LS and CECDAI with biomarkers and symptoms.
StudyAll patients with CD who underwent CE between March/2010 and February/2016 were included. LS and CECDAI were determined after analysis of each CE. In patients with small-bowel CD, C-reactive protein (CRP) and Harvey–Bradshaw index (HBI) were evaluated. Statistical analysis: descriptive statistics, Spearman's correlation coefficient and linear regression analysis. Significance: p<0.05.
ResultsFifty-three patients were included and the mean values obtained for LS were 1147±1453, CECDAI 11.3±6.9, CRP 0.92±1.5mg/dL and HBI 2.4±2.8. There was a very strong correlation between LS and CECDAI (rs=0.878; p<0.0001) and thresholds values of 135–790 in LS corresponded to 7.7–10.3 cutoff values in CECDAI, respectively. Neither CRP correlated with LS (rs=0.068; p=0.72) or CECDAI (rs=−0.004; p=0.98), nor HBI with LS (rs=−0.15; p=0.40) or CECDAI (rs=−0.10; p=0.23).
ConclusionCorrelation between the two CE activity scores was very strong, with LS thresholds of 135–790 corresponding to CECDAI values of 7.7–10.3. HBI and CRP had no correlation with CECDAI and LS.
la cápsula endoscópica (CE) posee la mayor sensibilidad cuando se trata de la evaluación de la mucosa del intestino delgado en la enfermedad de Crohn (EC). Las últimas líneas de orientación recomiendan el uso de puntuaciones homologadas de la CE para evaluar la actividad inflamatoria del intestino delgado en la EC. La puntuación de Lewis (LS) y el índice de actividad de la cápsula endoscópica para la enfermedad de Crohn (CECDAI) son las puntuaciones homologadas actualmente disponibles, pero los estudios comparativos son escasos. Además, falta la correlación de estas puntuaciones endoscópicas con los biomarcadores y la actividad clínica. El objetivo de este estudio es comparar la LS con el CECDAI, determinar valores de corte para el CECDAI similares a los de la LS (135-790) y correlacionar LS y CECDAI con biomarcadores y síntomas.
Estudiose incluyó a todos los pacientes con EC en que se había realizado CE entre marzo de 2010 y febrero de 2016. Se establecieron LS y CECDAI después del análisis de cada CE. En pacientes con EC de intestino delgado, se evaluaron la proteína C-reactiva (CRP) y el índice de Harvey–Bradshaw (HBI). Análisis estadístico: estadística descriptiva, coeficiente de correlación de Spearman y análisis de regresión lineal. Significado: p < 0,05.
ResultadosSe incluyó a 53 pacientes y los valores medios obtenidos de la LS fueron 1147 ± 1453; del CECDAI, 11,3 ± 6,9; de la CRP, 0,92 ± 1,5 mg/dl, y del HBI, 2,4 ± 2,8. Hubo una correlación muy fuerte entre LS y CECDAI (rs=0,878; p <0,0001) y los valores umbrales de 135-790 en la LS correspondieron a valores de corte de 7,7 a 10,3 en el CECDAI. Ni la CRP correlacionó con la LS (rs=0,068; p=0,72) o con el CECDAI (rs=−0,004; p=0,98), ni el HBI con la LS (rs=−0,15; p=0,40) o con el CECDAI (rs=−0,10; p=0,23)
Conclusiónla correlación entre las dos puntuaciones de actividad de la CE fue muy fuerte, con umbrales de la LS de 135-790 correspondientes a valores del CECDAI de 7,7-10,3. El HBI y la CRP no tenían correlación con el CECDAI y la LS.
Since its introduction into clinical practice in 2001, capsule endoscopy (CE) has achieved an important role in the study of numerous small-bowel disorders, including obscure gastrointestinal bleeding, Crohn's disease (CD), small-bowel tumors, polyposis syndromes and celiac disease.1–4
CD is a chronic inflammatory disorder which may involve any segment of the gastrointestinal tract.5,6 The small-bowel is affected in up to 80% of patients with CD, and is the only segment involved by the disease in up to one-third of the cases.5–9 Since a single gold standard for the diagnosis of CD is not currently available, the diagnosis is confirmed by clinical evaluation and a combination of endoscopic, histological, radiological, and/or biochemical exams.10
Small-bowel CE should be reserved for patients with suspected CD despite negative evaluations with ileocolonoscopy and imaging exams.1,10 In patients with known CD, CE should be reserved to assess the extent and location of the disease in cases with unremarkable or nondiagnostic findings from cross sectional imaging of the small-bowel, if deemed to influence patient management.1 CE represents the non-invasive method with the highest sensitivity in the evaluation of small-bowel mucosa in CD, with a miss rate for ulcers of only 1%, and a negative predictive value ranging from 96% to 100%.1,7,11
Recent recommendations advocate the employment of validated CE scores for the assessment of small-bowel inflammatory activity in CD, allowing a standardized description of lesions and an objective assessment of severity and follow-up.1,7 Lewis score (LS) and Capsule Endoscopy Crohn's Disease Activity Index (CECDAI) are the two validated scores currently available, but comparative studies are scarce.11,12 Moreover, evidence concerning the correlation of these endoscopic scores with biomarkers and clinical activity in small-bowel CD is lacking.
The primary aim of this study was to compare LS with CECDAI and to determine cutoff values for CECDAI similar to those of LS (135 and 790). The second aim was to correlate LS and CECDAI with biomarkers and symptoms.
Materials and methodsPatientsAll patients with CD who underwent CE in our department between March 2010 and February 2016 were included. Patients with obstructive symptoms or a suspected stenosis in a cross-sectional imaging exam, and patients taking nonsteroidal anti-inflammatory drugs in the 30 days preceding the CE were excluded.
Data regarding demography, smoking habits, disease duration, Montreal classification and therapy for CD by the time of CE was analyzed. In patients with CD confined to the small-bowel, biomarkers using C-reactive protein (CRP) and clinical assessment using the Harvey–Bradshaw index (HB) were determined with an interval gap of these determinations and CE performance not exceeding 30 days (and only in patients with no change on maintenance therapy).
Capsule endoscopiesCE were performed using the Mirocam® CE system (Mirocam, IntroMedic, Seoul, Korea) following the protocol of our department4 which consists of capsule ingestion with a glass of water at 8a.m. after a clear liquid diet the day before the procedure and an overnight fast without prior bowel preparation; performance of real-time views 1 and 2h after CE ingestion; resumption of normal daily activities in an outpatient basis (except for hospitalized patients) and ingestion of an oral light diet 4h after CE ingestion; removal of the recorder 12h after CE ingestion or earlier if real-time viewing confirms that the device has already reached the colon. All patients gave their informed consent for the procedure.
All CE were reviewed at a fixed rate by one author (AP) to evaluate findings, first gastric image, first duodenal image and first cecal image or last small-bowel frame in case of incomplete CE. Small-bowel transit time (SBTT) was defined as the time elapsed between the first duodenal image and the first cecal image or the last small-bowel frame in case of incomplete CE images.
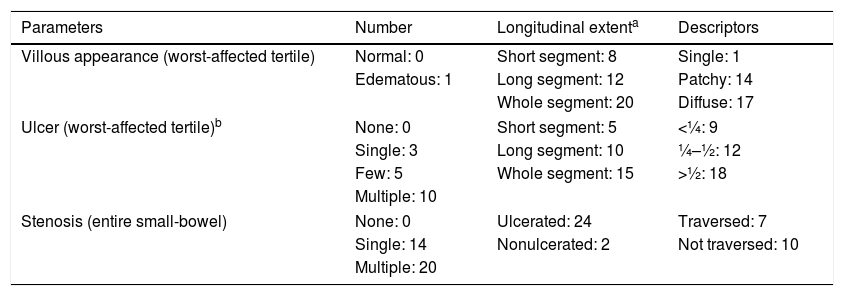
To calculate the LS (Table 1), the SBTT was first divided into three equal parts (tertiles). For each tertile, a subscore was determined based on the extension and distribution of edema, as well as the number, size and distribution of ulcers. Stenoses were evaluated considering the entire length of the small-bowel.7,11 LS was obtained by summing the maximum tertile score {[(Villous parameter×extent×descriptor)+(Ulcer parameter×extent×size)] for tertile 1 or [(Villous parameter×extent×descriptor)+(Ulcer parameter×extent×size)] for tertile 2 or [(Villous parameter×extent×descriptor)+(Ulcer parameter×extent×size)] for tertile 3}+(Stenosis number×ulcerated×traversed).11 The score ranges between 8 and 4800 points and values <135 include normal to clinically non-significant mucosal inflammatory defects, values between 135 and 790 correspond to mild disease, and values ≥790 include moderate-to-severe activity.13,14
Lewis score.
| Parameters | Number | Longitudinal extenta | Descriptors |
|---|---|---|---|
| Villous appearance (worst-affected tertile) | Normal: 0 | Short segment: 8 | Single: 1 |
| Edematous: 1 | Long segment: 12 | Patchy: 14 | |
| Whole segment: 20 | Diffuse: 17 | ||
| Ulcer (worst-affected tertile)b | None: 0 | Short segment: 5 | <¼: 9 |
| Single: 3 | Long segment: 10 | ¼–½: 12 | |
| Few: 5 | Whole segment: 15 | >½: 18 | |
| Multiple: 10 | |||
| Stenosis (entire small-bowel) | None: 0 | Ulcerated: 24 | Traversed: 7 |
| Single: 14 | Nonulcerated: 2 | Not traversed: 10 | |
| Multiple: 20 | |||
Lewis score: Score of the worst-affected tertile [(villous parameter×extent×descriptor)+(ulcer number×extent×size)]+stenosis score (number×ulcerated×traversed).
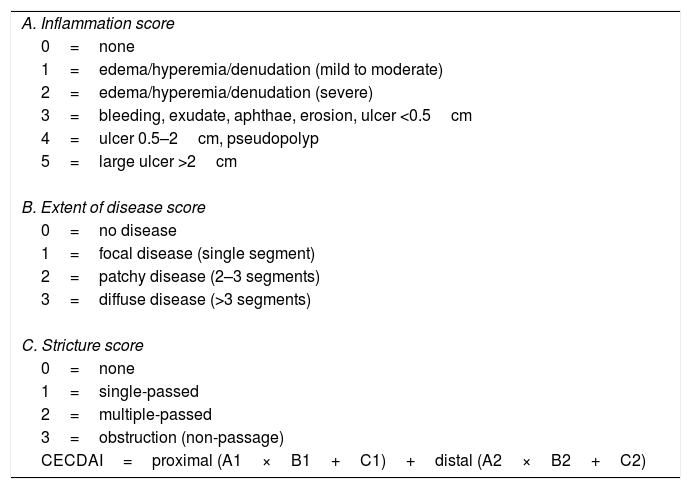
To calculate the CECDAI (Table 2), the SBTT was first divided into two equal parts and each segment was evaluated according to the degree of inflammation (A, 0–5 points), extent of disease (B, 0–3 points) and strictures (C, 0–3 points).7,12 The final score was calculated by adding the two segmental scores: proximal ([A1×B1]+C1)+distal ([A2×B2]+C2).7,12 The score ranges from 0 to 36. The authors of the CECDAI have not defined cutoff values, but higher CECDAI levels indicate increasing severity of mucosal inflammation.14
Capsule Endoscopy Crohn's Disease Activity Index.
| A. Inflammation score |
| 0=none |
| 1=edema/hyperemia/denudation (mild to moderate) |
| 2=edema/hyperemia/denudation (severe) |
| 3=bleeding, exudate, aphthae, erosion, ulcer <0.5cm |
| 4=ulcer 0.5–2cm, pseudopolyp |
| 5=large ulcer >2cm |
| B. Extent of disease score |
| 0=no disease |
| 1=focal disease (single segment) |
| 2=patchy disease (2–3 segments) |
| 3=diffuse disease (>3 segments) |
| C. Stricture score |
| 0=none |
| 1=single-passed |
| 2=multiple-passed |
| 3=obstruction (non-passage) |
| CECDAI=proximal (A1×B1+C1)+distal (A2×B2+C2) |
The Mirocam® CE system does not have an automatic way to calculate neither the Lewis score nor the CECDAI. To overcome this limitation, the authors applied the formula of each score in an Excel® sheet, allowing automatic calculation after manual introduction of each variable, as explained above.
The CE variables that were recorded included SBTT, LS and CECDAI.
Statistical analysisDescriptive statistics were used to describe variables – mean and standard deviation for continuous variables and proportions for categorical variables. Continuous variables were compared using Spearman's correlation coefficient. Spearman's correlation coefficient compares the correlation between two variables, and values can range between −1 and 1, according to the strength of the correlation. Value equal to 1, represent a perfect correlation among the two compared variables. Linear regression analysis was used to determine CECDAI cut-off values corresponding to the 130–790 cut-offs established for LS. A p-value ≤0.05 was considered to indicate statistical significance. The Statistical Package for Social Sciences version 20.0 (IBM Corp., Armonk, New York, USA) was used for data entry and data analysis.
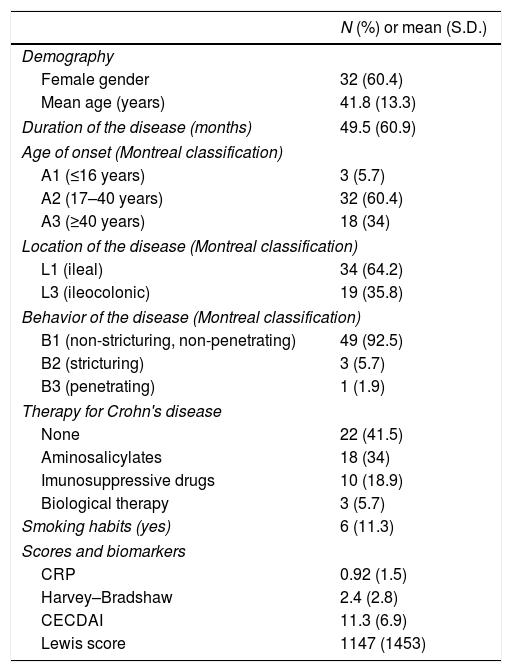
ResultsCharacteristics of patients and CE procedures are summarized in Table 3. Fifty-three patients were included, with the majority of the study population being female 60.4% (n=32), with a mean age of 41.8 years.
Characteristics of patients with Crohn's disease submitted to capsule endoscopy.
| N (%) or mean (S.D.) | |
|---|---|
| Demography | |
| Female gender | 32 (60.4) |
| Mean age (years) | 41.8 (13.3) |
| Duration of the disease (months) | 49.5 (60.9) |
| Age of onset (Montreal classification) | |
| A1 (≤16 years) | 3 (5.7) |
| A2 (17–40 years) | 32 (60.4) |
| A3 (≥40 years) | 18 (34) |
| Location of the disease (Montreal classification) | |
| L1 (ileal) | 34 (64.2) |
| L3 (ileocolonic) | 19 (35.8) |
| Behavior of the disease (Montreal classification) | |
| B1 (non-stricturing, non-penetrating) | 49 (92.5) |
| B2 (stricturing) | 3 (5.7) |
| B3 (penetrating) | 1 (1.9) |
| Therapy for Crohn's disease | |
| None | 22 (41.5) |
| Aminosalicylates | 18 (34) |
| Imunosuppressive drugs | 10 (18.9) |
| Biological therapy | 3 (5.7) |
| Smoking habits (yes) | 6 (11.3) |
| Scores and biomarkers | |
| CRP | 0.92 (1.5) |
| Harvey–Bradshaw | 2.4 (2.8) |
| CECDAI | 11.3 (6.9) |
| Lewis score | 1147 (1453) |
CD was restricted to the small-bowel in 64.2% (n=34) of patients and in the remaining 35.8% (n=19) the activity was affected small-bowel and colon. The majority of patients (n=49; 92.5%) had a non-structuring and non-penetrating CD.
Mean values obtained for CRP and for HBI were 0.92±1.5mg/dL and 2.4±2.8, respectively.
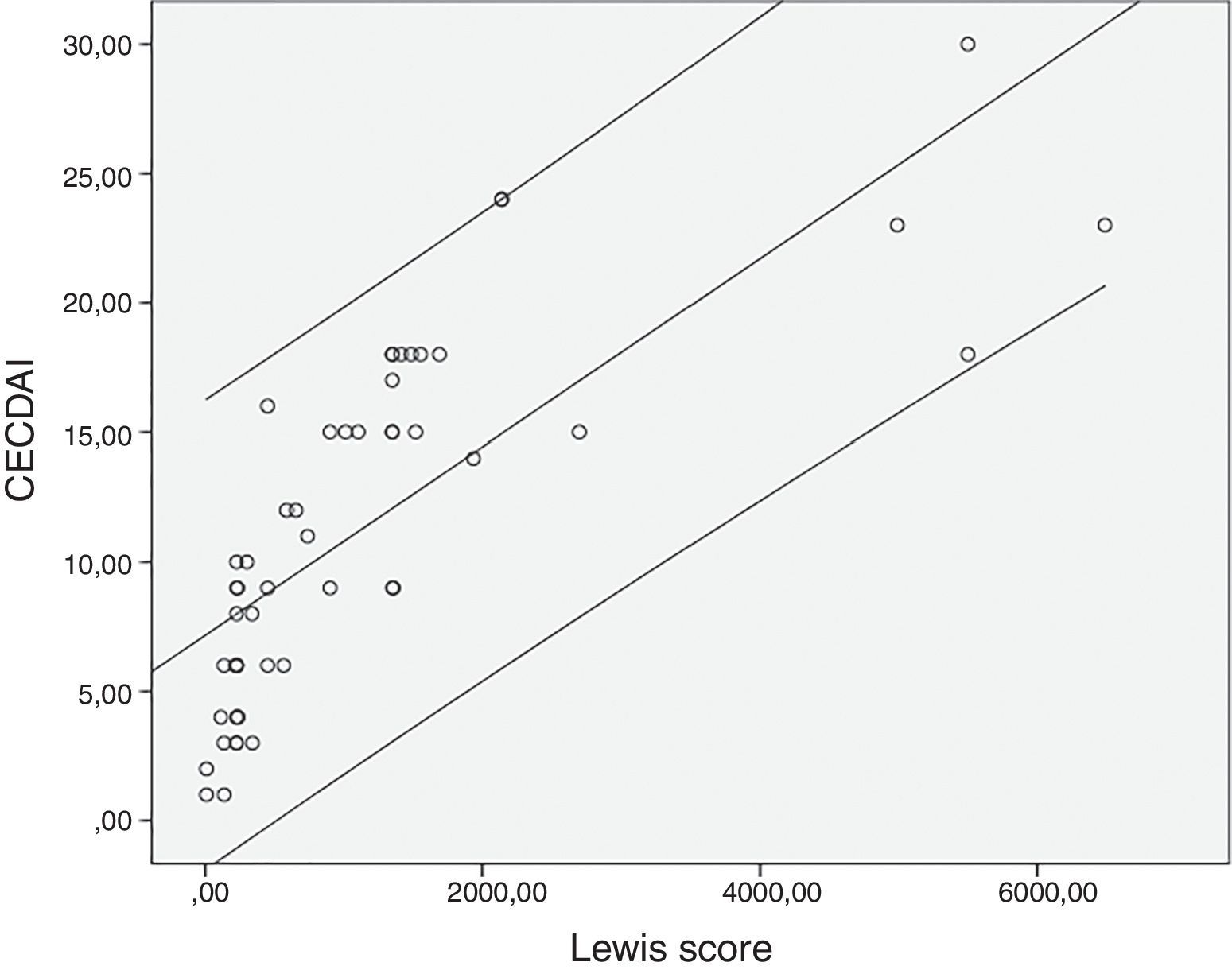
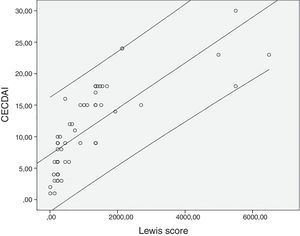
The mean value for LS was 1147±1453 and 11.3±6.9 for CECDAI. There was a very strong correlation between LS and CECDAI (rs=0.878; p<0.0001) (Fig. 1). In linear regression analysis, thresholds values of 135 and 790 in LS corresponded to 7.7 and 10.3 cutoff values in CECDAI, respectively, with an accuracy of 62.3%.
Values of CRP showed no correlation with LS (rs=0.068; p=0.72) or CECDAI (rs=−0.004; p=0.98). The same occurred with the correlation among HBI and the CE scores, as there was no correlation of HBI with LS (rs=−0.15; p=0.40) or CECDAI (rs=−0.10; p=0.23).
DiscussionCE has a very high sensitivity due to its capability for detecting mucosal inflammatory defects in the small-bowel often missed by other procedures including small-bowel follow-through, computed tomography enterography or magnetic resonance enterography.6,7,9,11,13,15–17 Moreover, CE has the advantage of detecting lesions in the proximal small-bowel segments beyond the reach of ileocolonoscopy, which remains the first-line exam in suspected CD, providing an additional information in negative ileocolonoscopies.1,9 Nevertheless, CE also has a relatively low specificity as up to 13.8% of healthy subjects have been found to have mucosal breaks and small erosions in the SB and an inability of tissue sampling, which may increase the risk of inaccurate diagnosis of CD.6,13,15,16 Moreover, until recently the lack of an accurate and reproducible scoring system to assess the severity of small-bowel CD that could be used in clinical practice and in research trials was another limitation of CE.12,16,18 Therefore, the need of developing an accurate score for estimation of the mucosal inflammatory activity in CE was crucial.6,18 Recent guidelines recommend the use of validated endoscopic scoring indices for the assessment of small-bowel inflammatory activity in patients with CD undergoing CE.1,7 These scores aim to standardize the description of lesions in CE reports thus increasing interobserver agreement and providing a reproducible method for assessment of endoscopic activity. That could be used to stratify disease severity, guide the decision in the appropriate medical management and to monitor the response to therapy and evaluate mucosal healing.1,7,9,18 Nevertheless, the results of these scoring systems are not diagnostic due to the lack of specificity of the evaluated parameters and should instead be integrated in the appropriate clinical context.1,19 LS and CECDAI are the two validated scores currently available to assess the inflammatory changes in small-bowel mucosa.6,9,11,12 In LS, the number of lesions (ulcers/mucosal breaks) is taken into consideration; mucosal edema and ulcers represent two distinct parameters; thresholds of inflammatory activity are defined to determine the severity of the disease; and an automatic calculation is available and incorporated in the RAPID READER® workstation of PillCam® CE.7,9,11,13,14 In CECDAI, mucosal edema and ulcers represent a continuum of the inflammatory changes which are evaluated in the “A” subscore; cutoff levels of inflammatory activity have not been validated yet; and no software is available for its automatic calculation, although this score is easier to calculate.7,12,14,18 Despite these differences, scarce studies compared both scores.14 In our study, there was a very strong correlation between these scores (rs=0.878; p<0.0001), and thresholds values of 135 and 790 in LS corresponded to 7.7 and 10.3 cutoff values in CECDAI, respectively. A previous study, reported a strong correlation between LS and CECDAI (rs=0.632; p<0.0001) and suggested different thresholds for inflammatory activity in CECDAI: 3.8–5.8.14 The risk of CE retention is a concern in established CD. To minimize this risk, the European Crohn's and Colitis Organization recommends the performance of cross sectional imaging or patency capsule in patients with established CD when CE is being considered to identify stenosis.20 In our department, we use the Mirocam® CE system which does not have a patency capsule (only available with the PillCam® CE system). Therefore, all the patients in our department who underwent capsule endoscopy had a previously cross sectional imaging to exclude stenoses. All patients with obstructive symptoms or stenoses detected in cross sectional imaging studies were excluded, as explained in the methods section. In our study, three patients presented capsule retention in stenoses. All cases were resolved with conservative treatment.
In our study, no correlation was established between the levels of mucosal endoscopic activity in CE and the levels of CRP. Despite the limited number of studies, previous evidence also suggests a heterogeneity of results and CRP levels correlate only modestly with endoscopic disease activity, as low CRP levels have been reported in patients with clinically-active disease and, conversely, not all patients with elevated CRP have active disease.5,8,14,17,19 Currently, CRP is an adjunctive measure of inflammation for monitoring in CD rather than being a target of treatment in CD.19
The HBI is a clinical score in CD which is a simplified version of the CD Activity Index.5,16 In our study, LS and CECDAI had no correlation with the HBI, which is similar to previous studies which demonstrated that clinical disease activity scores were not reliable predictors of mucosal inflammation in small-bowel CD.5,6,11,17 On one hand, clinical symptoms are subjective and may reflect other concomitant disorders, namely irritable bowel syndrome.6,14,16,18 On the other hand, symptoms may depend more upon transmural inflammation and fibrosis rather than mucosal inflammation which is assessed by CE.5,14 Conversely, not all mucosal disease is associated with symptoms.6
Mucosal healing is a predictor of better prognosis in CD and is a therapeutic target in clinical practice.1,6,17,19 Therefore, an objective assessment of bowel inflammation is necessary and the current gold standard for monitoring mucosal healing is ileocolonoscopy.17,19 Nevertheless, it is an invasive procedure and only allows assessment of the terminal ileum.17 This highlights the need of a procedure which measures mucosal involvement in small-bowel CD and recent clinical trials are evaluating the potential role of CE in this purpose.1,6,21 Our study revealed that small-bowel endoscopic assessment provided the most reliable information regarding the degree of mucosal healing/inflammation, as there was no correlation among the endoscopic scores, clinical scores and biomarkers. This aspect emphasizes the importance of CECDAI and LS in the measurement of disease activity.
The main limitations of this study are the retrospective nature and the small sample size.
In conclusion, correlation between the two CE activity scores was very strong, with LS thresholds of 135 and 790 corresponding to CECDAI values of 7.7 and 10.3. HBI and CRP had no correlation with endoscopic activity scores in small-bowel CD.
Author contributionsAna Ponte: Conception and design of the study, analysis and interpretation of data and drafting the article.
Rolando Pinho: Conception and design of the study, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content.
Adélia Rodrigues: Acquisition of data.
Joana Silva: Analysis and interpretation of data.
Jaime Rodrigues: Analysis and interpretation of data.
Mafalda Sousa: Analysis and interpretation of data.
João Carvalho: Final approval of the version to be submitted.
FundingThe authors declare no source of funding for this article.
Conflicts of interestThe authors declare no conflict of interest for this article.