We have carefully read the article by Cárdenas-Jaén et al.,1 recently published in this journal, which describes an interesting study of the prognostic value of gastrointestinal symptoms and complications in patients hospitalised for COVID-19.
One of the complications addressed is abnormal liver function or liver injury, defined as hypertransaminasemia with or without alteration of other liver function parameters. In this study, the prevalence of new-onset abnormal liver function was 32.1%, attributable to COVID-19 in 85% of these patients. The authors note that abnormal liver function was associated with severe COVID-19, but not with adverse events such as intensive care unit admission, hospital stay or mortality.
The actual prognostic value of abnormal liver function in COVID-19 patients has been the subject of debate. Several studies with large sample sizes, including meta-analyses and systematic reviews, suggest that abnormal liver function acts as an independent predictor of poor prognosis.2,3
To answer this hypothesis, we conducted a single-centre retrospective study of patients hospitalised with COVID-19 respiratory involvement, excluding those with known liver disease. We initially conducted a preliminary analysis of 302 inpatients up to 15 May 2020, the results of which were published.4 We subsequently extended the inclusion period until 1 March 2021, obtaining a large sample size (2075 patients), the results of which are presented in this letter.
Our definition of abnormal liver function or liver injury was identical to Cárdenas-Jaén et al.,1 while severe COVID-19 was defined as respiratory failure (SpO2 <93% breathing room air and/or PaO2/FiO2 <300). We used multiple logistic regression and t-test (for hospital stay) adjusted for age, sex, standardised Charlson index, alcohol consumption and presence of unknown non-alcoholic fatty liver disease (using the hepatic steatosis index [HSI] and Fibrosis-4 [FIB-4] index calculated in the 12 months prior to admission). p < 0.05 was deemed to be significant.
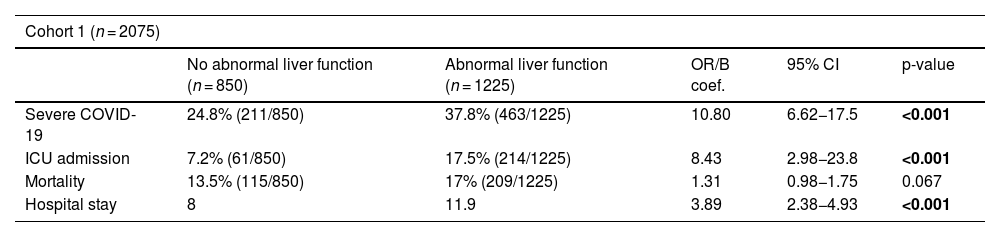
A high prevalence of abnormal liver function was recorded at baseline: 41.6% (863/2059) for AST and 27.4% (568/2075) for ALT, while 59.1% (1225/2075) exhibited at least one elevated liver biochemistry parameter. AST and ALT elevations were mild (average peak values <2 times their upper limit of normal), with results similar to those recorded by Cárdenas-Jaén et al.1 Like these authors, we also found an association between abnormal liver function and severe COVID-19. However, in contrast to them, we also observed that abnormal liver function was significantly associated with a higher likelihood of admission to the intensive care unit and hospital stay and, non-significantly, mortality (Table 1). In addition, when analysing each liver biochemistry parameter independently, we found that elevated AST significantly increased mortality (odds ratio [OR] 1.03, 95% confidence interval [CI]: 1.01–1.04, p = 0.043).
Results of the two cohorts of adult patients, without known liver disease, hospitalised for COVID-19 respiratory involvement.
| Cohort 1 (n = 2075) | |||||
|---|---|---|---|---|---|
| No abnormal liver function (n = 850) | Abnormal liver function (n = 1225) | OR/B coef. | 95% CI | p-value | |
| Severe COVID-19 | 24.8% (211/850) | 37.8% (463/1225) | 10.80 | 6.62−17.5 | <0.001 |
| ICU admission | 7.2% (61/850) | 17.5% (214/1225) | 8.43 | 2.98−23.8 | <0.001 |
| Mortality | 13.5% (115/850) | 17% (209/1225) | 1.31 | 0.98−1.75 | 0.067 |
| Hospital stay | 8 | 11.9 | 3.89 | 2.38−4.93 | <0.001 |
| Cohort 2 (n = 228) | |||||
|---|---|---|---|---|---|
| No abnormal liver function (n = 94) | Abnormal liver function (n = 134) | OR/B coef. | 95% CI | p-value | |
| Severe COVID-19 | 6.0% (8/94) | 42.6% (57/134) | 23.50 | 2.69−205.40 | 0.004 |
| ICU admission | 2.1% (2/94) | 19.4% (26/134) | 15.40 | 2.01−10.00 | 0.011 |
| Death | 4.3% (4/94) | 10.4% (14/134) | 8.20 | 0.84−79.80 | 0.070 |
| Hospital stay | 4 | 8.5 | 4.50 | 2.34−12.50 | <0.001 |
Association between abnormal liver function and severe COVID-19 adverse events (versus non-severe COVID-19), intensive care unit (ICU) admission, mortality and hospital stay (days). Cohort 1 includes patients hospitalised during the first 12 months of the pandemic.
(up to 01/03/2021) with SARS-CoV-2 vaccination rate of 0.9%. Cohort 2 is later (from 01/10/2021 to 20/01/2022) and only includes patients vaccinated for SARS-CoV-2. Multiple logistic regression and t-test results adjusted for age, sex, Charlson index, alcohol consumption and non-alcoholic fatty liver disease.
Statistically significant results (p < 0.05) are highlighted in bold.
n: number of patients; OR: odds ratio; CI: confidence interval; ICU: intensive care unit.
Furthermore, to minimise the impact of confounding factors, we repeated the statistical analyses excluding patients with pre-admission AST/ALT elevation (2.4%, 49/2075) or with suspected pharmacological hepatotoxicity during hospitalisation (5.5%, 114/2064), without this affecting the results described above. Finally, we assessed the possible effect of SARS-CoV-2 vaccination on these associations by repeating the study in a later cohort (from 1 October 2021 to 20 January 2022) of 228 vaccinated patients, obtaining similar results to those of the previous cohort (Table 1).
The development of abnormal liver function in COVID-19 is postulated to be due to the direct cytopathic effect by SARS-CoV-2 and, primarily, by the hyperactivity of the immune response triggered by the virus and leading to the cytotoxic storm.5 In this context, abnormal liver function is probably a manifestation of a more advanced systemic COVID-19 inflammatory stage, which would justify its negative prognostic value. As mentioned by Cárdenas-Jaén et al.1 in their study, the absence of these prognostic associations could be due to the fact that they included systemic inflammatory markers in the multivariate statistical analysis.
Nor should we forget that the inherent limitations of observational studies have not allowed sufficient scientific evidence to be gathered to make firm recommendations. However, based on our results and those published in the literature, we consider that the occurrence of abnormal liver function is relevant and could be considered to be an early unfavourable prognostic factor in patients hospitalised for COVID-19.
FundingA grant was awarded from the Fundación del Consorcio Hospital General Universitario de Valencia [Valencia General University Hospital Consortium], Spain, in the category "Award for the best SARS-CoV-2 or COVID-19 research project" in 2020.
Conflicts of interestThe authors declare that they have no conflicts of interest.