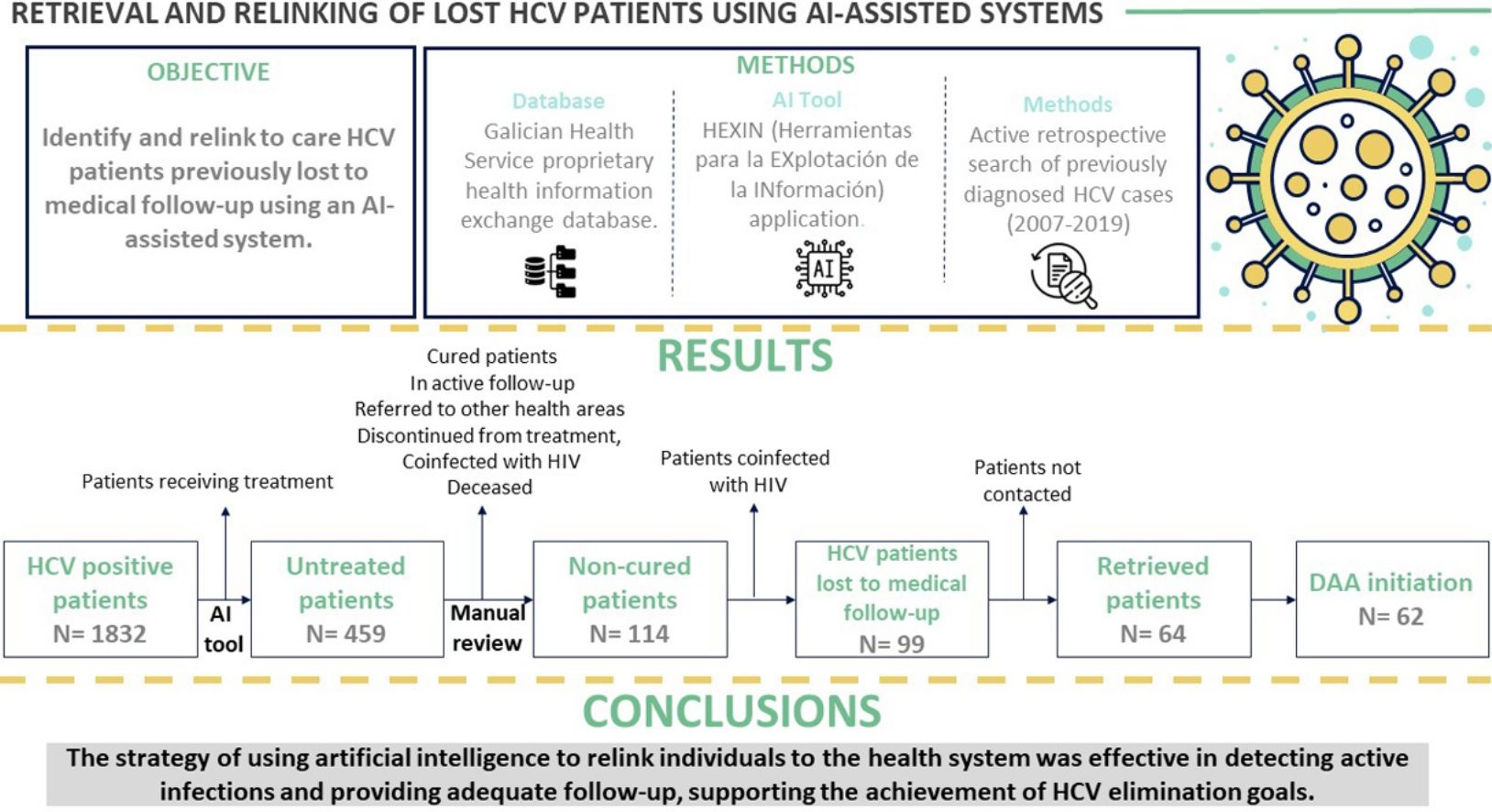
Direct-acting antivirals (DAAs) to treat hepatitis C virus (HCV) infection offer an opportunity to eliminate the disease. This study aimed to identify and relink to care HCV patients previously lost to medical follow-up in the health area of Pontevedra and O Salnés (Spain) using an artificial intelligence-assisted system.
Patients and methodsActive retrospective search of previously diagnosed HCV cases recorded in the Galician Health Service proprietary health information exchange database using the Herramientas para la EXplotación de la INformación (HEXIN) application.
Results and conclusionsOut of 99 lost patients identified, 64 (64.6%) were retrieved. Of these, 62 (96.88%) initiated DAA treatment and 54 patients (87.1%) achieved a sustained virological response. Mean time from HCV diagnosis was over 10 years. Main reasons for loss to follow-up were fear of possible adverse effects of treatment (30%) and mobility impediments (21%). Among the retrieved patients, almost one in three presented advanced liver fibrosis (F3) or cirrhosis (F4) at evaluation. In sum, HCV patients lost to follow-up can be retrieved by screening past laboratory records. This strategy promotes the achievement of HCV elimination goals.
Los antivirales de acción directa (AAD) representan una oportunidad para erradicar el virus de la hepatitis C (VHC). Este estudio se centró en identificar y revincular al sistema sanitario a pacientes con VHC que habían perdido previamente el seguimiento médico en el área sanitaria de Pontevedra y O Salnés (España), utilizando un sistema asistido por inteligencia artificial.
Pacientes y métodosSe realizó una búsqueda retrospectiva activa de casos diagnosticados de VHC en las bases de datos del Servicio Gallego de Salud, empleando la aplicación de inteligencia artificial Herramientas para la Explotación de la Información (HEXIN).
Resultados y conclusionesDe los 99 pacientes identificados como perdidos, se logró recuperar a 64 (64,6%). De estos, 62 (96,88%) comenzaron tratamiento con AAD y 54 (87,1%) alcanzaron una respuesta virológica sostenida. El tiempo promedio desde el diagnóstico de VHC fue superior a 10 años. Las principales causas de interrupción del seguimiento fueron el temor a efectos adversos del tratamiento (30%) y dificultades de desplazamiento (21%). Casi un tercio de los pacientes recuperados presentaban fibrosis hepática avanzada (F3) o cirrosis (F4) en el momento de la evaluación. En conclusión, es posible recuperar a los pacientes con VHC perdidos en el seguimiento mediante el análisis retrospectivo de registros de laboratorio, lo cual es fundamental para alcanzar los objetivos de eliminación de la enfermedad.
Despite the advances made in recent years, the hepatitis C virus (HCV) continues to be a global health problem. According to data from the World Health Organisation (WHO), an estimated 58 million patients have chronic HCV infection worldwide, representing 0.75% of the global population.1 For this reason, the WHO has recently set the target of HCV elimination by 2030,2 defined as an annual HCV incidence of ≤5 per 100,000 people not considered at risk and ≤2 per 100 persons who inject or who have ever injected drugs (PWID) and a reduction of HCV-related mortality to ≤2 per 100,000 people.2
In Spain, important advances in HCV elimination have been made through the National Strategic Plan for the Approach to Hepatitis C (PEACH).3 More than 135,000 people received HCV treatment between 2015 and 2019, placing Spain in an ideal position to achieve the WHO objectives. However, despite this progress, a 2019 seroprevalence study by the Spanish Ministry of Health indicated that 0.22% of the adult population between 20 and 80 years have active HCV infection.4 Therefore, according to these percentages, there are over 75,000 people with HCV in Spain. It is estimated that 29.4% of this population have not yet been diagnosed.4
To achieve the WHO objectives, the latest guidelines from the Spanish Ministry of Health encourage the active search for patients previously diagnosed with HCV but not treated.5 These guidelines are based on recommendations from the Spanish Association for the Study of the Liver (AEEH).6 Untreated patients with active HCV infection could be found among: (1) people with a positive antibody test that was not subsequently confirmed by RNA diagnosis of active HCV infection; (2) patients with confirmed viraemia but lost to medical follow-up; or (3) individuals who never received treatment with direct-acting antivirals (DAAs). The exact number of patients from each group is unknown today,4 but people with diagnosed viraemia would be the most accessible for early identification and treatment.
Several projects have been carried out to actively search for and engage with HCV infected patients, demonstrating the ability to recover a significant proportion of these patients and provide them with appropriate care and follow-up.7 At the European level, important regional studies have been performed in the Netherlands.8–10 In these studies, between 56% and 89% of individuals previously diagnosed with HCV were confirmed to still be actively infected at subsequent evaluations. In Spain, similar projects have also been carried out in different autonomous communities,11,12 confirming active infection in 28–63% of patients lost to follow-up. Since most patients are candidates to receive treatment, retrieval and relinkage strategies can significantly contribute to achieving the WHO objectives by way of micro-elimination campaigns at the regional level.7 In the case of Galicia, despite the higher prevalence of HCV compared to other Spanish regions (mainly due to an high number of PWID in the past),13 there are no initiatives for the identification and recovery of patients with HCV in this autonomous community to date.
The aim of this study was to identify and retrieve patients diagnosed with chronic HCV who were lost to follow-up in our health area and reincorporate them into the health system. Artificial intelligence technology facilitated a more effective and accurate identification of these patients. In addition to the possibility of offering them treatment, this type of project generates first-hand knowledge of the reasons behind loss to follow-up and the specific deficiencies in the HCV care pathway where the loss occurred.14 Some patients were never referred to a specialist doctor, while others were lost to medical follow-up at later stages.7 Pinpointing the weak points of the chain and the reasons for loss to follow-up will help optimise HCV elimination programmes.
Material and methodsStudy design, patient identification and retrievalThe study covered the population in the health area of Pontevedra and O Salnés (approximately 300,000 people) between 2007 and 2019. We conducted an active retrospective search of previously diagnosed HCV cases recorded in the Galician Health Service (SERGAS) proprietary health information exchange database (IANUS) using the HEXIN application (Herramientas para la EXplotación de la INformación). This software employs big data and artificial intelligence technologies for the analysis of clinical history data of microbiology, pharmacy and drug addiction units. The software was trained to identify patients with HCV who had been lost to follow-up, and algorithms were generated to select these patients according to the information contained in the clinical records of our health area.
Medical histories of patients identified were reviewed and analysed by the authors of the study. The following groups were excluded: (1) patients discontinued from HCV treatment after the introduction of DAAs for medical reasons, such as advanced age, comorbidities or palliative care; (2) patients treated in other health areas or in clinical trials; (3) patients in active clinical follow-up by gastroenterology or infectious diseases departments at our centre or in other health areas; (4) patients currently receiving treatment or pending confirmation of sustained virological response (SVR); and (5) patients with HIV coinfection, who were referred to the infectious diseases department of our hospital. The remaining cases were classified as patients with chronic HCV infection who had since been lost to follow-up in our health area and designated as candidates for DAA treatment by the gastroenterology department.
Patients were further categorised as having been lost to follow-up care after attending a first visit with a gastroenterologist or as having never been referred or linked to care post-diagnosis. The first group were contacted by telephone by a gastroenterologist or nurse, informing them of the situation and offering a new consultation appointment. For the second group, the respective primary care physician was notified and asked to refer the patient to the gastroenterology department.
For the retrieved patients, an initial evaluation was conducted to determine their eligibility for antiviral treatment. The decision to initiate treatment was based on current clinical practice. Follow-up care included regular monitoring to assess the efficacy of the treatment and manage any adverse effects, aiming for a sustained virological response (SVR). SVR was defined as undetectable HCV RNA determined by using Cobas Ampliprep Cobas TaqMan HCV 2.0/Cobas 6800 (Roche Diagnostics) real-time polymerase chain reaction-based assays assessed 12 weeks after treatment.
VariablesData collected included demographics (age, sex, history of drug use, background of psychiatric disorders) and clinical characteristics (referral history, years since diagnosis, previous unsuccessful treatment, and degree of liver fibrosis). The degree of liver fibrosis of retrieved patients was determined by elastography (Fibroscan®) and classified according to the METAVIR scale.15 Patients with advanced fibrosis or cirrhosis were classified according to the criteria of Ziol et al. (2005),16 and staging was completed with abdominal ultrasound. The degree of fibrosis for patients in prisons was determined with serological markers using the fibrosis index based on four factors (FIB-4) alone.17 Once in consultation, patients received care in accordance with current clinical practice, and a survey was conducted to determine the reasons for non-treatment and loss to follow-up.
Statistical analysisThe descriptive analysis was performed using means and standard deviations for quantitative variables following a normal distribution, while median and interquartile ranges were used for those not following a normal distribution. For categorical variables, proportions with confidence intervals were used.
Ethical considerationsThe study was approved by the Research Ethics Committee of Pontevedra-Vigo-Ourense on 8 October 2020 (registration code 2020/047). This committee complies, both in its composition and standard operating procedures, with current Spanish and European legislation.
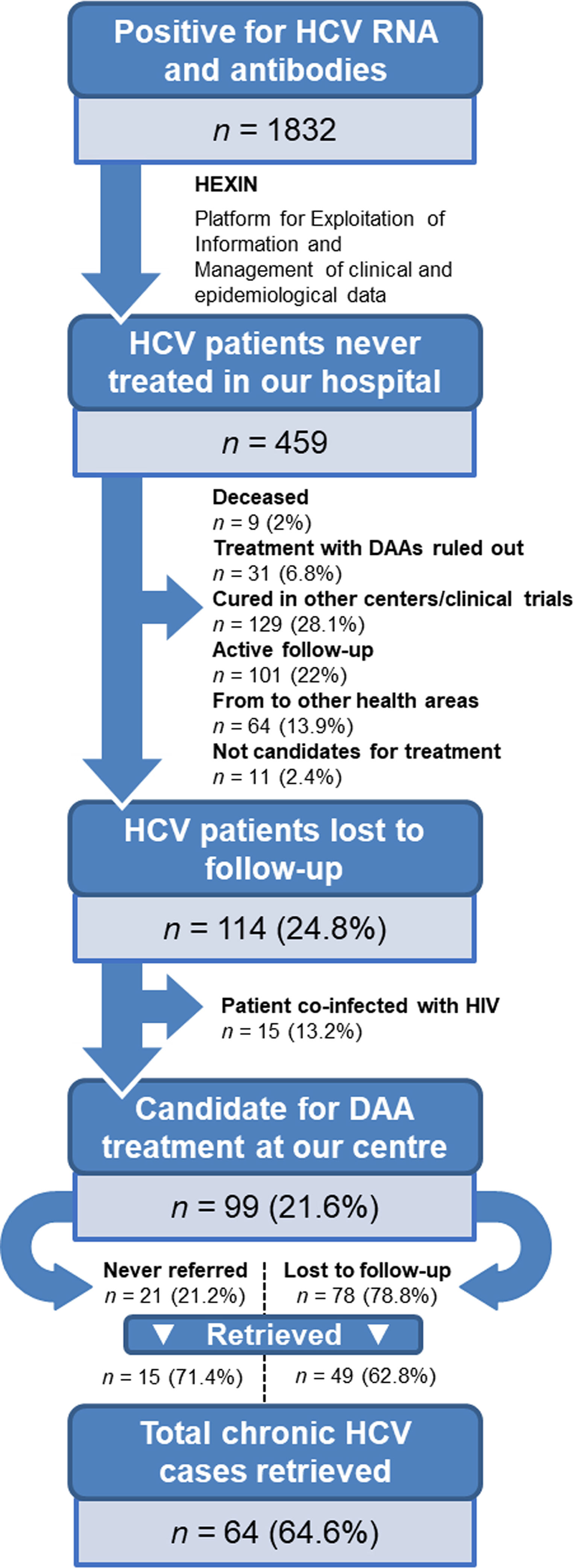
ResultsPatient identificationInitially, a total of 1832 patients with anti-HCV antibodies and HCV RNA-positive tests were identified. Using artificial intelligence software algorithms, we were able to reduce this number to 459 untreated patients (25.1%) by excluding those that were receiving treatment at our hospital. We then proceeded to manually review their clinical history to rule out patients who were cured, in active follow-up, referred to other health areas, discontinued from treatment after the introduction of DAAs in 2017, coinfected with HIV, or deceased (Fig. 1). This led to the identification of 114 non-cured patients who were lost to medical follow-up between 2007 and 2019. Of this group, 15 patients coinfected with HIV were referred to the infectious diseases’ unit. Finally, a total of 99 patients (21.6%) with chronic HCV infection were identified as lost to medical follow-up who would be candidates for DAA treatment by the gastroenterology department. We reached out to these patients but were not able contact 35 (35.4%) of them. The remaining 64 (64.6%) were retrieved for follow-up.
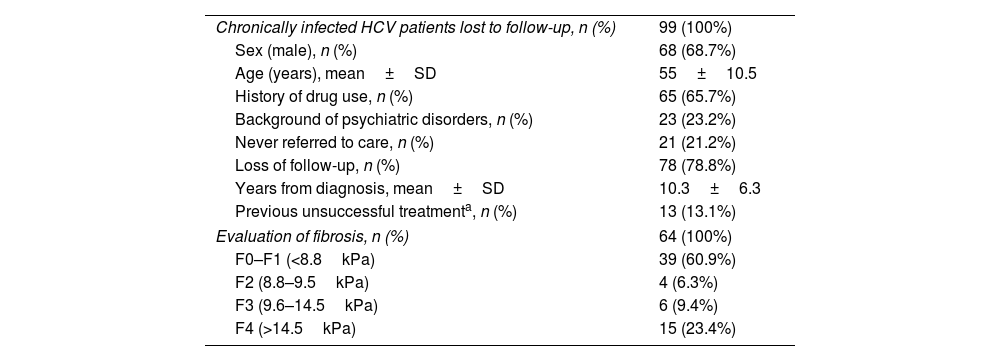
Demographic and clinical characteristicsThe demographic and clinical characteristics of the 99 patients identified as lost to medical follow-up are depicted in Table 1. The patient profile is mostly male (68.7%) with a mean age of 55±10.5 years. Around two thirds of patients (65.7%) had a history of drug use. Regarding loss to medical follow-up, 21.2% were never referred by the doctor who requested the test, and the remaining 78.8% attended the first visit but were lost to follow-up during subsequent hepatology consultation. Only 13 patients (13.1%) in this subgroup had previously tried HCV treatment without achieving a cure. The mean time from HCV diagnosis to relinkage to care was over 10 years.
Demographic characteristics of patients with chronic HCV patients lost to medical follow-up.
| Chronically infected HCV patients lost to follow-up, n (%) | 99 (100%) |
| Sex (male), n (%) | 68 (68.7%) |
| Age (years), mean±SD | 55±10.5 |
| History of drug use, n (%) | 65 (65.7%) |
| Background of psychiatric disorders, n (%) | 23 (23.2%) |
| Never referred to care, n (%) | 21 (21.2%) |
| Loss of follow-up, n (%) | 78 (78.8%) |
| Years from diagnosis, mean±SD | 10.3±6.3 |
| Previous unsuccessful treatmenta, n (%) | 13 (13.1%) |
| Evaluation of fibrosis, n (%) | 64 (100%) |
| F0–F1 (<8.8kPa) | 39 (60.9%) |
| F2 (8.8–9.5kPa) | 4 (6.3%) |
| F3 (9.6–14.5kPa) | 6 (9.4%) |
| F4 (>14.5kPa) | 15 (23.4%) |
HCV: hepatitis C virus; SD: standard deviation.
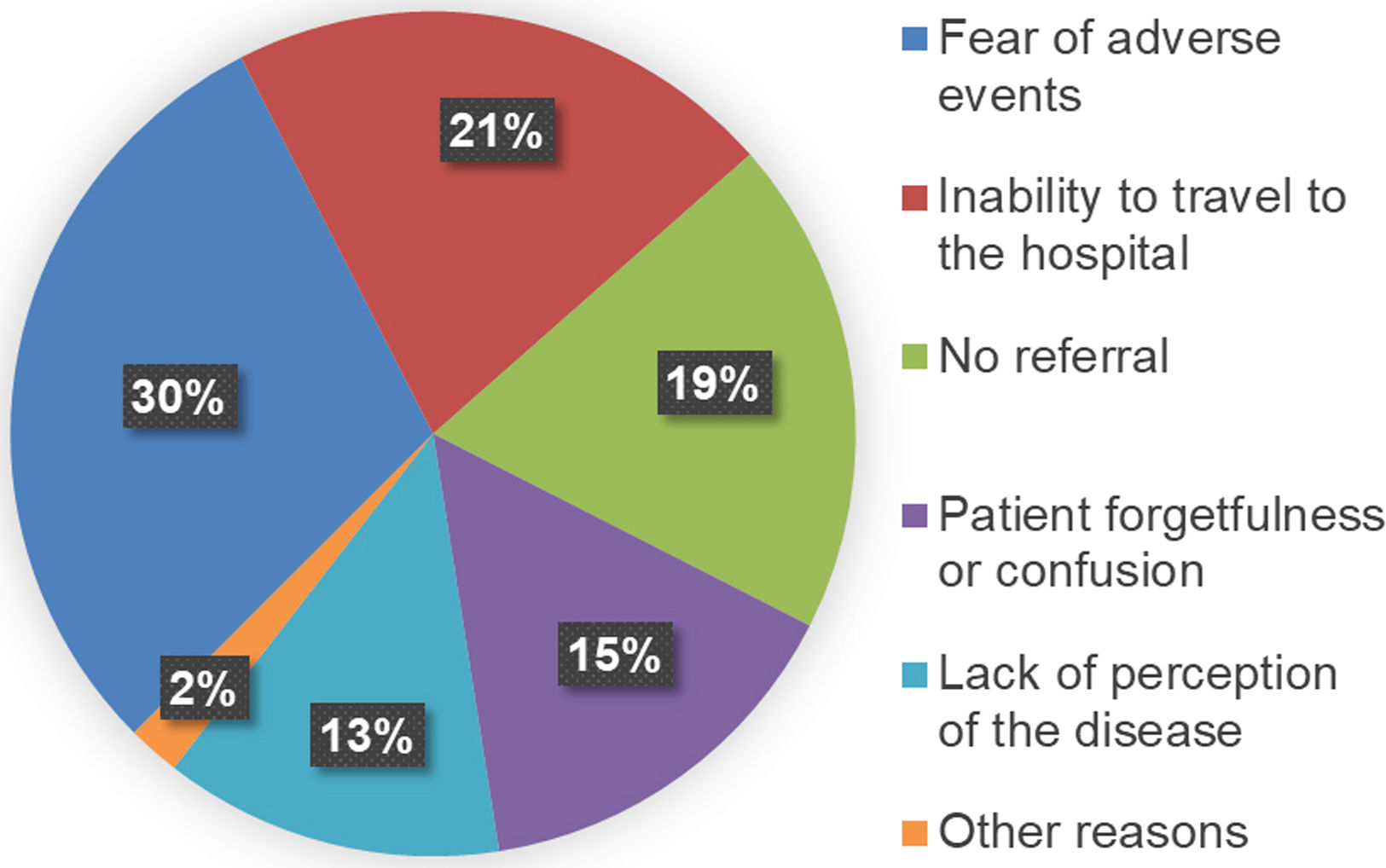
The 64 patients retrieved at our centre were surveyed to enquire why they had previously discontinued medical follow-up (Fig. 2). The first cause was fear of the possible adverse effects of DAA treatment (30%), followed by mobility impediments (21%). Some patients (13%) reported a lack of perception of the disease, while 15% attributed the failure to attend follow-up to forgetfulness and confusion about the treatment. Finally, 19% reported lack of referral. When analysing the degree of liver fibrosis of these patients (Table 1), 60.9% were at stage F0–F1, 6.3% at stage F2, 9.4% at stage F3, and 23.4% at advanced stage F4. Only 2 of the 23 patients (8.7%) with psychiatric histories were successfully recovered to offer them an antiviral treatment.
Treatment outcomesAmong the 64 patients retrieved, 62 (96.9%) began DAA treatment and 54 (87.1%) achieved a sustained virological response. Two patients (3.1%) refuse the treatment. Eight patients (14.8%) were lost to follow-up during the treatment period.
DiscussionOur active search for chronically infected HCV patients resulted in the identification of 114 non-cured patients who were lost to medical follow-up between 2007 and 2019. The strategy of using artificial intelligence to relink individuals to the health system was not only effective in detecting active infections and providing adequate follow-up, including DAA therapy, but also efficient in reducing the workload of reviewing clinical documentation by humans. Of the 99 candidates for treatment at the gastroenterology department (i.e., those with no HIV coinfection), 64 patients (64.6%) were retrieved and started treatment, most of them (87.1%) achieving SVR (by intention-to-treat analysis), reaching 100% when we take into account the 54 patients for whom we have adequate follow-up up to 12 weeks post-treatment completion. The remaining 35 patients (35.4%) were not retrieved because we could not contact them, suggesting that future efforts to recover patients lost to follow-up could benefit from the support of other areas. No differences were found between the group of retrieved patients and the group of non-retrieved patients regarding the characteristics listed in Table 1. To our knowledge, this project is the first HCV retrieval effort ever conducted in Galicia.
In our study, the use of the AI-assisted approach significantly contributed to obtain a high engagement rate (64%) which was considerably higher than the reported by other engagement activities of approximately 30%,9,10 despite some Spanish studies have reported recovery rates as high as 45%.12 These results can be explained by at least two factors. The first one is that the COVID-19 pandemic resulted in a thorough update of the contact data for all individuals served by the Galician Health Service, which undoubtedly facilitated the recovery process. Secondly, the use of an AI tool in the process of finding and retrieving chronic HCV patients lost to follow-up. By fully integrating and automating the screening process of patient records, AI eliminated possible human errors and captured all necessary information about patients. As such, healthcare personnel could spend more time and efforts on outreach and engagement with patients rather than spending much time reviewing data manually. Furthermore, the large data processing efficiency of the AI could easily identify prospective patients and ensure early and accurate intervention. Therefore, it appears that incorporating AI technology has a great potential for effectively guiding patient re-engagement and augmenting the effectiveness of interventions in chronic disease management.
When analysing the reasons for loss to follow-up, 58% were attributable to fear of possible adverse effects, lack of perception of the disease, or forgetfulness/confusion about the treatment. As described in previous studies,18 our data suggest that educational initiatives to raise awareness about HCV infection severity and treatment possibilities among the population at risk could help reduce the number of people lost to follow-up. We also observed that over 20% of the patients were never referred to a specialist doctor post-diagnosis. The lack of referral could be due to multiple factors, such as the lack of effective HCV treatments in the past. However, HCV awareness campaigns should also aim to provide more information to healthcare professionals to guarantee referral when they see a patient who is a candidate for DAA treatment.18
Early HCV diagnosis, followed by treatment with current DAA therapy, is essential to achieve SVR and prevent patient progression to more severe stages of the disease.19 These stages are frequently characterised by liver complications, such as cirrhosis and hepatocarcinoma (HCC), leading to a potential need for liver transplant and risk of death.20 In our group of retrieved patients, almost one in three presented advanced liver fibrosis (F3) or cirrhosis (F4) at evaluation. This could be most probably explained by the long period since diagnosis, which was over ten years on average. Pursuing treatment for all HCV patients is therefore crucial to avoid the poor prognosis associated with advanced stages of the disease.21 On the other hand, the high rate of treatment initiation (96.88%) and SVR achievement (100% in all patients who attended the analytical control at 12 weeks after the completion of the treatment) among the recovered patients underscores the effectiveness of using artificial intelligence to identify and re-engage lost patients and highlights the importance of offering antiviral treatment to maximise the chances of curing HCV. It is noteworthy that, despite the high rate of recovery, some patients in this category exhibit a significant rate of dropout from follow-up, even after initiating treatment (8 out of 62 patients in our case). While we lack the treatment outcomes for these 8 individuals, providing them with the necessary medication to complete the entire duration of each treatment suggests that many may have achieved SVR.12
The sanitary restrictions set up in 2020 to reduce SARS-CoV-2 transmission were a barrier to WHO HCV elimination goals, since they resulted in the suspension of many hepatitis screening and linkage-to-care programmes. According to recent calculations by Blach et al. (2021),22 the approximate one-year hiatus in HCV screening, diagnosis and treatment could translate to an increase of up to 44,800 cases of HCC and 72,300 deaths from liver disease worldwide. In Spain, a mathematical simulation was also carried out to calculate the possible impact of the COVID-19 pandemic on HCV elimination in this country.23 A cohort of 15,859 patients was compared in two different scenarios: a non-COVID-19 scenario assuming the customary diagnosis and treatment of HCV, and another COVID-19 scenario with a delay of 18 months (March 2020–June 2021). The comparison showed an increase in the number of deaths from HCC and HCV-decompensated cirrhosis of 117 and 118, respectively. The loss of HCV follow-up during the COVID-19 pandemic would reinforce the need to carry out projects to recapture lost patients.
Our study has some limitations that must be acknowledged. Our findings were restricted to one health area. Therefore, the outcomes discussed here may not be applicable to other regions or countries with different sociodemographic or epidemiological features, or with alternative health system structures. There are also some limitations intrinsic to retrospective studies using administrative databases. Some information on the study population may be missing or incorrectly recorded due to possible inaccurate coding or possible variations in medical practice among professionals. Lastly, we restricted our search to patients with confirmed HCV infection, as indicated by positive antibody and RNA test result records. Our methodology therefore did not account for an unidentified number of patients with positive antibody test results who were tested before the implementation of reflex or single-step diagnosis at our centre in 2017, and who have therefore not undergone confirmatory RNA testing. Further research is required to address the needs of these patients.
In conclusion, by analyzing the big data from the different digital systems that integrate the electronic medical record, we show that artificial intelligence can identify and retrieve HCV patients who were lost medical follow-up more quickly and efficiently than the manual and costly review of human resources that involves the usual searches of positive cases in isolated databases. This strategy is beneficial from the individual's perspective, since achieving SVR by providing early DAA treatment minimises the risk of developing liver cirrhosis and HCC at a later stage. It is noteworthy that the recruitment dates of our study were limited to a period (2007–2019) before the advent of the COVID-19 pandemic. Nowadays, the attention of health systems should shift back to HCV elimination programmes to identify and retrieve patients who may have been lost to medical follow-up during the COVID-19 pandemic. From an epidemiological point of view, reducing the HCV reservoir in the population helps the advance towards the goals established by the WHO to eliminate the virus by 2030.
FundingWe received funding from the Gilead Sciences FOCUS programme to support viral hepatitis screening and linkage to the first medical appointment after diagnosis. FOCUS funding does not support activities beyond the first medical appointment and is agnostic as to how organisations approach subsequent patient clinical management. We received an economic award from the Foundation of the College of Physicians of Pontevedra.
Conflicts of interestAlba Carrodeguas and José Luis Gonzalez-Sánchez own stock in and are employees of Gilead Sciences. The remaining authors declare no conflicts of interest associated with the research, authorship, and publication of this article. Data collection and management were conducted independently, with additional oversight from independent data monitoring agencies.
Data sharing statementThe data that support the findings of this study are available on request from the corresponding author. Data are not publicly available due to privacy or ethical restrictions.
The authors thank the staff of the health area of Pontevedra and O Salnés for their contribution to this work. We also thank Anchel González Barriga and Vanessa Marfil Vives of Medical Statistics Consulting (Spain) for providing editorial support, in the form of medical writing and assembling tables based on authors’ detailed directions, collating author comments, copyediting, fact-checking, and referencing.