Multiple studies have reported strong associations between Helicobacter pylori (Hp) inflammation and gastric cancer (GC) development. Altered expressions of pro/anti-inflammatory cytokines have a crucial role in Hp and GC proliferation. Although there are many studies related to cytokines polymorphisms involvement in GC risk, the role of Interleukin-4 (IL-4) and Interleukin-6 (IL-6) in gastric inflammation process is not yet clarified.
AimThis study aimed to investigate the impact of common IL-4 and IL-6 polymorphisms in GC development risk among Portuguese population.
MethodsA total of 100 GC biopsies (50 with intestinal type, IGC, 50 with diffuse type, DGC) and 50 chronic gastritis cases, used as control group, were included in this case-control study. IL-4 and IL-6 common polymorphisms were genotyped by PCR-SSP, using commercially available kits.
ResultsIL-4 low producer genotypes, IL-4-590TT (OR=6.7; 95% CI 1.4–32.4) and IL-4-1098GG (OR=4.4; 95% CI 1.7–16.9) were found associated with IGC and DGC, respectively. We also verified that IL-4 TTT haplotype was linked with both IGC (OR=5.8; 95% CI 2.3–14.4) and DGC (OR=2.3; 95% CI 1.0–5.5) groups. Concerning IL-6 results, IL-6-174CG genotype showed a higher prevalence among IGC cases (OR=7.3; 95% CI 2.7–20.3), and IL-6-174CC (OR=3.8; 95% CI 1.7–8.7) showed upper prevalence within DGC subjects. Finally, IL-6-174/nt565CG haplotype showed a significant association with both IGC (OR=7.3; 95% CI 2.7–20.3) and DGC (OR=7.9; 95% CI 4.2–14.9).
ConclusionIL-6 and IL-4 expression variants seem to have an important role in GC risk mechanisms. This study provides preliminary evidence that IL-4 and IL-6 polymorphisms, although not directly linked to the disease, may be useful tools in the study of this multifactorial disease.
Múltiplos estudos têm referenciado fortes associações entre infeção/inflamação por Helicobacter pylori (Hp) e o desenvolvimento do cancro gástrico (CG). A alteração na expressão das citocinas pro/anti-inflamatórias desempenha um papel crucial na proliferação da Hp e do CG. Apesar de existirem vários estudos relacionados com os polimorfismos das citocinas envolvidos na progressão do CG, o papel da Interleukin-4 (IL-4) e Interleukin-6 (IL-6) no mecanismo de inflamação gástrica ainda não está totalmente esclarecido.
ObjetivoEste estudo teve como objetivo principal estudar o impacto dos polimorfismos comuns da IL-4 e IL-6 no risco de desenvolvimento do CG na população Portuguesa.
MétodosUm total de 100 biópsias de CG (50 do tipo intestinal, CGI, 50 do tipo difuso, CGD) e 50 casos de gastrite crónica, utilizados como grupo controlo, foram incluídos neste estudo de caso-controlo. Os polimorfismos da IL-4 e da IL-6 foram genotipados por PCR-SSP, utilizando kits comerciais disponíveis.
ResultadosOs genótipos de baixa produção da IL-4, IL-4 -590TT (OR=6,7; 95% CI 1,4 a 32,4) e IL-4 -1098GG (OR=4,4; 95% CI 1,7 a 16,9) encontram-se associados com o CGI e com o CGD, respetivamente. Também verificámos que o haplótipo IL-4 TTT encontra-se relacionado com ambos os grupos de CGI (OR=5,8; 95% CI 2,3 a 14,4) e CGD (OR=2.3; 95% CI 1,0 a 5,5). Considerando os resultados da IL-6, o genótipo IL-6-174CG apresentou uma elevada prevalência entre os pacientes com CGI (OR=7,3; 95% CI 2,7 a 20,3), e o IL-6 -174CC (OR=3,8; 95% CI 1,7 a 8,7) apresentou maior prevalência no grupo de CGD. Finalmente, o haplótipo IL-6 -174/nt565CG apresentou uma associação significativa com ambos os grupos de CGI (OR=7,3; 95% CI 2,7 a 20,3) e CGD (OR=7,9; 95% CI 4,2 a 14,9).
ConclusãoOs variantes de expressão da IL-6 e IL-4 parecem desempenhar um papel importante nos mecanismos de progressão do CG. Este estudo fornece evidências preliminares de que os polimorfismos da IL-4 e da IL-6, apesar de não estarem diretamente ligados a esta patologia, podem ser ferramentas úteis no estudo desta doença multifatorial.
Gastric cancer (GC) is one of the most important public health problem, with high mortality and poor survival prognosis worldwide.1–4 Like other types of cancers, GC is multifactorial resulting from many environmental and genetic factors interplay.3,5 Among those environmental factors, Helicobacter pylori (Hp) infection is identified as one of the major causes of GC development.6,7 It is well known that Hp infection causes extensive inflammation in gastric mucosa that could result in atrophic gastritis, intestinal metaplasia, dysplasia and cancer.8,9
Several works also reported that cytokine levels are deregulated in Hp-infected gastric tissue and are associated with precancerous lesions development.5,9 Cytokine expression levels could be influenced by polymorphic variants in specific gene regions that could differ among different individuals.10–14 Such knowledge indicate that immune responsiveness to cancer, and particularly to GC, can be influenced by specific pro/anti-inflammatory cytokine genotypes.8–14
Although there are several studies related to cytokines polymorphisms involvement in Hp infection and GC progression,15,16 namely IL-4 and IL-6,17,18 its role in gastric inflammation mechanisms is not yet clarified. IL-4 and IL-6 are involved in inflammation processes been responsible for inflammatory cascade activation. Those cytokines are very important at systemic level, regulating fibroblasts and epithelial cells and other molecules secretion (such as IL1-Ra, IL-10, IL-13, IL-1β, IL-8, etc.…).17,18 Recent studies indicate that IL-6, and maybe IL-4, are also involved in E-Cadherin carcinogenesis pathway among GC diffuse type patients.19,20
IL-4 is an important immunomodulatory cytokine,21,22 which regulates balance between T helpers (Th) 1 and Th2 immune response.17,18,21,22 There are several polymorphisms described in IL-4 gene, including the -1098 (T to G), -590 (C to T) and -33 (C to T) mutations, in promoter region.16,21–25 Those mutations form several haplotypes, namely: TCC (high producer), TTT (intermediate producer), GCC and GTT (low producers).21–24 These haplotypes are associated with differences in IL-4 expression and total immunoglobulin-E levels23,24 that could affect the risk of infection, autoimmunity and cancer development.16,21
IL-6 is also a central cytokine, involved in multiple physiological and pathophysiological processes, implicated in pathogenesis and cancer development. 26–32 As IL-4, IL-6 gene has several polymorphisms in the promoter region that can regulate its expression.17,26 Among them, the substitution of C to G at position -174 and the substitution of G for A at nucleotide 565 are the most studied.26–32 These mutations form 4 different haplotypes: GG, GA (high producers haplotypes), CG and CA (low producers haplotypes).28,30,31 IL-6 haplotypes are associated with differences in the expression level of IL-6,17,30 that can influence the risk cancer, and particularly GC.26–32
Since IL-4 and IL-6 polymorphisms are responsible for different cytokine expression levels among individuals, that consequently control the immune responsiveness to Hp-infection and GC, and also because the role of this two cytokines polymorphisms in GC pathological and histological progression has not been fully solved, our study aims to clarify these issues by coming across the relationship between IL-4 and Il-6 genotypes and GC types, as well as their interaction with other cytokines.
2Methods2.1Population50 Chronic Gastritis (CG) control samples (mean age of 57.2±17.4 years; 62% men and 38% women); 50 GC patients with intestinal type (mean age of 73.3±15.7 years; 62% men and 38% women) and 50 from diffuse type (mean age of 65±16.4 years; 60% men and 40% women) biopsies formalin-fixed paraffin-embedded (FFPE) were selected. The cases were randomly collected from 2009 to 2011 subjects from the archives of Institute of Anatomic Pathology, Faculty of Medicine of the University of Coimbra.
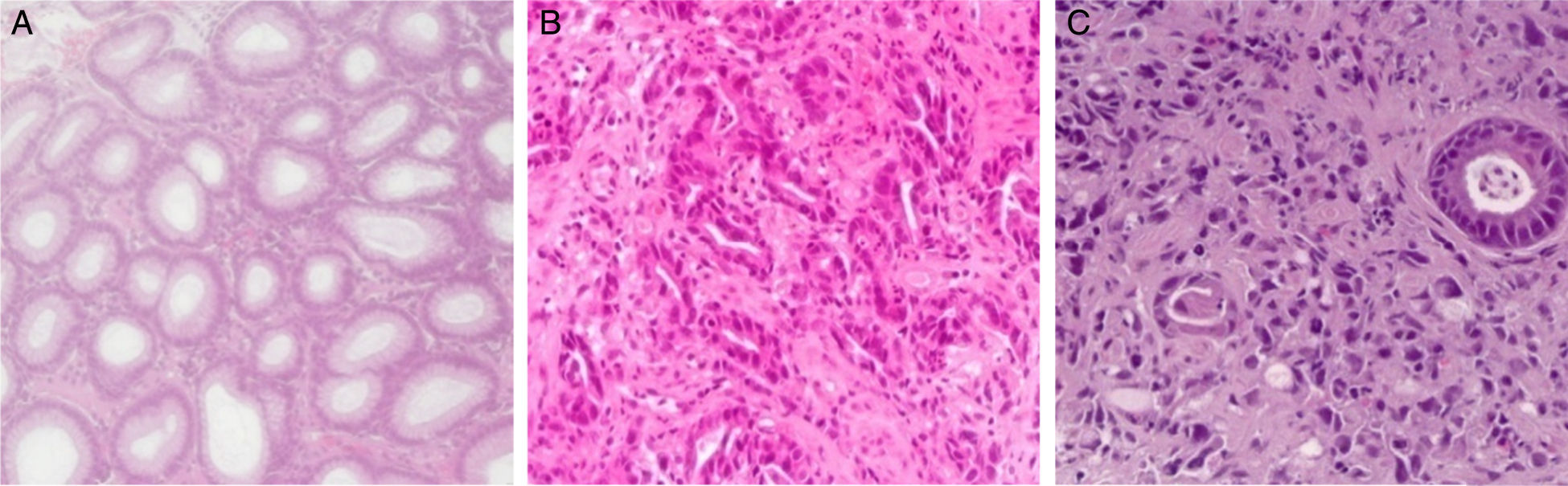
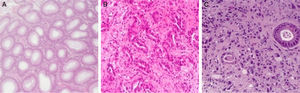
Gastritis samples were clinically identified as chronic using the Sydney updated scoring system (i.e., 0=none, 1=slight, 2=moderate, and 3=marked)33 and were selected according Hp-infection positivity, using hematoxylin-eosin staining technique (Fig. 1).34
GC samples were clinically identified as belonging to gastric antrum carcinomas and were selected according to the malignant cells availability with at least 60 malignant cells. The GC cases were classified according to Lauren's criteria as: intestinal type (IGC) and diffuse type (DGC) (Fig. 1).35
Subjects with alcohol and smoke clinical histories were excluded from the study. This study was supported and approved by local ethics committee (CIMAGO – Faculty of Medicine of the University of Coimbra, Coimbra, Portugal).
2.2DNA extractionEach biopsy was microdissected in order to separate normal from affected tissues and genomic DNA was extracted according QIAamp® DNA FFPE Tissue kit (QIAGEN, Califórnia, EUA) manufacturer protocol, using only the normal portion of the tissue. The DNA's quality and concentration values were evaluated using GeneQuant Pro (Biochrom, Cambridge, England).
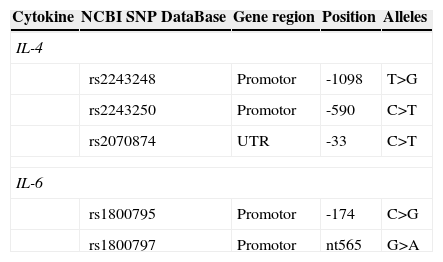
2.3Polymorphisms genotypingPolymorphisms genotyping (Table 1) were carried through commercial kits “Cytokine Box Kit” (Genebox, Cantanhede – Portugal) using PCR-SSP technique, these kits included internal, negative and positive controls form each sample. The amplification of IL-4 and IL-6 promotor mutations was performed using manufacturer protocol. The amplified PCR products were analyzed by electrophoresis with a SYBR Safe (Molecular Probes, Oregon – USA) 2% agarose gel and visualized in a UV transilluminator.
2.4Genotyping quality controlPrimers specificity evaluation was executed using DNA from IHWG Cytokine panel (from International Histocompatibility Working Group). Seven samples (14% of the total samples) from each group were re-analysed by Sanger sequencing technique, using capillary electrophoresis system (Applied Biosystem, Lifescience Technology, USA), having full genotyping correspondence.
2.5Statistical analysisIL-4 (-1098 T> G; -590 C> T; -33 C> T) and IL-6 (nt565 G> A; -174 C> G) frequencies were compared between groups (GCD versus CG; GCI versus CG) using the STATISTICA 14 (StatSoft, Inc., 2013) based on chi-square (2×2) test, χ2 and Exact Fisher test. The significance level was set at p<0.05, odds ratio (OR) and 95% confidence intervals (CI) for relative risks were also calculated for each variation.
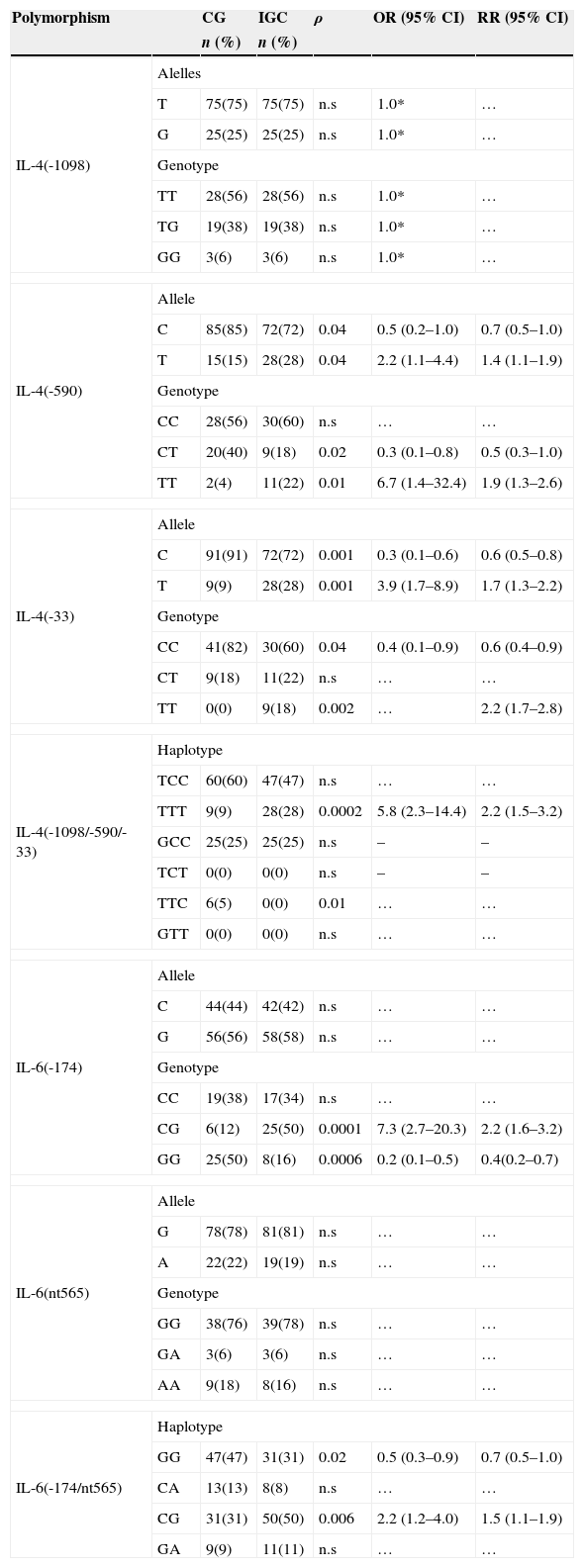
3Results3.1IL-4 -1098, -590 and -33 polymorphisms in intestinal gastric cancerIL-4 -590T (ρ=0.04; OR=2.2; 95% CI 1.1–4.4) and IL-4 -33T (ρ=0.001; OR=3.9; 95% CI 1.7–8.9) mutant alleles were more frequent among IGC subjects (Table 2). We also found a higher prevalence of IL-4 -590TT (ρ=0.01; OR=6.7; 95% CI 1.4–32.4) and IL-4 -33TT (ρ=0.002; RR=2.2; 95% CI 1.7–2.8) low producer genotypes between IGC patients (Table 2). This tendency was also verified in IL-4 TTT (ρ=0.0002; OR=5.8; 95% CI 2.3–14.4) mutated haplotype that showed high incidence in ICG group (Table 2).
IL-4 and IL-6 allele/genotype frequencies among CG and IGC groups.
| Polymorphism | CG | IGC | ρ | OR (95% CI) | RR (95% CI) | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| IL-4(-1098) | Alelles | |||||
| T | 75(75) | 75(75) | n.s | 1.0* | … | |
| G | 25(25) | 25(25) | n.s | 1.0* | … | |
| Genotype | ||||||
| TT | 28(56) | 28(56) | n.s | 1.0* | … | |
| TG | 19(38) | 19(38) | n.s | 1.0* | … | |
| GG | 3(6) | 3(6) | n.s | 1.0* | … | |
| IL-4(-590) | Allele | |||||
| C | 85(85) | 72(72) | 0.04 | 0.5 (0.2–1.0) | 0.7 (0.5–1.0) | |
| T | 15(15) | 28(28) | 0.04 | 2.2 (1.1–4.4) | 1.4 (1.1–1.9) | |
| Genotype | ||||||
| CC | 28(56) | 30(60) | n.s | … | … | |
| CT | 20(40) | 9(18) | 0.02 | 0.3 (0.1–0.8) | 0.5 (0.3–1.0) | |
| TT | 2(4) | 11(22) | 0.01 | 6.7 (1.4–32.4) | 1.9 (1.3–2.6) | |
| IL-4(-33) | Allele | |||||
| C | 91(91) | 72(72) | 0.001 | 0.3 (0.1–0.6) | 0.6 (0.5–0.8) | |
| T | 9(9) | 28(28) | 0.001 | 3.9 (1.7–8.9) | 1.7 (1.3–2.2) | |
| Genotype | ||||||
| CC | 41(82) | 30(60) | 0.04 | 0.4 (0.1–0.9) | 0.6 (0.4–0.9) | |
| CT | 9(18) | 11(22) | n.s | … | … | |
| TT | 0(0) | 9(18) | 0.002 | … | 2.2 (1.7–2.8) | |
| IL-4(-1098/-590/-33) | Haplotype | |||||
| TCC | 60(60) | 47(47) | n.s | … | … | |
| TTT | 9(9) | 28(28) | 0.0002 | 5.8 (2.3–14.4) | 2.2 (1.5–3.2) | |
| GCC | 25(25) | 25(25) | n.s | – | – | |
| TCT | 0(0) | 0(0) | n.s | – | – | |
| TTC | 6(5) | 0(0) | 0.01 | … | … | |
| GTT | 0(0) | 0(0) | n.s | … | … | |
| IL-6(-174) | Allele | |||||
| C | 44(44) | 42(42) | n.s | … | … | |
| G | 56(56) | 58(58) | n.s | … | … | |
| Genotype | ||||||
| CC | 19(38) | 17(34) | n.s | … | … | |
| CG | 6(12) | 25(50) | 0.0001 | 7.3 (2.7–20.3) | 2.2 (1.6–3.2) | |
| GG | 25(50) | 8(16) | 0.0006 | 0.2 (0.1–0.5) | 0.4(0.2–0.7) | |
| IL-6(nt565) | Allele | |||||
| G | 78(78) | 81(81) | n.s | … | … | |
| A | 22(22) | 19(19) | n.s | … | … | |
| Genotype | ||||||
| GG | 38(76) | 39(78) | n.s | … | … | |
| GA | 3(6) | 3(6) | n.s | … | … | |
| AA | 9(18) | 8(16) | n.s | … | … | |
| IL-6(-174/nt565) | Haplotype | |||||
| GG | 47(47) | 31(31) | 0.02 | 0.5 (0.3–0.9) | 0.7 (0.5–1.0) | |
| CA | 13(13) | 8(8) | n.s | … | … | |
| CG | 31(31) | 50(50) | 0.006 | 2.2 (1.2–4.0) | 1.5 (1.1–1.9) | |
| GA | 9(9) | 11(11) | n.s | … | … | |
CG-Chronic Gastritis; IGC-Intestinal Gastric Cancer; n-Absolute Number; ρ-Probability; n.s-Non-Statiscally Significant; OR-Odds Ratio; RR-Relative Risk; CI-confidence Interval; *- Reference.
IL-6-174CG intermediate producer genotype showed a higher prevalence in IGC patients (50% vs. 12%; ρ=0.0001; OR=7.3; 95% CI 2.7–20.3) (Table 2). Additionally, IL-6 -174/nt565CG, low/intermediate producer haplotype, were founded associated with IGC patients group (ρ=0.006; OR=2.2; 95% CI 1.2–4.4) (Table 2).
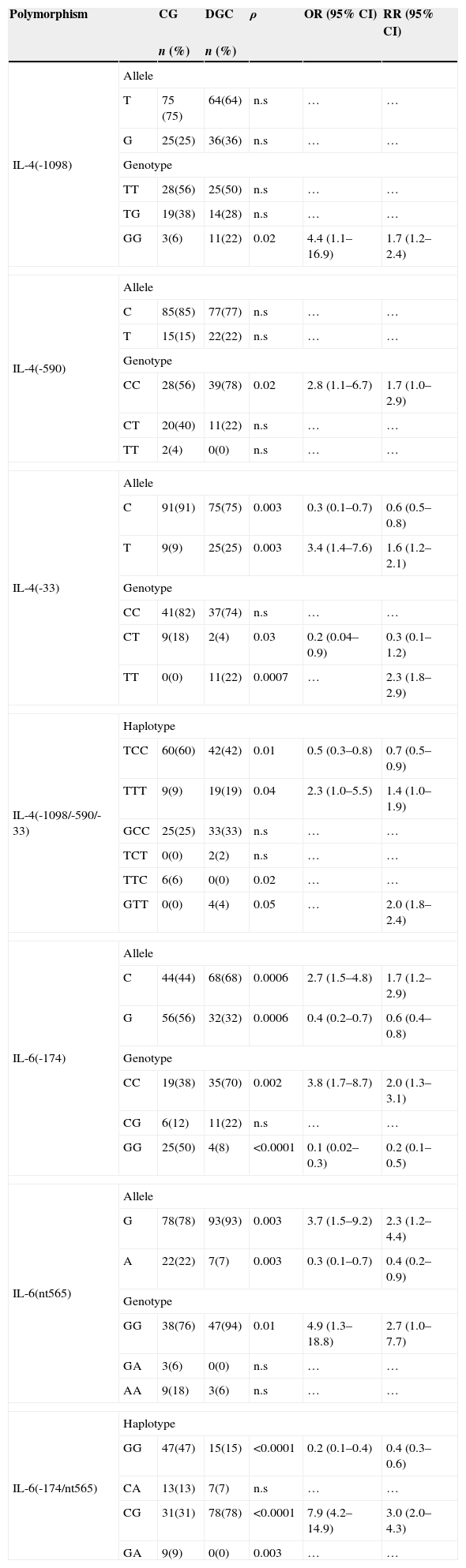
3.3IL-4 -1098, -590 and -33 polymorphisms in diffuse gastric cancerIL-4 -33T mutant allele (ρ=0.003; OR=3.4; 95% CI 1.6–7.6) was more frequent between DGC group (Table 3). We also found a higher prevalence of IL-4 -1098GG (ρ=0.02; OR=4.4; 95% CI 1.7–16.9) and IL-4 -33TT (ρ=0.0007; RR=2.3; 95% CI 1.8–2.9) low producer genotypes among DGC set (Table 3). This tendency was also verified in IL-4 TTT (ρ=0.04; OR=2.3; 95% CI 1.0–5.5) mutated haplotype that showed high incidence in ICG group (Table 3).
IL-4 and IL-6 allele/genotype frequencies among CG and DGC groups.
| Polymorphism | CG | DGC | ρ | OR (95% CI) | RR (95% CI) | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| IL-4(-1098) | Allele | |||||
| T | 75 (75) | 64(64) | n.s | … | … | |
| G | 25(25) | 36(36) | n.s | … | … | |
| Genotype | ||||||
| TT | 28(56) | 25(50) | n.s | … | … | |
| TG | 19(38) | 14(28) | n.s | … | … | |
| GG | 3(6) | 11(22) | 0.02 | 4.4 (1.1–16.9) | 1.7 (1.2–2.4) | |
| IL-4(-590) | Allele | |||||
| C | 85(85) | 77(77) | n.s | … | … | |
| T | 15(15) | 22(22) | n.s | … | … | |
| Genotype | ||||||
| CC | 28(56) | 39(78) | 0.02 | 2.8 (1.1–6.7) | 1.7 (1.0–2.9) | |
| CT | 20(40) | 11(22) | n.s | … | … | |
| TT | 2(4) | 0(0) | n.s | … | … | |
| IL-4(-33) | Allele | |||||
| C | 91(91) | 75(75) | 0.003 | 0.3 (0.1–0.7) | 0.6 (0.5–0.8) | |
| T | 9(9) | 25(25) | 0.003 | 3.4 (1.4–7.6) | 1.6 (1.2–2.1) | |
| Genotype | ||||||
| CC | 41(82) | 37(74) | n.s | … | … | |
| CT | 9(18) | 2(4) | 0.03 | 0.2 (0.04–0.9) | 0.3 (0.1–1.2) | |
| TT | 0(0) | 11(22) | 0.0007 | … | 2.3 (1.8–2.9) | |
| IL-4(-1098/-590/-33) | Haplotype | |||||
| TCC | 60(60) | 42(42) | 0.01 | 0.5 (0.3–0.8) | 0.7 (0.5–0.9) | |
| TTT | 9(9) | 19(19) | 0.04 | 2.3 (1.0–5.5) | 1.4 (1.0–1.9) | |
| GCC | 25(25) | 33(33) | n.s | … | … | |
| TCT | 0(0) | 2(2) | n.s | … | … | |
| TTC | 6(6) | 0(0) | 0.02 | … | … | |
| GTT | 0(0) | 4(4) | 0.05 | … | 2.0 (1.8–2.4) | |
| IL-6(-174) | Allele | |||||
| C | 44(44) | 68(68) | 0.0006 | 2.7 (1.5–4.8) | 1.7 (1.2–2.9) | |
| G | 56(56) | 32(32) | 0.0006 | 0.4 (0.2–0.7) | 0.6 (0.4–0.8) | |
| Genotype | ||||||
| CC | 19(38) | 35(70) | 0.002 | 3.8 (1.7–8.7) | 2.0 (1.3–3.1) | |
| CG | 6(12) | 11(22) | n.s | … | … | |
| GG | 25(50) | 4(8) | <0.0001 | 0.1 (0.02–0.3) | 0.2 (0.1–0.5) | |
| IL-6(nt565) | Allele | |||||
| G | 78(78) | 93(93) | 0.003 | 3.7 (1.5–9.2) | 2.3 (1.2–4.4) | |
| A | 22(22) | 7(7) | 0.003 | 0.3 (0.1–0.7) | 0.4 (0.2–0.9) | |
| Genotype | ||||||
| GG | 38(76) | 47(94) | 0.01 | 4.9 (1.3–18.8) | 2.7 (1.0–7.7) | |
| GA | 3(6) | 0(0) | n.s | … | … | |
| AA | 9(18) | 3(6) | n.s | … | … | |
| IL-6(-174/nt565) | Haplotype | |||||
| GG | 47(47) | 15(15) | <0.0001 | 0.2 (0.1–0.4) | 0.4 (0.3–0.6) | |
| CA | 13(13) | 7(7) | n.s | … | … | |
| CG | 31(31) | 78(78) | <0.0001 | 7.9 (4.2–14.9) | 3.0 (2.0–4.3) | |
| GA | 9(9) | 0(0) | 0.003 | … | … | |
CG-Chronic Gastritis; DGC-Diffuse Gastric Cancer; n-Absolute Number; ρ-Probability; n.s-Non-Statistically Significant; OR-Odds Ratio; RR-Relative Risk; CI-confidence Interval; *- Reference.
IL6 -174C (ρ=0.0006; OR=2.7; 95% CI 1.5–4.8) and IL-6 -174CC (ρ=0.002; OR=3.8; 95% CI 1.7–8.7), low producers variants, showed upper prevalence within DGC set. On other hand, IL-6 nt565G and nt565GG, high producers variants, were more prevalent in DGC subjects (ρ<0.01; OR>3.7; 95% CI 1.3–18.8). Concerning IL-6 haplotypes results, low/intermediate producer haplotype, 174/nt565CG, showed a high incidence in DGC set (78% vs. 31%, ρ<0.0001; OR=7.9; 95% CI 4.2–14.9) (Table 3).
4Discussion4.1IL-4 polymorphisms in gastric cancerIL-4 plays a central role in Th cells maturation contributing to Th2 phenotype differentiation.36 In this process, IL-4 can increase anti-inflammatory cytokines production (such as IL-10, IL-13 and itself)36 and suppress pro-inflammatory cytokines production (such as IL-1β and IL-8).37–39 Moreover, activation of IL-4 pathway can lead to cell proliferation, cell growth or apoptosis depending on the variety of stimuli39 Therefore, genetic variations responsible for different IL-4 expression levels, and consequently for Th cells differentiation, may be critical in determinate the pro-or anti-tumor immune response.36–43 Moreover, IL-4 impact in GC remains unclear, since some studies associate this molecule with GC prevalence,25,39 while other studies show no association between them.38,40,42 In this study we have evaluated the impact of IL-4 polymorphisms in IGC and DGC patients and found significant correlations between them. These results support the idea that IL-4 can have an important role in GC development, namely by contributing for Th2 differentiation and immune modulation.
4.2IL-4 polymorphisms role in intestinal gastric cancerIGC is characterized by a unique glandular pattern, resembling the gastrointestinal tract glands (Fig. 1B), and by a relatively well-defined/sequential progression.35,43,44 The evolution of these tumors begins with gastritis occurrence, that could progresses for mucosa atrophy (atrophic gastritis), followed by intestinal metaplasia, dysplasia and carcinoma.35,43 IGC is often described as a result of persistent chronic inflammation, Hp-dependent.3,35,44 In this sense, IL-4 polymorphisms can influence this cancer type susceptibility and risk.
In this work we have found that the presence of IL-4 mutant alleles (IL-4 -590T and IL-4 -33T) are correlated with IGC occurrence. Contrasting, the high production variants (IL-4 -590C, IL-4 -33C and IL-4 -33CC) are correlated with CG prevalence. As IL-4 plays a central role in Th cells maturation to Th2 phenotype, decreases in this molecule production results in Th2 response reduction.24,41,42 This molecule can act as IFN, IL-1 and TNF inhibitor, decreasing the cells pro-inflammatory responses. It also been reported than IL-4 may contribute to cell-mediated immunity.38–42 Those processes can be responsible for increased gastric mucosa deregulated proliferation, triggered by pro-inflammatory cytokines (such as IL-1β and IL-8).24,38–42 Cellular deregulation/proliferation can be the main cause of tumor development in IGC patients. These findings are in agreement with those previously described by Wu and collaborators,39 in 2003, which refer the low IL-4 production (IL-4 -590TT genotype) as responsible for the development of gastric carcinoma and high production (IL-4 -590CC genotype) as responsible for the development of peptic ulcer.39
4.3IL-4 polymorphisms role in diffuse gastric cancerDGC is characterized by individualized neoplastic cells, variable stroma and sometimes signet ring cells (Fig. 1C).45–48 This kind of cancer develops subsequently to chronic infection, without going through atrophic gastritis and intestinal metaplasia steps.48 DGC has a great predisposition for intra and transmural growth, is highly metastatic and has poor clinical outcome.45–48 As in IGC, IL-4 polymorphisms can influence DGC susceptibility.
In this study, we have seen that IL-4 cytokine low production variants particularly, IL-4 -33T allele, IL-4 -1098GG and IL-4 -33TTgenotypes, IL-4 -1098/-590/-33 TTT and IL-4 -1098/-590/-33 GTT haplotypes, are correlated with DGC prevalence. Simultaneously, IL-4 -33C allele, IL-4-590CC and IL-4 -33CT genotypes and IL-4 -1098/-590/-33 TCC haplotype (associated with high IL-4 production) are correlated with chronic gastritis incidence. Resuming, IL-4 high production appears to have a protective effect in DGC, while low production of this cytokine seams to increase the DGC risk. Consequently, decreased IL-4 production leads to gastric mucosa tissue breakdown increase and tumor development, triggered by Th2 pathway absence (downregulating IL-10, IL-13).24,42–44,49 Although there are no studies on IL-4 polymorphisms that compare individuals with CG and DGC, these findings are conflicting with previous studies involving polymorphisms responsible for IL-4 high production on gastric cells, maybe duo to sample and/or population features.25,49 However, the results obtained in this work are consistent with those obtained for IGC.
4.4IL-6 polymorphisms in gastric cancerIL-6 is a cytokine with dual role in the immune system, whose role in carcinogenesis is not yet fully understood.26–32 This molecule can promote tumor growth by inhibiting cancer cells apoptosis and inducing tumor angiogenesis.30–32 IL-6 is also very important at systemic level, stimulating fibroblasts and epithelial cells to secrete IL1-Ra and promoting down-regulation of anti-inflammatory activity.30,31,50 IL-6 are also involved NF-κB down-modulation activities by E-cadherin in GC.19 E-cadherin can rise IL-6 and TNF expression leading to reduced cell apoptosis and increased cell survival.19 These molecules can also mediate inflammation associated cancer development mechanisms, including Hp infection. For those reasons, high IL-6 levels are associated with worse prognosis in advanced GC cases.27,51,52 Polymorphisms in IL-6 promoter region, particularly at -174 position, have great study interest because it has been reported its association with cancer prevalence and/or prognosis.26,30,31,50,52–55 In spite this association, IL6 role in GC development/predisposition remains doubtful with conflicting results.54,55 In this work, IL6 -174G>C and nt565 G>C polymorphisms showed an important association among IGC, DGC and CG sets. These findings supports the idea that IL-6 contributes to GC carcinogenic development process, mostly by regulating pro- and/or anti-inflammatory activities in Hp-infected tissue.
4.5IL-6 polymorphisms4.5.1Role in intestinal gastric cancerSince IGC is normally described as a result of continuous chronic inflammation,35,43–45Hp-dependent, and IL-6 regulate inflammatory responses,26–32 it can be expected that IL-6 polymorphisms can influence ICG risk. In this study, the incidence of IL-6 low producers’ genotype (IL-6 -174CG) and haplotype (IL-6 -174/nt565 CG) were correlated with IGC set. On the other hand, IL-6 high producers’ genotype (IL-6 -174GG) and haplotype (IL-6 -174/nt565GG) showed a significant association with CG prevalence. Our results suggest that IL-6 over-expression have a protective role in IGC subjects, while its down-regulation appears to increase IGC predisposal. Our data are only corroborated by Kamangar et al55 work, where IL-6 low producer genotype (IL-6 -174CG) showed a higher risk for GC predisposal.55 Even though, there might be several possible mechanisms underlying this results, including sample outcomes, since, GC development occurs from both environmental and genetic factors interactions. Nevertheless, it is well known that the decreased on IL-6 production results in macrophages anti-tumor activity and tumor cells lyses full drop.56 On the other hand, since IL-6 is Th3 cytokine, down-regulation of this molecule may also trigger an increase of Th1 response (stimulating TNF, IL-1 and IL-8 expression) that leads to uncontrolled inflammation and, consequently, to gastric carcinogenesis.26–32,50,52–56
4.6IL-6 polymorphisms role in diffuse gastric cancerOnce DGC is defined as a result of chronic inflammation,36,46–48 and IL-6 is involved in E-cadherin19 and inflammatory pathways,52–56 we can estimated an important role of IL-6 polymorphisms in DGC risk. This hypothesis was corroborated with our results, since we have found a high correlation between DGC subjects and IL-6 low production variants (IL-6 -174C allele, IL-6 -174 CC genotype and IL-6 -174/nt565CG haplotype). Additionally, in this work, IL-6 high production polymorphisms (IL-6 -174G allele, IL-6 -174 GG genotype and IL-6 -174/nt565GG and GA haplotypes) showed a significant association with CG occurrence. As in IGC, the IL-6 over-expression was correlated with DGC protection, while its low production seams to increase the risk of DGC occurrence.50 IL-6 involvement in DGC can also be explained by E-cadherin pathway, since E-cadherin can influence IL-6 and TNF expression modulating cell apoptosis, cell survival, cell migration, and inflammation associated gastric cancer development.19 In this way, as in IGD, a decrease of IL-6 production is associated with an increase of Th1 (upregulating TNF, IL-1 and IL-8) and a decrease of Th2 activities (downregulating IL-10, IL-13 and IL-4)50,52–56 that could lead to a deregulation in chronic inflammation process.17,18 Alternatively to IL-6 dual Th role in inflammation process, these results could also be explained by its activity in macrophages anti-tumor responses regulation.26–32,50,52–56 These findings are consistent with some results previously described for diffuse type32 and with those obtained for the intestinal type.55
4.7Study limitationsThere might be several possible mechanisms underlying all association studies, such as, the results from interplay of both environmental and genetic factors, which can be responsible for analysis default. The sample size of the present study (50 DCG, 50 IGC and 50 controls) might not be large enough to detect small effect of low penetrance mutations. The combine effect of multiple genes/mutations can provide more reliable information for genetic contribution to risk of GC. We cannot completely exclude the effects of the other conditions (i.e. age, weight, gender, diet type, etc.…) and residual confounding attributable to the measurement error (namely, unicentric characters, lack of assess of Hp status in GC subjects, etc.…). It is essential a large approaching study with large sample size to confirm our outcomes. Still, the present study provides preliminary evidence that IL-6 and IL-4 expression variants, may contribute to the risk of GC in Portuguese Population, and may be useful tools in the study of this multifactorial disorder. Nevertheless, large approaching studies with large sample size are essential to confirm our outcomes.
5ConclusionsCytokines studied in this work seem to play an important role in both DGC and IGC development risk. It was clear that IL-4 and IL-6 polymorphisms could make individuals more and/or less susceptible to GC, independently of its histological type. Although some of these data have not yet been described, these findings raise important questions about cytokines activity in gastric cancer genesis. IL-4 and IL-6 expression variants can manipulate some of tumor development mechanisms. Those molecules high production variants appear to have a protective role in both IGC and DGC, while the low production modifications seem to increase GC susceptibility. Still, his kind of study is quite important in countries like Portugal, with high levels of gastric disease, since we can improve the GC individual risk determination using cytokine polymorphisms as a tool. Moreover, this study provides preliminary evidence that cytokine polymorphism (namely, IL-4 and IL-6) determination could be useful to follow up patients and relatives in our population. Nevertheless, large approaching studies with large sample size are essential to confirm our outcomes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.