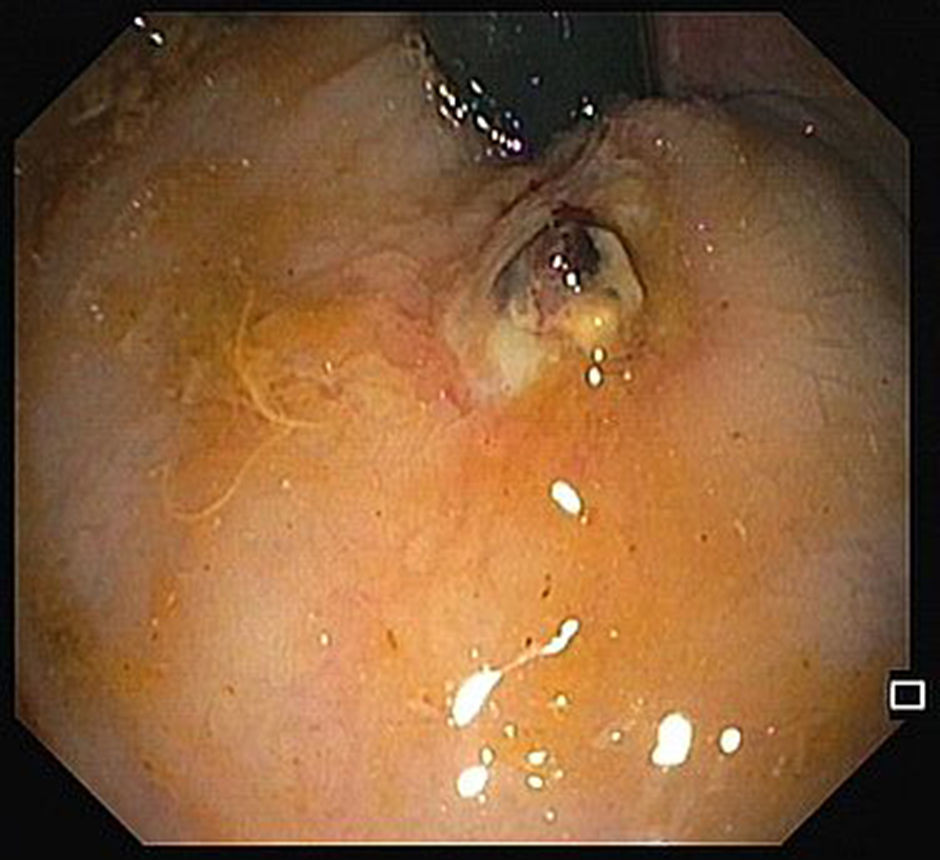
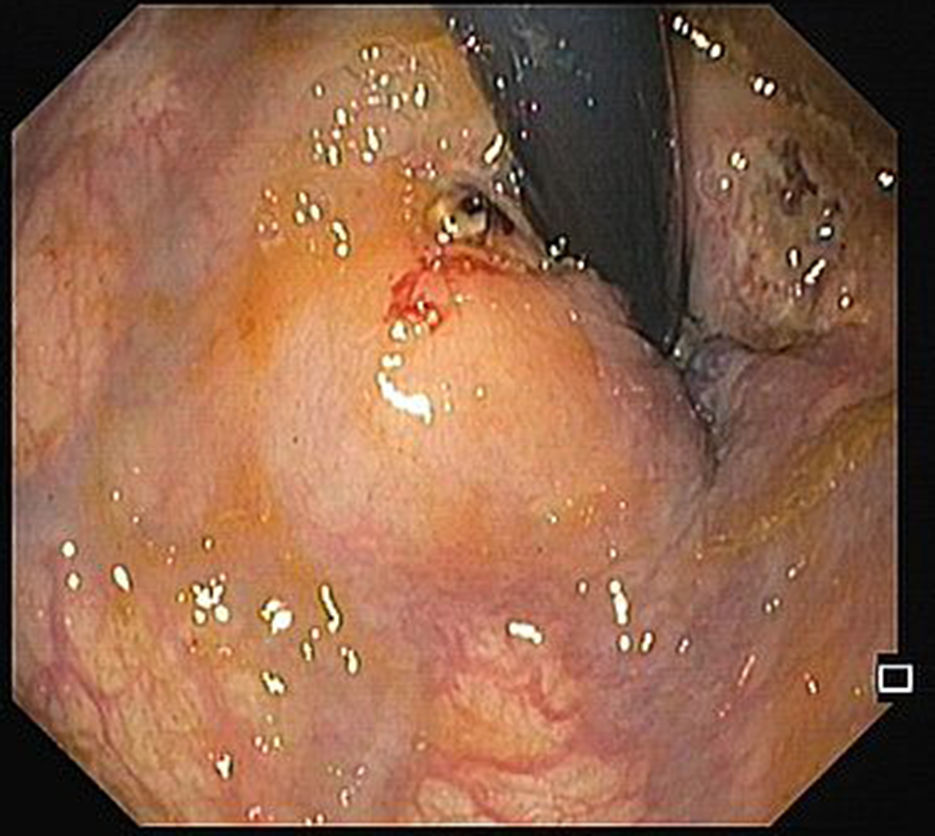
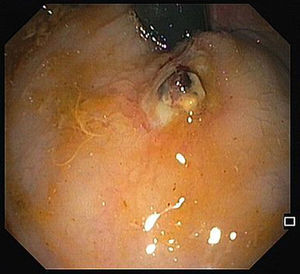
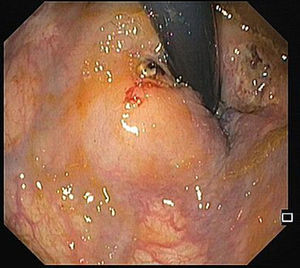
A 68-year-old male was admitted due to severe rectal bleeding. His past medical history was remarkable for an endoscopic submucosal dissection of a rectal adenoma in 2010 and hemorrhoidal disease, being submitted to two rubber ligations 16 days before. He was not under anticoagulant or antiplatelet therapies. On admission, he was hypotensive (blood pressure 95/45mmHg), with cardiac rate of 90bpm. Blood analysis showed a 4g/dL drop of hemoglobin (14.8–9.8g/dL). After hemodynamic stabilization, endoscopy was performed, that revealed absence of blood in the rectal lumen, with fecal content of normal coloration. On retroflexion, 2 ulcers were visible. One of them had a large vessel, probably arterial (Fig. 1), that was certainly the origin of the rectal bleeding. Due to the presence of the ulcer, banding was not possible, and the localization was not suitable for hemoclips placement since its proximity to the anal verge. Injection on the vessel or around it was not possible due to the presence of the ulcer. Therefore, a total of 8cc of 1% polidocanol was injected in the submucosa, in the four quadrants of the ulcer (Fig. 2). Patient was discharged 72h later, with normal blood pressure, bowel transit and no recurrence of rectal bleeding.
The risk of bleeding after hemorrhoidal band ligation is about 1–2%,1 and it usually occurs immediately after the procedure or 3–10 days, after the rubber band and the ligated tissue fall. The presence of rectal ulcers after banding is rare, and there are a few case reports of massive bleeding in patients taking antiplatelet agents.2 Our patient was not under antiplatelets, and massive rectal bleeding occurred after 16 days. The ulcer consequent to ligation exposed a large vessel, and due to the inability of other more conventional treatments and high risk of bleeding, polidocanol injection around the ulcer was attempted, with an excellent outcome.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.