Two subpopulations of mast cells (MC) are found in the gut: the mucosal reactive MC (T lymphocytes dependent) and the submucosal constitutive MC (unrelated to the immune system).1 The former act in the regulation of immune host defence (food allergy, infections, inflammatory bowel disease (IBD)), tissue homeostasis (epithelial secretion and permeability), neuronal functions (peristalsis and pain) and brain–gut interaction.2,3 Submucosal MC are related to blood vessel growth and tissue remodeling.1
Activated mucosal MC release inflammatory mediators (histamine, serotonin, proteases and cytokines) direct to the enteric nervous system (ENS) and its effector motor response, causing diarrhea and abdominal pain.1,2
In healthy subjects, intestinal MC are less than 20 per high-power field (HPF).1 Higher values have been reported for IBD,3 irritable bowel syndrome (IBS),4 food allergy,5 idiopathic constipation6 and colonic diverticular disease.7
We describe two case reports of mastocytic enterocolitis, a recently defined condition, characterized by dense MC infiltrate within the mucosa of the gastrointestinal tract.1
A 21-year-old female presented to our Unit with a six months history of non-bloody diarrhea, abdominal pain and weight loss; she denied recent use of drugs. A previous colonoscopy revealed hyperaemia of the descending colon, with histological examination showing moderate lymphoplasmacellular infiltration of the lamina propria with an increased number of eosinophils (over 60×10 HPF). Mesalazine 800mg tid was administered for 2 months, without benefit.
Laboratory tests showed normal levels of blood count, acute phase reactants, anti-tissue transglutaminase antibodies (AbtTG) and serum tryptase; fecal calprotectine, stool culture and parasitologic examination also proved negative.
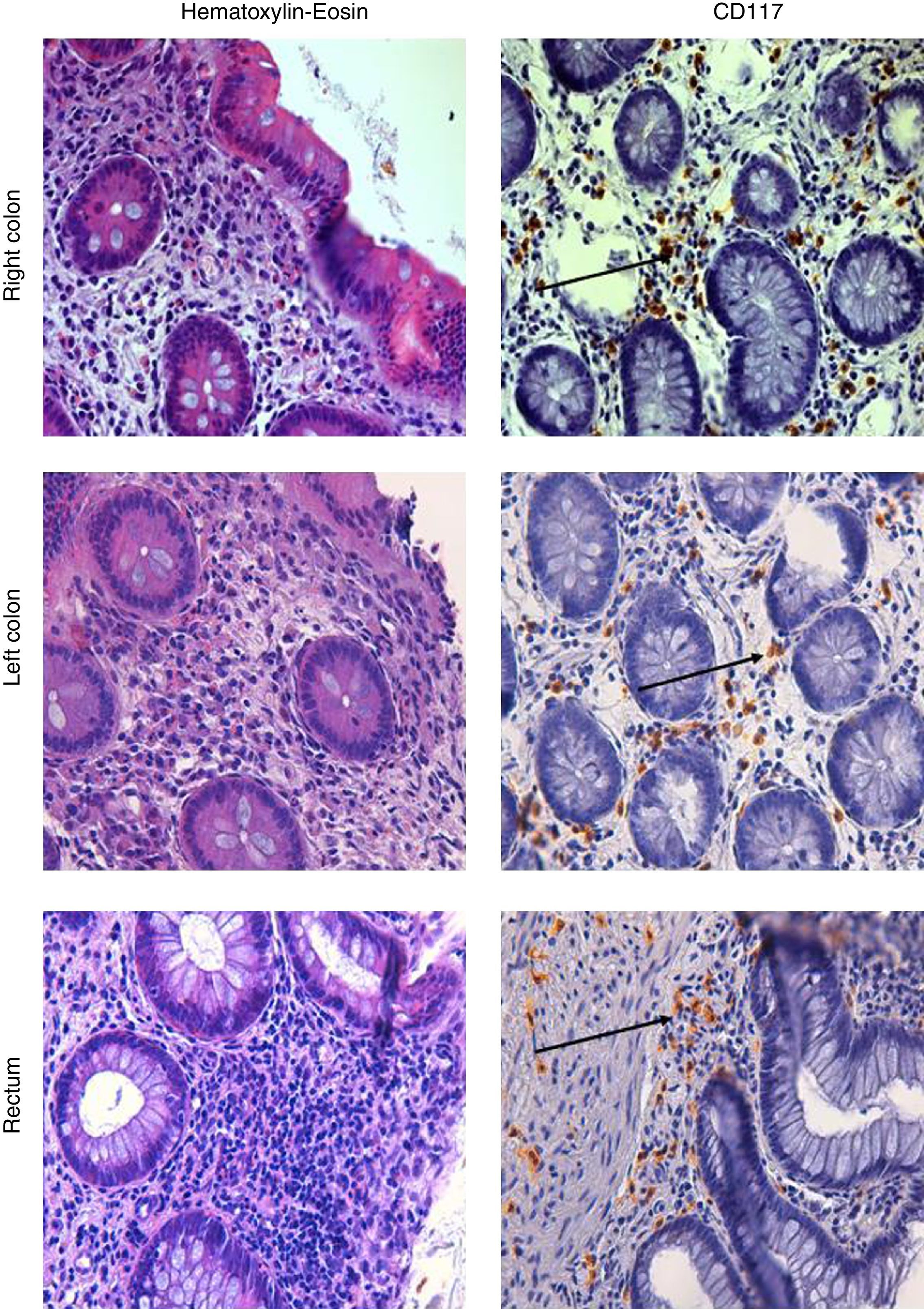
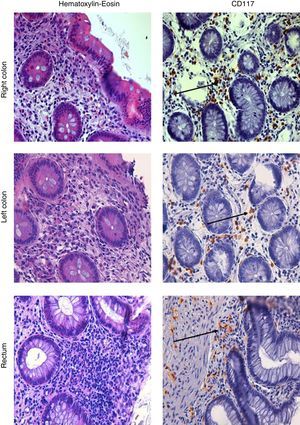
A further ileocolonoscopy showed no lesions. Histology of the colorectal biopsies, however, revealed MC colitis, with diffuse positivity for CD117 (18, 33 and 34 MCs per HPF, in the right colon, the left colon and the rectum, respectively) and an increased number of eosinophils (Fig. 1).
Upper endoscopy was negative for macroscopic lesions; histological examination, however, revealed MC infiltration in the duodenum, antrum and fundus of the stomach, and distal esophagus (43, 24, 25 and 43 MC per HPF, respectively). Systemic mastocytosis was ruled out by normal histological findings for the bone marrow biopsy, as was any abdominal or pelvic alteration by a CT scan. Dermatological examination found no evidence of cutaneous lesions.
The patient was first treated with histamine H1 and H2 receptor antagonist (Ranitidine 150mg bid and Cetirizine 250mg bid) and a granule membrane stabilizing agent (chromoglycate sodium 200mg qid). This resulted in some benefit. Treatment with metilprednisolone 16mg bid was then started and led to a prompt resolution of the symptoms. One year later she suffered a relapse and was treated with a cycle of beclomethasone dipropionate 10mg/day for 6 weeks which resolved the symptoms.
A 57 year-old woman presented to our Unit for episodic bloody stools for 2 months. She had undergone a kidney transplant operation 22 years before as treatment of polycystic kidney disease and she suffered from hypertension. She had been taking cyclosporine 100mg/day, prednisone 5mg/day, beta-blocker, calcium-channel blockers, furosemide, aspirin and omeprazole. A previous colonoscopy revealed hyperaemia of the right colon and diverticula of the left colon, with histological examination showing mild chronic inflammation with lymphoplasma cells, eosinophils and superficial epithelial erosions.
She was treated with mesalamine 1600mg bid and rifaximin 400mg bid. Results of screenings for bacterial, viral and parasitological infections were negative; serum levels of acute-phase reactants, AbtTG and fecal calprotectine were normal. Histological examination following an upper endoscopy revealed a mild chronic inflammation without H. pylori infection or MC infiltration.
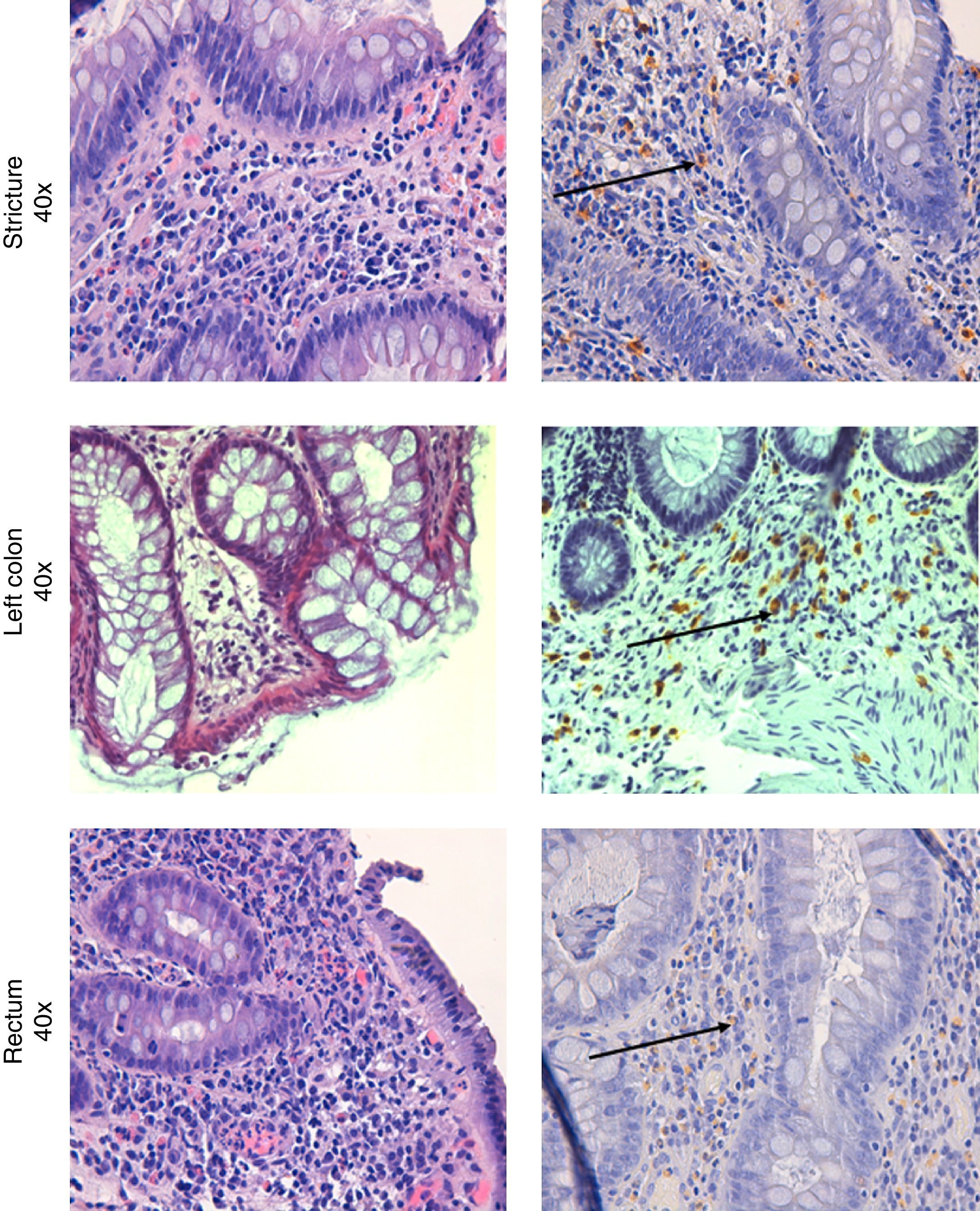
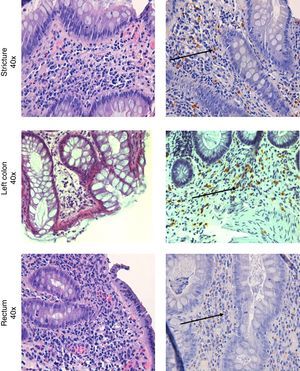
Colonoscopy was repeated, confirming the presence of the diverticula, without any other macroscopic lesions. Histological examination of the colorectal biopsies revealed edema and inflammatory infiltration of the lamina propria, an increased number of eosinophils and MC infiltration of the muscolaris mucosa and the submucosa in both the left and right colon (35 and 20 MC per HPF, respectively) (Fig. 2). Histological examination of a bone marrow biopsy ruled out systemic mastocytosis. An abdominal and pelvis CT scan, carried out two months before, detected the presence of diverticula in the left colon with mild thickening of the sigmoid wall, hypoplasia of both kidneys, and an ectopic transplanted normal kidney in the right iliac fossa; no other lesions were found.
Therefore mast cell colitis was hypothesized as a result of these tests and Beclomethasone dipropionate 5mg bid was administered, leading to a complete resolution of the symptoms. At 6 month follow-up, she reported no further complaints.
Mastocytic enterocolitis was first described in a group of patients with chronic intractable diarrhea, comparing duodenal and colonic MC concentrations of these patients with those of other patients suffering from known intestinal disorders causing chronic diarrhea (celiac disease and IBS), and also with those of a healthy control group. Histological examination of duodenal and colonic biopsies revealed the following mean concentrations of MC per HPF for the three groups: 25.7±4.5 (study group); 12.4±2.3 (group with known diarrheal disorders); 13.3±3.5 (healthy control group). 70% of patients with chronic intractable diarrhea were found to have significantly increased MC concentrations in their duodena and colons,1 a value above 20 per HPF may be regarded as signifying a pathological increase.1,4 As in the study of Sethi et al., the mean value of MC found in the women with chronic diarrhea of unknown etiology was 30 per HPF, in our cases we considered this cut-off for the diagnosis of MC colitis.8
The infiltration of mucosal MC in mastocytic enterocolitis is thought to be a reactive gut-specific phenomenon from unknown stimuli, not associated with systemic or cutaneous mastocytosis.1 Drugs could be a potential cause of MC colitis. One of the 2 patients described above was taking Cyclosporine, which is a well known cause of eosinophilic colitis,9 whereas it is less clear if it also may affect MC intestinal infiltration.
Unlike systemic mastocytosis, defined by specific criteria, mastocytic enterocolitis has no consistent clinical determinants; it should be considered in patients with intractable diarrhea having no abnormality other than an increased concentration of intestinal MC.
It should be pointed out that at routine hematoxylin and eosin staining, MC number may appear normal, while it can result increased by immunohistochemical analysis for MC tryptase.5,10 This, however, can lead to underestimation of the number of degranulated MCs, since released secretory granule tryptase does not stain.
A stain for c-kit (CD117), the surface MC receptor for stem cell factor or qPCR measurement of histidine decarboxylase or tryptase gene expression, can reduce the underestimation of degranulated MC.11
It has been shown that mast cell concentrations should be best evaluated from biopsies taken from the left side of the colon.8 Nevertheless, besides the number, the degree of MC activation is the most relevant finding leading to gastrointestinal symptoms.
In 67% of cases, symptoms improve with drugs affecting MC mediator function and release, such as histamine H1 and H2 receptor antagonists (cetirizine, ranitidine) and granule membrane stabilizing agents (cromolyn sodium).1 Glucocorticoids decrease tissue MC concentration by downregulating the tissue stem cell factor produced by fibroblasts and required for the differentiation, migration, maturation, and survival of local MCs.12
Mastocytic enterocolitis should be considered in patients with intractable diarrhea and unremarkable histological findings, often classified as diarrhea-predominant IBS, but which could benefit from specific treatments.
All authors have contributed to the following actions: acquisition of data (AC, AV, AP, LS, AM), drafting the article (AC, GL, GF), revising the manuscript for intellectual content (GC, GB, VV), final approval of the submitted manuscript (all authors).