An 84-year-old woman presented with a 2-day history of jaundice, fever and abdominal pain. Physical examination showed scleral icterus and right upper quadrant tenderness without inspiratory arrest at palpation (absent Murphy's sign). Laboratory workup revealed leukocytosis (12.4×103μL), elevated C-reactive protein (8.3mg/dL) and cholestasis (bilirubin 5.4mg/dL, alkaline phosphatase 893U/L, gamma-glutamyl transferase 1143U/L) with elevated liver enzymes (aspartate aminotransferase 231U/L, alanine aminotransferase 178U/L). Abdominal ultrasound demonstrated a scleroatrophic gallbladder with cholelithiasis and an impacted large gallstone in the common bile duct with dilated common and intrahepatic bile ducts.
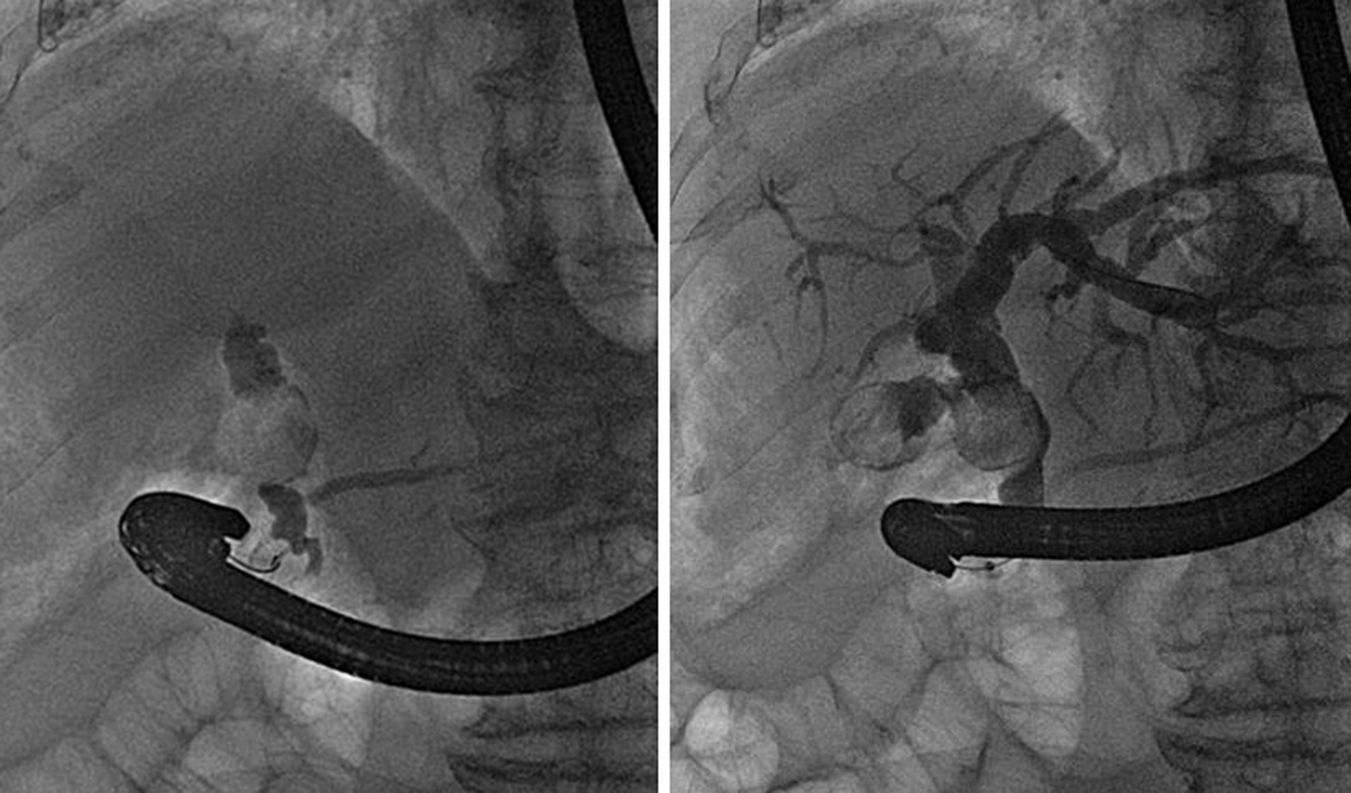
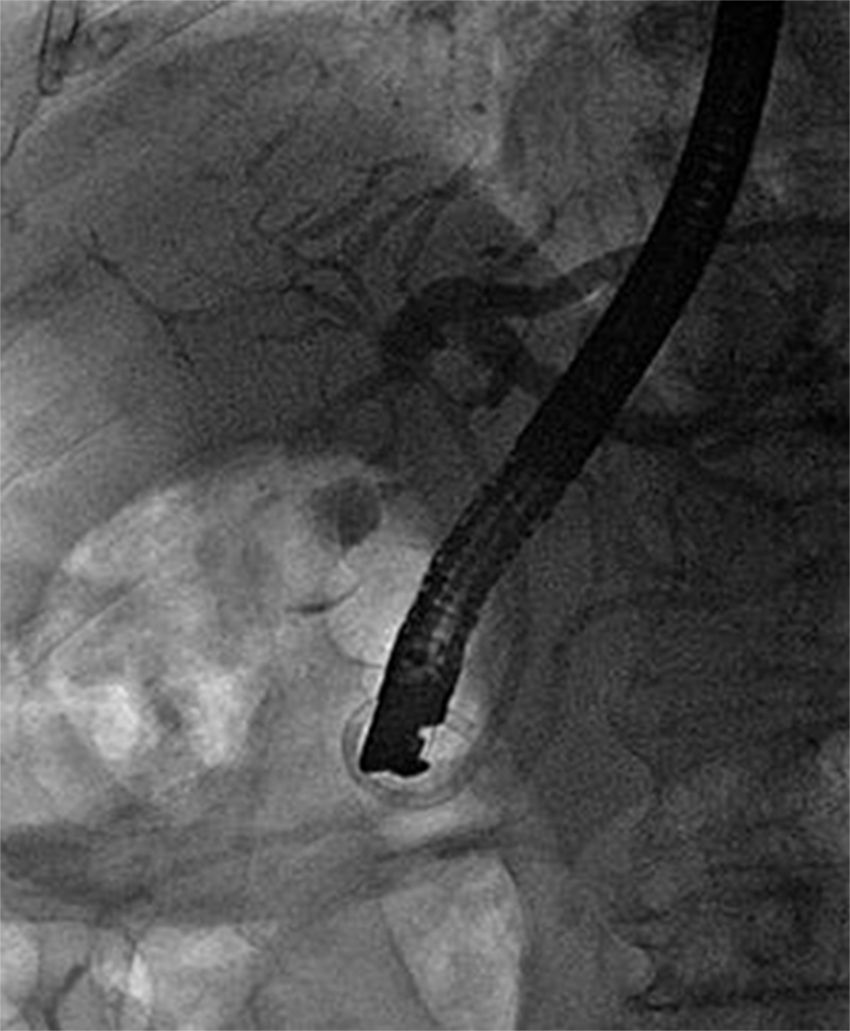
We performed an endoscopic retrograde cholangiopancreatography (ERCP) that clearly showed common hepatic duct compression by a large gallstone (20mm) impacted in the cystic duct (Fig. 1), compatible with the diagnosis of Mirizzi syndrome. Successful biliary decompression was performed by internal stenting (Fig. 2) with subsequent patient referral to surgery (cholecystectomy plus closure of the fistula).
The Mirizzi syndrome refers to common hepatic duct obstruction caused by an extrinsic compression from an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder.1 The majority of the patients present the clinical triad of jaundice, fever, and right upper quadrant pain, showing in the laboratory evaluation elevations in the serum concentrations of alkaline phosphatase and bilirubin.2
The Mirizzi syndrome is part of the differential diagnosis of obstructive jaundice and therefore the diagnostic approach usually begins with ultrasonography complemented by ERCP or magnetic resonance cholangiography.
A useful classification system takes into account the presence and extent of a cholecystobiliary fistula, due to erosion of the anterior or lateral wall of the common bile duct by impacted stones.3
Surgery is the mainstay of therapy for Mirizzi syndrome.4 ERCP treatment can be effective as a temporizing measure before surgery and can be definitive treatment for unsuitable surgical candidates.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.