The risk of iatrogenic perforations in colonoscopy is not negligible. Experience with endoscopic closure of perforations is increasing and new devices for this purpose are being released, making endoscopy a therapeutic option. National data regarding iatrogenic perforations is scarce and the burden of iatrogenic perforations in out-hospital procedures is poorly characterized in the literature.
ObjectiveEvaluation of iatrogenic perforations rate during colonoscopy, their characteristics, management and prognosis.
MethodsRetrospective study of all patients with perforations secondary to in-hospital and non-hospital colonoscopies treated in a tertiary hospital between 01-01-2006 and 01-10-2014. Demographic, endoscopic, radiological and therapeutic data were analyzed.
ResultsFifty-three perforations were identified, 20 occurring in colonoscopies performed in non-hospital environment (45% with therapeutic procedures) and 33 occurring in-hospital procedures (73% in therapeutic colonoscopies; representing 0.12% of all colonoscopies carried out in-hospital). Patients: male in 56%, average age of 71 years, history of previous abdominopelvic surgery in 31% and diverticulosis in 10%. Colonoscopy: elective in 93%, under deep sedation in 21%, with less than excellent/good bowel preparation in 56%. A resident was the first performer in 10 cases. Perforations: average size of 21mm (4–130mm), diagnosed during the procedure in 51% of cases and occurred in rectum-sigmoid transition in 58.5%. Regarding therapeutics, all patients with perforation occurring in non-hospital colonoscopies were managed by surgery. Concerning treatment of those in our unit: 2-conservative, 12-endoscopic (10 successfully), 21-surgical (including the 2 cases with failure of the endoscopic approach). Comparing endoscopic treatment (n=10, G1) versus surgery (n=21; G2): perforation size – 9mm (G1) versus 28mm (G2); perforation location – 7/10 in rectum-sigmoid (G1) versus 8/21 in rectum-sigmoid and 10/21 transverse/ascending colon/hepatic angle (G2). Morbidity: 1 infection in G1 and 13 complications in G2 (infection, hemorrhage, fistula). Mortality: no deaths in G1 and 2 deaths at 30 days due to septic shock in G2.
ConclusionPerforations in colonoscopy are rare in our clinical practice. Endoscopic closure was effective, though limited to perforations found during the procedure. The mortality was relatively low and endoscopic management did not seem to worsen it. An additional effort is necessary in order to detect perforations during colonoscopy.
O risco de perfuração iatrogénica na colonoscopia não é negligenciável. A comercialização de dispositivos para o encerramento endoscópico de perfurações e a experiência para esse efeito têm aumentado, tornando a endoscopia uma opção terapêutica. Dados nacionais referentes a perfurações iatrogénicas escasseiam e o impacto das perfurações em colonoscopias realizadas em ambiente extra-hospital encontra-se mal caracterizado.
ObjetivoAvaliação da taxa de perfurações ocorridas durante colonoscopia, características tratamento e prognóstico.
MétodosEstudo retrospetivo com todos os doentes com perfuração secundária a colonoscopia realizada intra/extra-hospital tratados num hospital terciário entre 01-janeiro-2006 e 01-outubro-2014. Análise dos dados demográficos, endoscópicos, radiológicos, terapêuticos.
ResultadosIdentificaram-se 53 perfurações, 20 em colonoscopias realizadas em ambiente extra-hospitalar (procedimentos terapêuticos associados em 45%) e 33 em exames intra-hospitalares (73% em colonoscopias terapêuticas; representando 0,12% de todas as colonoscopias realizadas em regime hospitalar). Doentes: sexo masculino em 56%, idade média 71 anos, cirurgia abdomino-pélvica prévia em 31% e diverticulose cólica em 10%. Colonoscopia: eletiva em 93%, sob sedação em 21%, com preparação intestinal inferior a excelente/boa em 56%. Um interno participou como executante em 10 casos. Perfurações: tamanho médio 21mm (4–130mm), detetadas durante o procedimento em 51%, localizadas na transição recto-sigmoide em 58,5%. Os doentes com perfurações ocorrendo em regime extra-hospitalar foram tratados cirurgicamente. Relativamente às opções terapêuticas dos doentes com perfurações ocorridas na nossa unidade: 2-conservadora, 12-endoscópica (10 com sucesso), 21-cirúrgica (incluindo os 2 casos com falência da abordagem endoscópica). Comparando a abordagem endoscópica (n=10, G1) versus cirúrgica (n=21, G2): tamanho da perfuração 9mm (G1) versus 28mm (G2); localização da perfuração–7/10 no recto-sigmóide (G1) versus 8/21 no recto-sigmóide e 10/21 no transverso/ângulo hepático/ascendente (G2). Morbilidade: 1 infeção (G1) e 13 complicações (G2) (infeção, hemorragia, fístula). Mortalidade: 0 mortes aos 30 dias em G1 e 2 em G2.
ConclusãoAs perfurações na colonoscopia são comprovadamente raras na nossa prática clínica. O encerramento endoscópico foi eficaz, embora limitado às perfurações detectadas durante o exame. A morbimortalidade foi relativamente baixa, não agravando com a abordagem endoscópica. Um esforço adicional é necessário para detetar perfurações durante a colonoscopia.
Colonoscopy is the gold standard test for the prevention of colorectal cancer through the diagnosis and treatment of colorectal neoplastic lesions.1 Although invasive, colonoscopy is generally well tolerated and considered safe, being associated with a low risk of iatrogenic injuries. Perforation, although rare, is a serious complication with significant morbidity and mortality – from 25 to 53% and 0 to 26% respectively.2,3 The iatrogenic perforation rate (0.05–0.39%)4 varies widely whether considering diagnostic (0.03–0.8%) or therapeutic procedures (0.15–3%).3
The ideal approach to perforations is still uncertain because there are no randomized trials conducted for this purpose.2 Traditionally, surgery was the first-line therapy in most of these cases. However, endoscopy has also become a therapeutic option with the increasing experience in the endoscopic closure of perforations and the release of more and better dedicated devices,4–8 allowing a reasonable resolution rate in complications occurring in diagnostic colonoscopies (17–48%) and even better results with respect to those occurring during therapeutic procedures (72–79%).9
With the increasing number of endoscopic screening programs, greater accessibility to colonoscopy and the progressive expansion of the indications for therapeutic endoscopy, the number of perforations may eventually increase.2 In this context, an effective endoscopic management of iatrogenic perforations is crucial to preserve a favorable cost–benefit ratio.
In this study, a cohort of patients with perforation secondary to colonoscopy treated in one of the largest tertiary and teaching hospitals in Portugal was analyzed, determining the frequency and the characteristics of the perforations and its respective management and prognosis. National data regarding this subject is scarce and Centro Hospitalar e Universitário de Coimbra (CHUC) attends a large number of patients and performs various complex therapeutic interventions. Additionally, the burden of iatrogenic perforations in out-hospital procedures, poorly characterized in the literature, were included in this study, allowing a better clarification of its impact in the absolute number of iatrogenic perforations. These results may eventually help to identify potential gaps in prevention, diagnosis and treatment of perforations, in order to reduce its incidence and associated complications.
2Methods2.1Patients and settingRetrospective analysis of all patients with iatrogenic perforation secondary to in-hospital and non-hospital colonoscopies treated at CHUC between January 1st 2006 and October 1st 2014.
All male and non-pregnant female patients, 18 years or older, treated in CHUC with perforation secondary to colonoscopy were included. Patients were identified from the electronic medical records of hospitalized patients, using the terms “perforation” and “colonoscopy”. Children and pregnant-woman were excluded from the study.
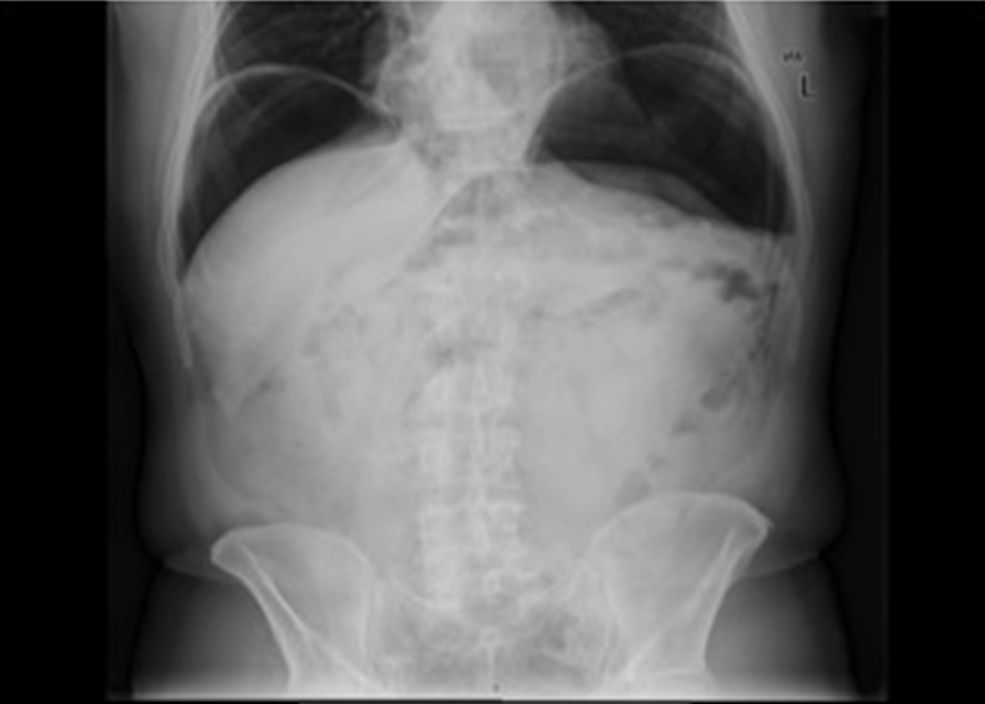
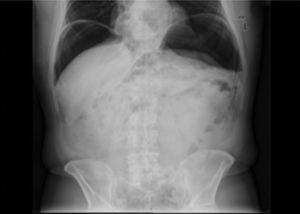
2.2Outcomes and definitionsThe main outcome of this study was to define the frequency of iatrogenic perforations during colonoscopy. Secondary outcomes were the characterization of these perforations and determination of their respective management and prognosis. Iatrogenic perforation was defined as the perforation occurred during or after colonoscopy in the absence of any other possible cause, such as abdominal trauma. Perforations detected during colonoscopy, with visualization of a defect involving four layers of the colon wall, or after the examination, through the documentation of free air on abdominal plain x-ray or tomography10 were considered.
Demographic and clinical data (gender, age, history of diverticulosis or previous abdominopelvic surgery, comorbidities – coronary heart disease, congestive heart failure, peripheral artery disease, cerebrovascular disease, dementia, chronic obstructive lung disease, connective tissue disease, peptic ulcer disease, chronic liver disease, diabetes, hemiplegia, chronic kidney disease, leukemia, lymphoma, metastatic disease, AIDS), as well as endoscopic (elective/emergency procedure, with or without moderate-to-deep sedation with propofol by anesthesiologist, quality of bowel preparation and participation of a resident, diagnostic or therapeutic procedure if complemented with polipectomy, endoscopic mucosal resection, endoscopic hemostasis, colon dilation or stent placement), radiological and therapeutic (endoscopic and surgical) data analysis was performed.
2.3Statistical analysisStatistical analysis was performed using SPSS 20.0 (Statistical Package for the Social Sciences, IBM Corporation, Armonk, NY, USA). Data calculated to characterize the study population were expressed as descriptive statistics namely mean, median, standard deviation and range (minimum and maximum). The qualitative variables were expressed as through absolute (number of cases) and relative frequencies (as a percentage). The association of two categorical variables was tested through the Chi-Square test or Fisher Exact test.
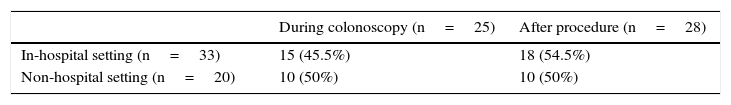
3Results3.1Local and patientsFifty-three cases of perforation were identified: 20 occurred in colonoscopies performed in non-hospital setting and the other 33 in-hospital setting. The perforations occurring in in-hospital setting represented 0.12% of all colonoscopies performed during the study time period (n=21,481).
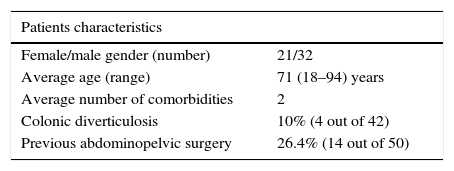
Demographic data regarding the patients suffering from iatrogenic perforations are shown in Table 1.
3.2Characteristics of perforationsPerforations occurred in elective colonoscopies in 93% of cases (51 out of 53 cases). In 21% of the cases (9 out of 41 cases), the colonoscopy was performed under moderate-to-deep sedation. Bowel preparation was less than excellent or good in 56% of cases (30 cases) (subjective semi-quantitative evaluation – no validated bowel preparation cleansing scale was routinely used). In 10 cases (30.3% of in-hospital procedures), a resident of Gastroenterology participated in the procedure as the first performer.
The average size of iatrogenic perforation was 21mm (4–130mm).
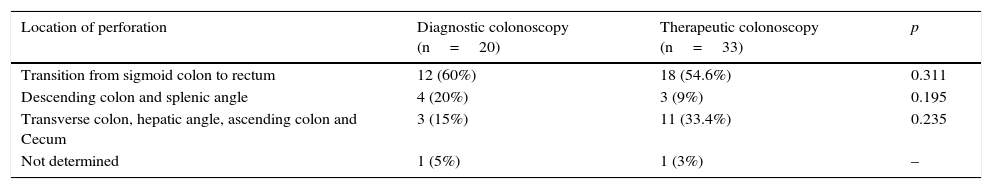
Regarding location of perforations: 31 occurred in the transition from sigmoid colon to rectum (58.5%), 5 in the descending colon (9.4%), 2 in the splenic angle (3.8%), 7 in the transverse colon (13.2%), 1 in the hepatic angle (1.9%), 1 in the ascending colon (1.9%) and 5 in the cecum (9.4%). In 1 case there was insufficient information in the files to determine the location of the perforation.
Perforations occurred in therapeutic procedures in 9 (45%) of non-hospital colonoscopies versus 24 (72.7%) of in-hospital colonoscopies. All non-hospital therapeutic colonoscopies complicated with perforation were related to polipectomy procedures. Regarding in-hospital setting: 18 perforations occurred after endoscopic mucosal resection, 4 after polypectomy and 2 after argon plasma coagulation.
As Table 2 shows, there was no correlation between the location of perforation and the type of colonoscopy (diagnostic versus therapeutic).
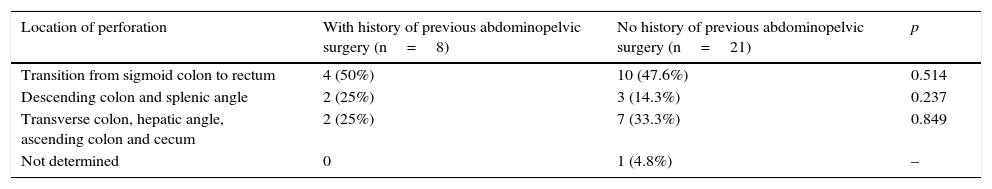
Information regarding previous abdominopelvic surgery was obtained in 50 of these patients: 14 had previous surgery (26.4%). Table 3 compares the location of perforation according to the history of abdominopelvic surgery.
Location of perforations according to the type of colonoscopy (diagnostic versus therapeutic).
| Location of perforation | Diagnostic colonoscopy (n=20) | Therapeutic colonoscopy (n=33) | p |
|---|---|---|---|
| Transition from sigmoid colon to rectum | 12 (60%) | 18 (54.6%) | 0.311 |
| Descending colon and splenic angle | 4 (20%) | 3 (9%) | 0.195 |
| Transverse colon, hepatic angle, ascending colon and Cecum | 3 (15%) | 11 (33.4%) | 0.235 |
| Not determined | 1 (5%) | 1 (3%) | – |
Twenty-five (47.2%) perforations were diagnosed during the endoscopic procedure, 14 after the procedure but in the first 24h and 11 after 24h of the procedure. In 3 cases it was not possible to determine the moment of diagnosis with the available information. No significant differences were found comparing perforations detected during or after colonoscopy according to setting of delivery of care (p=0.426) – Table 4. In the 28 cases of perforations diagnosed after the procedure, 14 (50%) were detected in the first 24h.
Location of perforations according to history of previous abdominopelvic surgery.
| Location of perforation | With history of previous abdominopelvic surgery (n=8) | No history of previous abdominopelvic surgery (n=21) | p |
|---|---|---|---|
| Transition from sigmoid colon to rectum | 4 (50%) | 10 (47.6%) | 0.514 |
| Descending colon and splenic angle | 2 (25%) | 3 (14.3%) | 0.237 |
| Transverse colon, hepatic angle, ascending colon and cecum | 2 (25%) | 7 (33.3%) | 0.849 |
| Not determined | 0 | 1 (4.8%) | – |
Perforations occurring in non-hospital setting were exclusively treated surgically and will not be object of further evaluation.
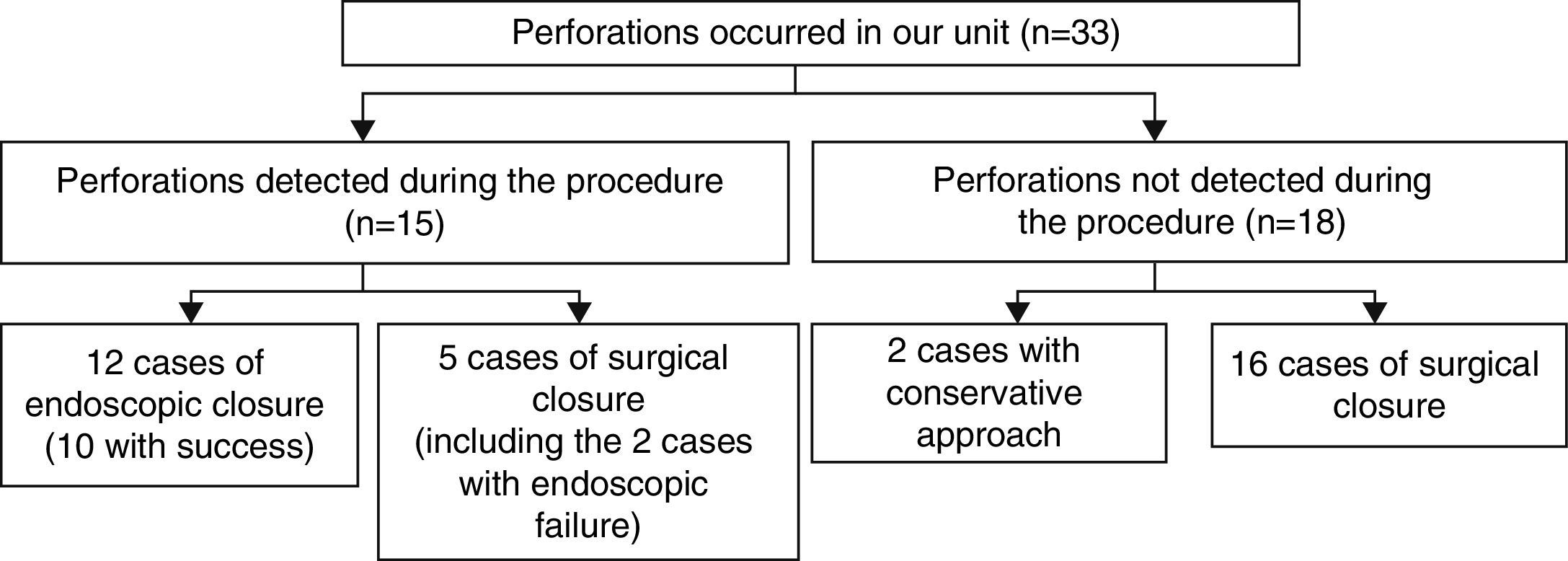
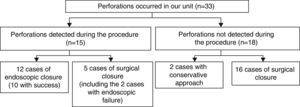
In perforations occurring in our unit (n=33) non-surgical treatment, including conservative approach was attempted in 14 cases, being successful in 12 cases (36.3%). Considering only perforations following therapeutic procedures (24 cases), the rate of effective non-surgical treatment raises (10 cases; 41.7%). Subdividing the perforations according to the time of diagnosis (Fig. 1):
- (A)
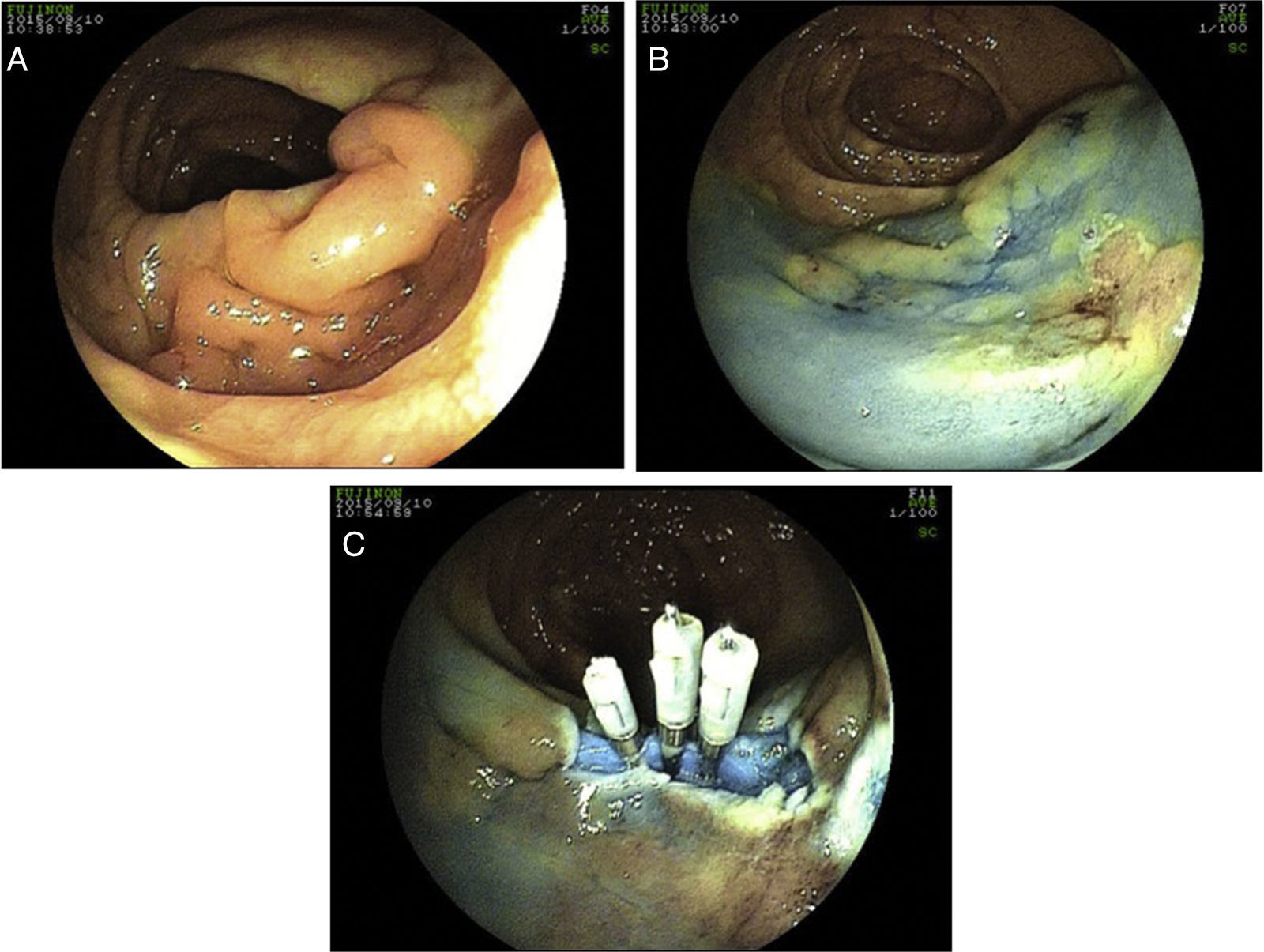
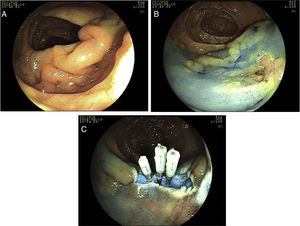
15 perforations have been detected during colonoscopy, 12 of which occurred in therapeutic procedures. Five perforations were managed surgically, 2 of which after failure of endoscopic clipping. The surgical approach was laparotomy in 100% of cases. In 3 cases (60%) a segmental resection of the colon was performed, with the remaining cases being over sewed. Endoscopic closure was successfully performed using clips (Olympus reusable EZ clip system with HX-11OUR fixing device with either HX-610-090L or HX-610-135L long clips in 9 cases – Figs. 2 and 3 – and an Over-The-Scope-Clip – OTSC – Ovesco System Set 12/6t OTSC Anchor 200.100 in one case). The number of clips used depended on the size of the perforation, but the average number was around 4 clips per perforation. All patients were hospitalized for surveillance, under null diet, intravenous fluids and broad-spectrum antibiotics.
- (B)
18 perforations were detected after colonoscopy, 2 of which were submitted to conservative therapy. In one case presence of free air in the abdomen was documented after development of abdominal pain, fever and increased inflammatory parameters on the same day of a diagnostic colonoscopy. Given the condition of the patient (elderly with multiple co-morbidities, including refractory celiac disease with marked cachexia due to severe malabsorption syndrome) and after discussion with the surgery team, a conservative therapeutic approach was chosen. The patient was placed on a nil per os diet, intravenous fluids and broad-spectrum antibiotics with a favorable clinical response. In the other case, the patient was asymptomatic. Free air in the abdomen was incidentally found 3 days after a diagnostic procedure during staging for additional disease (rectum adenocarcinoma). The remaining sixteen cases were addressed surgically: 14 by laparotomy and 2 by laparoscopy. In 13 (81.3%) cases a segmental resection of the colon was made.
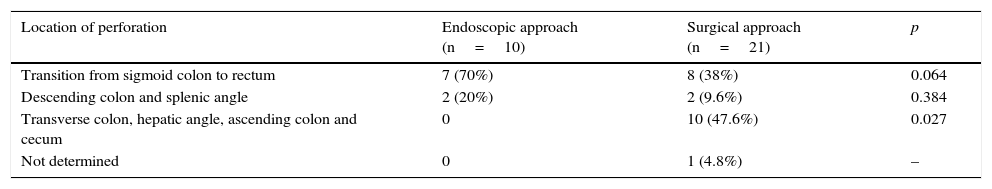
Comparing the endoscopic (n=10, G1) versus surgical (n=21, G2) approaches:
- •
Perforations addressed surgically had larger size (G1 – 9mm G2 – 28mm; p=0.014).
- •
Perforations treated in G1 mainly occurred in the rectum-sigmoid (7 of 10) and in G2 in the rectum-sigmoid (8 of 21) and transverse colon/hepatic angle/ascending colon (10 of 21), as shown in Table 5.
Table 5.Location of perforations according to the therapeutic approach (endoscopy/surgery).
Location of perforation Endoscopic approach (n=10) Surgical approach (n=21) p Transition from sigmoid colon to rectum 7 (70%) 8 (38%) 0.064 Descending colon and splenic angle 2 (20%) 2 (9.6%) 0.384 Transverse colon, hepatic angle, ascending colon and cecum 0 10 (47.6%) 0.027 Not determined 0 1 (4.8%) –
Comparing the same groups previously described regarding endoscopic (n=10, G1) versus surgical (n=21, G2) approaches in terms of complications, there was 1 infection in G1 and 13 complications in G2 – 8 infections (3 of the surgical wound, 4 abdominal infection and 1 respiratory infection), 4 hemorrhages and one iatrogenic enteric fistula. Five ostomies were constructed.
3.6MortalityIn the endoscopic group no deaths at 30 days were recorded. In G2, there were two deaths due to septic shock. A patient aged 75 years with multiple co-morbidities, including liver cirrhosis Child-Pugh C, underwent endoscopic mucosal resection of a sessile lesion (tubulovilous adenoma with low grade dysplasia) with 30mm diameter in the splenic angle complicated with perforation detected three days later. Bowel preparation in the examination was deficient. He was surgically treated (colostomy), but nevertheless evolved into abdominal and respiratory septic shock. The second patient, a 78-year old female, underwent endoscopic mucosal resection of a 55mm sessile lesion (serreated adenoma) in the cecum complicated with perforation. Endoscopic clipping was tried without success. The patient was referred to surgery (right hemicolectomy). However, she evolved unfavorably with respiratory septic shock and died;
On average, admissions lasted 6 days in G1 and 12 days in G2.
4DiscussionIatrogenic perforations in colonoscopy are rare events in our clinical practice, occurring in 0.12%, a percentage that is within the lower limit of the rates published in the literature.9 Moreover, 18 in 24 (75%) of cases occurred in procedures associated with a higher risk of complication (endoscopic mucosal resection).
Perforations occurred mainly in patients with advanced age. Patients over 65 years have 4–6 times higher risk of colonic perforation, comparing with younger patients.3,11 Possible reasons for this increased rate include loss of mechanical strength of the colon wall and the fact that these patients have more often endoscopic findings requiring therapeutic intervention.3
The transition from sigmoid colon to rectum was the most common location of perforation, both in the therapeutic and diagnostic procedures. Iatrogenic perforations often occur in the rectum-sigmoid transition because of redundancy, luminal narrowing and/or post-surgical adhesions12 and because of the direct pressure that is often exerted on the rectosigmoid wall.3 There is a growing evidence of an increased risk of perforations in therapeutic procedures.3 In addition to the classical mechanisms associated with perforations in diagnostic procedures – mechanical damage and barotrauma – endoscopic interventions alone can be responsible for the perforations.3 The thermal effect of endoscopic interventions (such as polypectomy, endoscopic mucosal resection, endoscopic submucosal dissection or argon plasma coagulation) increases the likelihood of perforation.3 This study confirmed the rectum-sigmoid colon transition as the most frequent sites of perforation. The descending colon was also often affected in diagnostic colonoscopies, while in therapeutic procedures perforations also occurred frequently in transverse colon and cecum. The wall thickness in these locations may justify these rates.
Previous abdominopelvic surgery has been reported as a risk factor to perforation,3 but in our series, the rate of perforations in patients with past history of abdominopelvic surgery was relatively low. The history of abdominopelvic surgery did not affect the location of perforation. There were also no cases related to retroflexion.
In this series, in almost a third of cases a trainee was involved as the first performer; however, being an academic hospital, this is quite frequent and a deeper analysis on the relevance of this factor was no the subject of this work. Colonoscopy by a training fellow is believed to increase rate of colonic perforation. However, published studies have been unable to demonstrate any significant impact of trainee endoscopist on the increased rates of colonic perforation13 and the Society of American Gastrointestinal Endoscopic Surgeons Colonoscopy Study Outcome Group reported that there was no association between experience of the endoscopist and complications.13
A relatively low percentage of colonoscopies have been performed under moderate-to-deep sedation, a controversial factor that has been implicated in this risk of perforation.14,15 Although there is a lack of strong data providing it as a risk factor and the fact that it does not apply to perforations due to therapeutic procedures (that accounted for 62.3% of the cases), it may have eventually contributed to the low percentage of perforations in this series.
In perforations detected during colonoscopy, endoscopic closure was effective in 66.6% of cases (10 out of 15). Recent developments in endoscopic accessory devices have boosted the use of clips as a promising strategy in addressing iatrogenic perforations,4–8 with Jovanovic et al5 and Magdeburg et al7 being the first to address on early endoscopic repair during endoscopy. Initially developed as a therapeutic modality for bleeding peptic ulcers,16,17 endoscopic clips have been reported as effective in the closure of gastric and colonic perforations. Efficacy of endoscopic closure of the perforations occurring during diagnostic procedures varies from 17 to 48% and from 72 to 79% in therapeutic colonoscopies.9 Classically, this approach is successful in small perforations (less than 10mm diameter), but successful reports in larger perforations (up to 3cm) using OTSC have also been published.18 Higher success rates may also be expected with newer clipping devices with wider opening and possibility of repositioning. In our clinical practice, clips are being routinely to close mucosal defects, including larger ones in endoscopic mucosal resections, where OTSC are also being employed.
One of the two patients who died was an old man with multiple co-morbidities including hepatic cirrhosis Child-Pugh C presenting with a low-grade neoplasia and was submitted to a therapeutic procedure (endoscopic mucosal resection) with a higher risk of adverse events. A reflection on the benefit versus risk should always be undertaken before referral or decision to treat in these circumstances, in which a conservative approach may be more advised, since severe adverse events will inevitably lead to a worse prognosis.
A high rate of poor bowel preparation was documented, and the two patients who died had poor bowel preparation. Endoscopic resections should be avoided in this circumstances and an additional effort should be make in order to improve this situation. Despite this fact, the morbimortality was relatively low, with rates at the lower limit of the values reported in the literature2,3 – 38% of surgical versus 10% of endoscopic morbidity; 9.5% of surgical 30-day mortality–and the endoscopic approach did not seem to worsen it. Perforations that required surgery had higher morbidity and mortality. The fact that the perforations treated by surgery tend to be larger and detected later, increasing the risk of peritonitis and sepsis, probably predisposed to a worse prognosis.
Laparoscopy emerged in the last decade as a therapeutic possibility in early-detected perforations including those with endoscopic failure, because of its many advantages: less postoperative complications, shorter lengths of hospital stay, smaller incision size and lower labor withdrawal time.19 However, this technique was only adopted in the latest 2 patients in this series. The areas of perforation were easily localized and there was not extensive inflammation neither fecal contamination.
The rate of perforations diagnosed during the procedure or in the first 24h (73.6%) is within the interval rates referred in the literature (65.1–78%)3 and the endoscopic or surgical perforation closure was frequently successful and without major morbidity. However, a significant number of perforations were diagnosed more than 24h after procedure (14 cases; 26.4%), some already presenting with abdominal sepsis, increasing the need for surgery and probably incurring a worse prognosis. One of the reasons for delayed diagnosis of perforation may be related to the high percentage of therapeutic endoscopic procedures. Perforations in this context are usually smaller, hindering its detection.3 Moreover, the patients could initially have gone to primary care units before being referenced to this tertiary hospital, postponing its diagnosis and approach. The relevance of failing to recognize a perforation is exponential by the increasing number of negligence litigations all over the world, Portugal not being an exception. To give an indicative example, it was recently reported in a recent resolution of a Portuguese judge condemning a Portuguese gastroenterologist for a perforation occurring during a colonoscopy. Knowledge of the factors leading to preventable patient injury is needed to develop optimal risk prevention strategies for reducing malpractice risk related to gastrointestinal endoscopy. In fact, it is already stated in European guidelines that (i) each center shall implement a written policy regarding the management of iatrogenic perforations; (ii) in case of endoscopically identified perforation, the endoscopist shall report all its characteristics, endoscopic treatment that might have been possible and whether carbon dioxide or air has been used for insufflation; (iii) symptoms or signs suggestive of iatrogenic perforation after an endoscopic procedure shall be carefully evaluated and documented.2 Following these risk prevention strategies will not only reduce the diagnostic and therapeutic delays and improve the outcomes, but also decrease the number of malpractice claims related to gastrointestinal practice.
One of the limitations of the study is the absence of information regarding the total number of colonoscopies performed in non-hospital setting, making it impossible to calculate the rate of iatrogenic perforations in this situation. Another limitation is related to the lack of data regarding the characteristics of the procedures non-complicated with perforations. Both limitations are due to the retrospective design of the study and to electronic system deficiencies, hampering the analysis of possible risk factors for this endoscopic complication.
5ConclusionsPerforations in colonoscopy are rare in our clinical practice. A careful selection of the indication for treatment, differentiation in advanced therapeutic endoscopy and guidance of endoscopists in formation are needed in order to further minimize the risk of perforation.
Endoscopic closure was effective, though limited to perforations found during the procedure. The mortality was relatively low and endoscopic management did not seem to worsen it. An additional effort is necessary in order to improve the detection of perforations during the procedure and increase the likelihood of endoscopic resolution, minimizing peritoneal contamination (and progression to peritonitis) and improving surgical conditions in case this approach is required.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.