Empagliflozin plays a beneficial role in individuals with type 2 diabetes at high risk of cardiovascular complications. This study aimed to assess the prevalence of individuals with type 2 diabetes who required empagliflozin based on clinical guidelines between the years 2022 and 2023.
Material and methodsThis study was a descriptive-analytical cross-sectional study conducted on a target population of patients with type 2 diabetes. Patient data, including demographic characteristics, smoking status, hypertension, hyperlipidemia, renal insufficiency, retinopathy, and proteinuria, were collected. The indication for prescribing empagliflozin was determined based on the risk of cardiovascular complications.
ResultsA total of 398 individuals with type 2 diabetes with a mean age of 58.4 years were examined. Overall, 87.4% of the patients had an indication for empagliflozin prescription. The indication for empagliflozin prescription was significantly higher in men, individuals with hyperlipidemia, those over 55 years of age, obese individuals, and smokers. The mean age, body mass index, and triglyceride levels were higher in candidates for empagliflozin prescription. Male candidates for empagliflozin had significantly higher rates of smoking and systolic blood pressure compared to females.
ConclusionsThe findings of this study demonstrated that a significant percentage of individuals with type 2 diabetes had an indication for empagliflozin prescription based on clinical and laboratory criteria.
La empagliflozina tiene un papel beneficioso en las personas con diabetes tipo 2 con alto riesgo de complicaciones cardiovasculares. Este estudio tuvo como objetivo evaluar la prevalencia de pacientes con este padecimiento que requerían empagliflozina según las guías clínicas entre los años 2022 y 2023.
Material y métodosSe trata de un estudio transversal descriptivo-analítico realizado en una población objetivo de personas con diabetes tipo 2. Se recogieron los datos de los pacientes, incluyendo las características demográficas, el hábito tabáquico, la hipertensión, la hiperlipidemia, la insuficiencia renal, la retinopatía y la proteinuria. La indicación para prescribir empagliflozina se determinó en función del riesgo de complicaciones cardiovasculares.
ResultadosSe examinaron un total de 398 individuos con diabetes tipo 2 con una edad media de 58,4 años. En general, 87,4% de estos tenía una indicación para la prescripción de empagliflozina, la cual fue significativamente mayor en los hombres, aquellos con hiperlipidemia, obesidad, los mayores de 55 años y los fumadores. La edad media, el índice de masa corporal y los niveles de triglicéridos fueron mayores en los candidatos a la prescripción de este medicamento. Los candidatos masculinos a este fármaco tenían tasas significativamente más altas de tabaquismo y presión arterial sistólica, en comparación con las mujeres.
ConclusionesLos resultados de este estudio demostraron que un porcentaje significativo de personas con diabetes tipo 2 tenía una indicación para la prescripción de empagliflozina según los criterios clínicos y de laboratorio.
Diabetes is a widespread chronic disease that is expected to affect 629 million people by 2045.1,2 According to statistics, the annual number of deaths related to this disease will reach approximately 1.59 million people by 2025.3 It can cause many deaths and complications, especially in obese individuals.4 Metformin is the first-line drug for type 2 diabetes, but it may not be enough to control blood sugar levels.5,6 Other drugs can be added to metformin, depending on the patient's condition and preferences. Empagliflozin is one of these drugs, which has benefits for the heart, kidneys, and weight.7,8 It works by blocking glucose reabsorption in the kidneys and increasing glucose excretion in the urine.9,10 It is absorbed quickly, binds to proteins, and has a short half-life.11 Empagliflozin has been shown to improve glycemic control, lower blood pressure, reduce body weight, and lower the risk of heart failure hospitalization and cardiovascular death in patients with type 2 diabetes and established cardiovascular disease.12 Empagliflozin has also been shown to reduce the risk of sustained decline in kidney function, end-stage kidney disease, cardiovascular death, and hospitalization in patients with chronic kidney disease at risk of progression.13 Empagliflozin is not recommended for patients with type 1 diabetes mellitus, as it may increase the risk of diabetic ketoacidosis (a serious condition where the body produces too much acid in the blood).14 A study has reported that the FDA accepted a supplemental New Drug Application (sNDA) for empagliflozin for the treatment of type 2 diabetes in children 10 years and older. If approved, empagliflozin would be a new oral treatment option for pediatric patients with type 2 diabetes.15 Empagliflozin can reduce the risk of death from cardiovascular causes (such as heart attack or stroke) by 38% compared to placebo. This effect was mainly driven by a lower rate of sudden cardiac death.16 Empagliflozin can improve the symptoms and quality of life of people with heart failure, as well as their physical functioning and exercise capacity.17 In Iran, limited studies have been conducted on the use and importance of empagliflozin in individuals with type 2 diabetes. Considering the drug's effects in reducing cardiovascular mortality and morbidity in people with type 2 diabetes who are at high risk of cardiovascular disease, attention to the benefits of this medication and its targeted use based on clinical guidelines is of significant importance. Given the above-mentioned factors and the limited research conducted on this topic in Iran, a study has been designed to investigate the prevalence of individuals with type 2 diabetes who require empagliflozin based on clinical guidelines among patients visiting healthcare centers in Southeast of Iran in the year (2022–2023).
Patients and methodsThe present study is a cross-sectional descriptive-analytical study that was conducted with the aim of determining the frequency on 398 patients with type 2 diabetes who needed empagliflozin drug in those referred to health centers in Southeast of Iran (Kerman). The patients were recruited in Outpatient clinics in Kerman. They were consecutively recruited between December 6, 2022, and June 5, 2023. In this study, the prevalence of people who should receive empagliflozin was considered. The inclusion criteria were having type 2 diabetes, being 50 years or older, and having consent to complete the data collection form. Out of 600 patients who were assessed for eligibility, 202 were excluded for various reasons, such as not meeting the inclusion criteria, declining to participate, or being lost to follow-up. The exclusion criteria were all non-diabetic individuals, patients with type 1 diabetes or gestational diabetes, and individuals who did not have consent to participate in the study. According to the doctor's advice, the patients may have been treated for glycemic or non-glycemic purposes. More women than men participated in the study (72% vs. 27%), which may reflect the higher prevalence and incidence of diabetes among women in Kerman. Sampling was done by available sampling method. In this Sampling, the researchers chose a sample of participants based on convenience or accessibility, rather than using a random or systematic method. This type of sampling is also known as purposive sampling or non-probability sampling. It is often used when the population is hard to reach or when there are time or cost constraints. Then patients entered the study after obtaining informed consent and maintaining confidentiality of information.
To collect data, a data collection form includes sociodemographic and anthropometric data (age, gender, body mass index), comorbidities and associated cardiovascular risk factors (hypertension, hyperlipidemia, smoking, etc.), clinical data (time from diabetes onset, target organ damage microvascular (retinopathy, proteinuria) and macrovascular), blood pressure (in mmHg), biologic parameters (glomerular filtration rate and lipid profile) is completed. Based on the international guidelines, two categories of patients can be treated with empagliflozin: (1) patients with type II diabetes that are at very high risk of cardiovascular disease. This category includes 3 subsets:
- a.
Patients with atherosclerotic cardiovascular disease (ASCVD): in this study, 110 people (27.6%) had this disease.
- b.
Patients with target organ damage (TOD) (either chronic kidney disease, retinopathy and proteinuria): It included 108 people (27.1%) with TOD, including 22 people (5.5%) with CRF, 72 people (18.1%) with retinopathy, and 58 people (14.6%) with proteinuria.
- c.
Having three or more major risk factors, including age over 55 years, hyperlipidemia, smoking and obesity: age over 55 years (260 people or 65.3%), hyperlipidemia (234 people or 58.8%), smoking (34 people or 8.5%), and obesity (108 people or 27.1%). A total of 202 people (50.8%) were candidates for prescribing this drug.
(2) Patients with type II diabetes that are at high risk of cardiovascular disease, who are those that have had diabetes for more than 10 years and do not have any of the high-risk factors.
Statistical analysis was performed using SPSS 26 software. Descriptive statistics indices including frequency, percentage, mean and standard deviation were used to describe the research data and analytical statistics indices including Chi-square test and independent t-test were used for statistical analysis. P-value less than 0.05 was considered statistically significant.
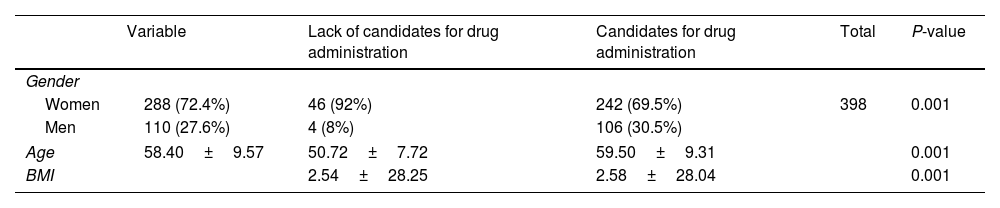
ResultsThe study included a total of 398 patients with type 2 diabetes, of whom 288 (72.4%) were women and 110 (27.6%) were men. The average age of patients was (58.40%±9.57) years old. The results of the study showed that 348 (87.4%) of the patients with type 2 diabetes needed empagliflozin. Among the candidates for empagliflozin, 69.5% were women and 30.5% were men. Among men, 106 out of 110 men (96%) were candidates for empagliflozin, while among women, 242 out of 288 women (84%) were candidates for empagliflozin. This observation is due to the higher number of women in the study sample. The difference between men and women was statistically significant (P<0.001). The average age of the candidates for empagliflozin (59.5 years old) was higher than that of those who did not need empagliflozin (50.72 years old), and this difference was statistically significant (P<0.001). The average body mass index (BMI) of the candidates for empagliflozin (28.04) was higher than that of those who did not need empagliflozin (25.28), and this difference was statistically significant (P<0.001) (Table 1).
Demographic characteristics of empagliflozin candidate and non-candidate patients.
| Variable | Lack of candidates for drug administration | Candidates for drug administration | Total | P-value | |
|---|---|---|---|---|---|
| Gender | |||||
| Women | 288 (72.4%) | 46 (92%) | 242 (69.5%) | 398 | 0.001 |
| Men | 110 (27.6%) | 4 (8%) | 106 (30.5%) | ||
| Age | 58.40±9.57 | 50.72±7.72 | 59.50±9.31 | 0.001 | |
| BMI | 2.54±28.25 | 2.58±28.04 | 0.001 | ||
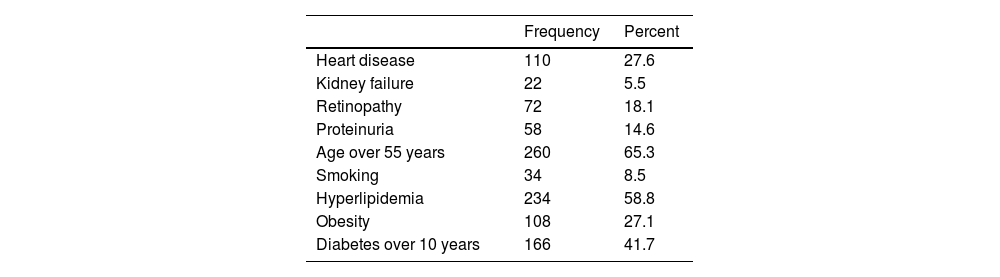
The second category includes patients at high risk of cardiovascular disease who have had diabetes for more than 10 years and do not have any of the high-risk factors. A total of 84 people (21.1%) had diabetes for more than 10 years without any of the high-risk factors. The distribution of all prescription criteria is presented in Table 2.
Determining the frequency of criteria related to the indication of prescribing the drug empagliflozin in people with type 2 diabetes.
| Frequency | Percent | |
|---|---|---|
| Heart disease | 110 | 27.6 |
| Kidney failure | 22 | 5.5 |
| Retinopathy | 72 | 18.1 |
| Proteinuria | 58 | 14.6 |
| Age over 55 years | 260 | 65.3 |
| Smoking | 34 | 8.5 |
| Hyperlipidemia | 234 | 58.8 |
| Obesity | 108 | 27.1 |
| Diabetes over 10 years | 166 | 41.7 |
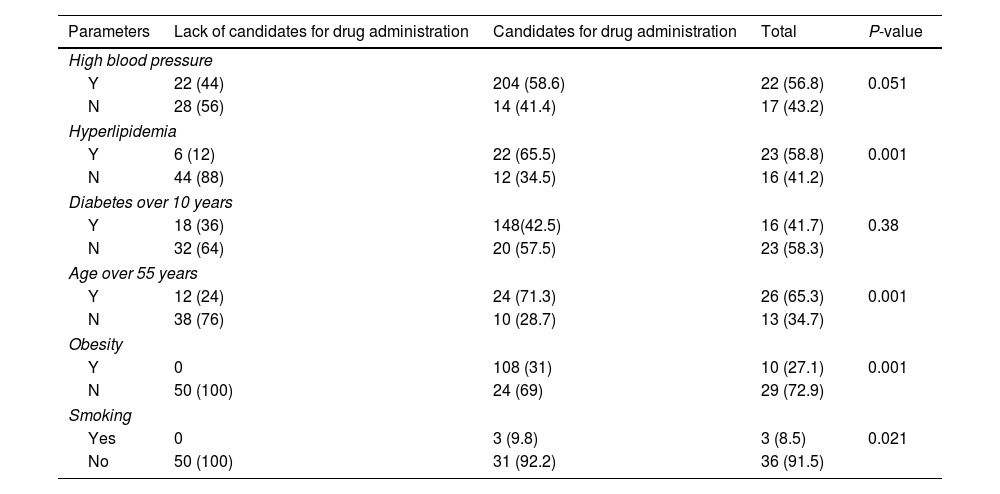
By comparing clinical characteristics of patients candidates and non-candidates for empagliflozin treatment, significant differences were found regarding high blood pressure (P=0.051), hyperlipidemia (P=0.001), over 55 years old (P=0.001), obese (P=0.001) and smokers (P=0.021) (Table 3).
Determining the frequency of people with type 2 diabetes requiring empagliflozin based on different parameters.
| Parameters | Lack of candidates for drug administration | Candidates for drug administration | Total | P-value |
|---|---|---|---|---|
| High blood pressure | ||||
| Y | 22 (44) | 204 (58.6) | 22 (56.8) | 0.051 |
| N | 28 (56) | 14 (41.4) | 17 (43.2) | |
| Hyperlipidemia | ||||
| Y | 6 (12) | 22 (65.5) | 23 (58.8) | 0.001 |
| N | 44 (88) | 12 (34.5) | 16 (41.2) | |
| Diabetes over 10 years | ||||
| Y | 18 (36) | 148(42.5) | 16 (41.7) | 0.38 |
| N | 32 (64) | 20 (57.5) | 23 (58.3) | |
| Age over 55 years | ||||
| Y | 12 (24) | 24 (71.3) | 26 (65.3) | 0.001 |
| N | 38 (76) | 10 (28.7) | 13 (34.7) | |
| Obesity | ||||
| Y | 0 | 108 (31) | 10 (27.1) | 0.001 |
| N | 50 (100) | 24 (69) | 29 (72.9) | |
| Smoking | ||||
| Yes | 0 | 3 (9.8) | 3 (8.5) | 0.021 |
| No | 50 (100) | 31 (92.2) | 36 (91.5) | |
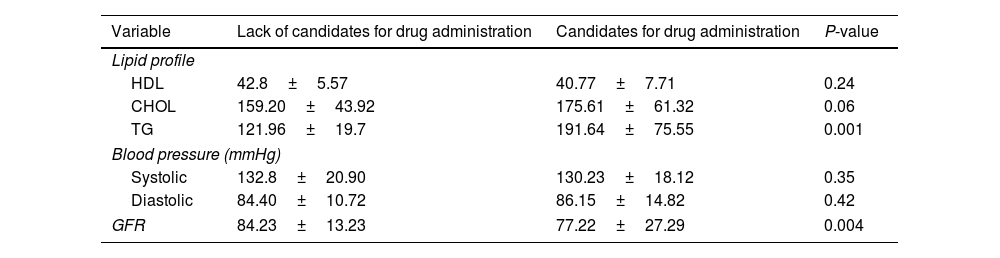
Regarding differences in the lipid profile and GFR, the average levels of lipid profile were higher (P=0.001), and the average levels of GFR were lower (P=0.001), for empagliflozin candidates compared with non-candidates (Table 4).
Determining the frequency of people with type 2 diabetes requiring empagliflozin on the level of lipid profile (HDL, CHOL and TG), blood pressure (systolic and diastolic) and glomerular filtration.
| Variable | Lack of candidates for drug administration | Candidates for drug administration | P-value |
|---|---|---|---|
| Lipid profile | |||
| HDL | 42.8±5.57 | 40.77±7.71 | 0.24 |
| CHOL | 159.20±43.92 | 175.61±61.32 | 0.06 |
| TG | 121.96±19.7 | 191.64±75.55 | 0.001 |
| Blood pressure (mmHg) | |||
| Systolic | 132.8±20.90 | 130.23±18.12 | 0.35 |
| Diastolic | 84.40±10.72 | 86.15±14.82 | 0.42 |
| GFR | 84.23±13.23 | 77.22±27.29 | 0.004 |
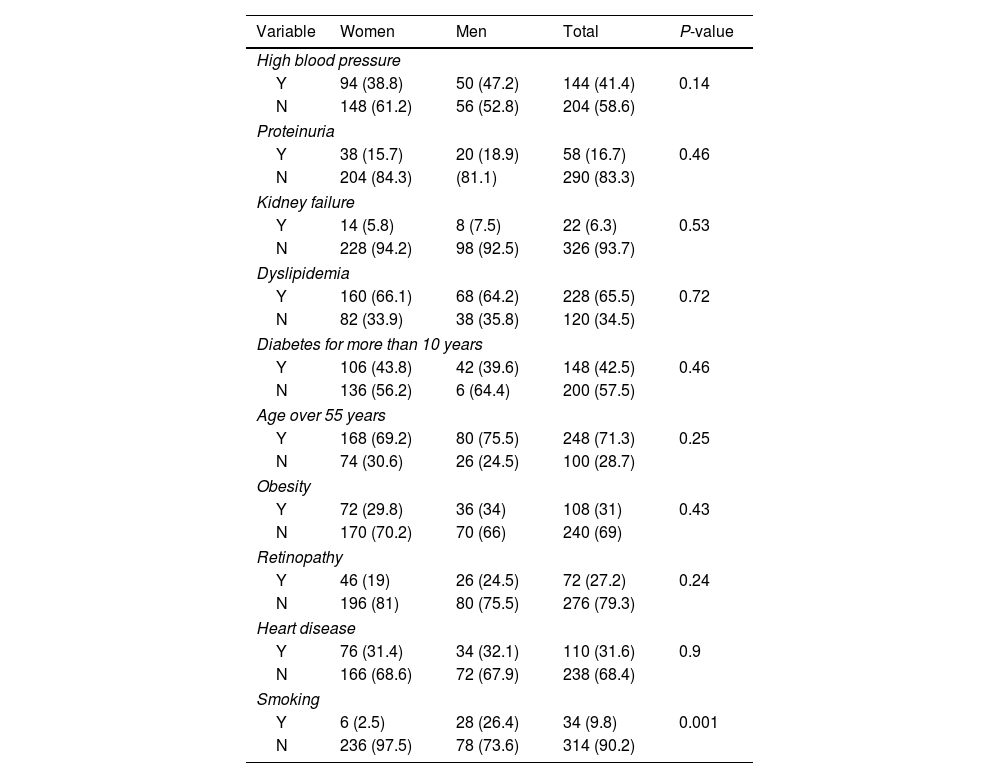
Focusing on candidates to be treated with empagliflozin, among the different variables, 6 people of the women and 28 people of the men smoked and this difference was statistically significant (P=0.001) (Table 5).
Determining the frequency of different variables in women and men with type 2 diabetes requiring empagliflozin according to gender.
| Variable | Women | Men | Total | P-value |
|---|---|---|---|---|
| High blood pressure | ||||
| Y | 94 (38.8) | 50 (47.2) | 144 (41.4) | 0.14 |
| N | 148 (61.2) | 56 (52.8) | 204 (58.6) | |
| Proteinuria | ||||
| Y | 38 (15.7) | 20 (18.9) | 58 (16.7) | 0.46 |
| N | 204 (84.3) | (81.1) | 290 (83.3) | |
| Kidney failure | ||||
| Y | 14 (5.8) | 8 (7.5) | 22 (6.3) | 0.53 |
| N | 228 (94.2) | 98 (92.5) | 326 (93.7) | |
| Dyslipidemia | ||||
| Y | 160 (66.1) | 68 (64.2) | 228 (65.5) | 0.72 |
| N | 82 (33.9) | 38 (35.8) | 120 (34.5) | |
| Diabetes for more than 10 years | ||||
| Y | 106 (43.8) | 42 (39.6) | 148 (42.5) | 0.46 |
| N | 136 (56.2) | 6 (64.4) | 200 (57.5) | |
| Age over 55 years | ||||
| Y | 168 (69.2) | 80 (75.5) | 248 (71.3) | 0.25 |
| N | 74 (30.6) | 26 (24.5) | 100 (28.7) | |
| Obesity | ||||
| Y | 72 (29.8) | 36 (34) | 108 (31) | 0.43 |
| N | 170 (70.2) | 70 (66) | 240 (69) | |
| Retinopathy | ||||
| Y | 46 (19) | 26 (24.5) | 72 (27.2) | 0.24 |
| N | 196 (81) | 80 (75.5) | 276 (79.3) | |
| Heart disease | ||||
| Y | 76 (31.4) | 34 (32.1) | 110 (31.6) | 0.9 |
| N | 166 (68.6) | 72 (67.9) | 238 (68.4) | |
| Smoking | ||||
| Y | 6 (2.5) | 28 (26.4) | 34 (9.8) | 0.001 |
| N | 236 (97.5) | 78 (73.6) | 314 (90.2) | |
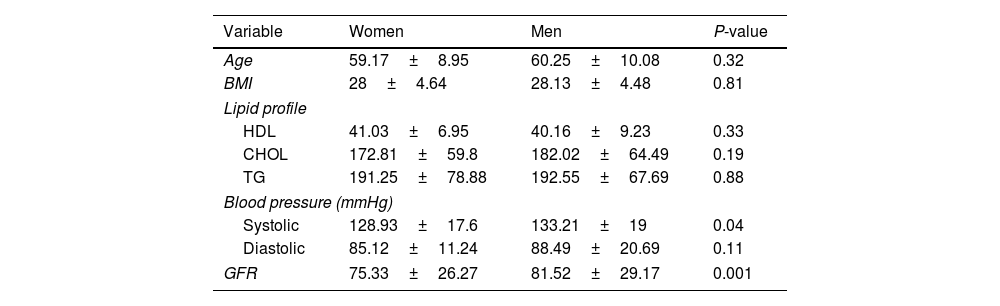
The mean blood pressure and GFR of women and men who were candidates for the drug empagliflozin were statistically significant, respectively (P=0.04) (P=0.001) (Table 6).
Determining the average of different variables in women and men with type 2 diabetes requiring empagliflozin.
| Variable | Women | Men | P-value |
|---|---|---|---|
| Age | 59.17±8.95 | 60.25±10.08 | 0.32 |
| BMI | 28±4.64 | 28.13±4.48 | 0.81 |
| Lipid profile | |||
| HDL | 41.03±6.95 | 40.16±9.23 | 0.33 |
| CHOL | 172.81±59.8 | 182.02±64.49 | 0.19 |
| TG | 191.25±78.88 | 192.55±67.69 | 0.88 |
| Blood pressure (mmHg) | |||
| Systolic | 128.93±17.6 | 133.21±19 | 0.04 |
| Diastolic | 85.12±11.24 | 88.49±20.69 | 0.11 |
| GFR | 75.33±26.27 | 81.52±29.17 | 0.001 |
In this study, we examined nearly 400 patients with type 2 diabetes to investigate the distribution of cardiovascular risk factors and indications for prescribing empagliflozin in these patients. The assessment of patients for empagliflozin prescription indications revealed that a total of 87.4% of individuals with type 2 diabetes had indications for empagliflozin prescription. Considering the high distribution of cardiovascular risk factors in our population, particularly among those with type 2 diabetes, it is expected that many of these patients, who meet the criteria for empagliflozin prescription, will benefit from this medication. Nowadays, cardiovascular diseases are considered the primary cause of disability and mortality in patients with type 2 diabetes. The current management and treatment of diabetes involve a comprehensive and patient-centered approach, aiming to control hyperglycemia and other risk factors for cardiovascular diseases, such as hypertension, obesity, and dyslipidemia.18,19 Among the pharmacological factors, sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the established antidiabetic medications used in the treatment of type 2 diabetes. However, gliflozins or flozins can be distinguished from other antidiabetic drugs because of their unique mechanism of action, independent of insulin (by increasing glucosuria). In addition to reducing plasma glucose levels, they also improve several other metabolic and hemodynamic abnormalities that are risk factors for cardiovascular diseases.20 Various clinical trials have demonstrated that empagliflozin significantly improves glycemic control, reduces weight, and improves blood pressure in patients with type 2 diabetes.21,22 Therefore, this medication has been proposed as a beneficial option for the treatment of diabetes, especially in individuals at risk of cardiovascular diseases.
Obesity is considered one of the risk factors for the development of cardiovascular diseases in individuals with type 2 diabetes, and it holds significant importance as one of the relevant criteria in assessing patients for empagliflozin prescription. The findings of the current study demonstrated that the mean body mass index (BMI) of patients eligible for empagliflozin prescription (28.04) was higher than that of patients who did not require empagliflozin prescription (25.28). Furthermore, in the group with indications for empagliflozin prescription, 31% of the patients were diagnosed with obesity. Previous studies have shown that empagliflozin not only has beneficial effects on glycemic control in patients with type 2 diabetes but also contributes to weight reduction. For example, Liakos et al., in a systematic review and meta-analysis analyzing data from over 6200 patients from 10 different studies, demonstrated that empagliflozin prescription was associated with a significant reduction in weight compared to placebo in patients with diabetes.23 In another study, Haring et al. demonstrated that adding empagliflozin to the diet therapy with metformin over a 24-week period resulted in a significant weight reduction compared to placebo. The weight changes with a 10mg dose of the drug were reported to be 2.08kg, with a 25mg dose being 2.46kg, and with the placebo only 0.17kg.21 Heise et al. state that the weight loss following empagliflozin prescription first occurs due to fluid loss and then due to caloric loss resulting from increased urinary glucose excretion.24 Indeed, dyslipidemia is also considered one of the important risk factors for cardiovascular diseases in individuals with type 2 diabetes, and as such, it is taken into account as one of the relevant criteria in assessing patients for empagliflozin prescription. Our study findings indicate that the mean triglyceride levels of patients eligible for empagliflozin prescription (191.64) were significantly higher than those of patients who did not require empagliflozin prescription (121.96), and overall, 228 individuals (65.5%) among the candidates for empagliflozin had hyperlipidemia compared to only 12% of individuals who were not candidates for the drug. Previous study findings have shown inconsistent results regarding the efficacy of this drug on lipid profiles. The findings of previous studies have shown inconsistent results regarding the efficacy of this drug on lipid profiles. For example, in their study, Roden et al. did not observe a significant difference in changes triglycerides or the LDL to HDL ratio during treatment with empagliflozin compared to placebo.25 Similarly, in another comprehensive study conducted by Zinman et al., a slight increase in both LDL and HDL was reported following empagliflozin treatment.26 This increase is believed to be due to hemoconcentration resulting from the use of these drugs, but the clinical significance of these changes is not yet fully understood. Overall, it seems that the cardiovascular protective effects of this drug may be independent of its direct effects on the lipid profile of patients.27
Kidney dysfunction is another organ impairment considered as a risk factor in prescribing empagliflozin to patients with diabetes. Our study showed that the mean glomerular filtration rate (GFR) of patients eligible for empagliflozin prescription (77.22) was lower than that of patients who did not require empagliflozin prescription (84.23), and this difference was statistically significant. Generally, degrees of kidney dysfunction occur in nearly 35% of patients with type 2 diabetes, and this condition is associated with increased mortality. Controlling the patient's blood glucose levels plays a crucial role in preventing the progression of kidney disorders. In a study conducted by Wanner et al., it was reported that patients receiving empagliflozin had a significantly lower risk of progressing toward macroalbuminuria or clinically relevant kidney-related outcomes, such as an increase in serum creatinine levels or the need for kidney dialysis, compared to the placebo group.28 Therefore, prescribing this drug can be effective in reducing the progression of kidney dysfunction in patients with diabetes. Various factors can contribute to these beneficial effects of empagliflozin. The drug leads to a decrease sodium reabsorption in the proximal tubule, resulting in an increase in sodium transfer to the macula densa. This can effectively activate tubuloglomerular feedback, leading to vascular regulation and a reduction in hyperfiltration. On the other hand, the effects of this medication on arterial stiffness and vascular resistance can also be involved in the protective effects of the drug on kidney function.28,29
Our study also demonstrated that among men with type 2 diabetes, 106 out of 110 men (96%) were candidates for receiving empagliflozin, whereas this proportion was 242 out of 288 women (84%) in females. This statistically significant difference indicates that men were significantly more likely to be candidates for empagliflozin compared to women. To investigate which of the risk factors have different frequency distributions between men and women, our study examined the frequency of each risk factor separately by gender. The results showed that 6 individuals (2.5%) of the women and 28 individuals (26.4%) of the men who participated in the study were candidates for empagliflozin and were smokers. This difference was statistically significant, indicating that smoking was significantly more prevalent among diabetic men compared to diabetic women. Furthermore, the mean systolic blood pressure among women who were candidates for drug prescription was 128.93±17.6, while it was 133.21±19 in men who were candidates for drug prescription. Therefore, it appears that smoking and high blood pressure are two important factors contributing to the increased risk of cardiovascular diseases in individuals with type 2 diabetes, and consequently, a higher frequency of empagliflozin prescription indication in men compared to women. This difference was statistically significant, indicating a significantly higher systolic blood pressure in men compared to women.
The findings from previous studies on the effectiveness of gliflozins also indicated that men had a higher frequency of drug prescription indications. For example, in the study by Wiviott and colleagues, nearly 63% of individuals with diabetes who were candidates for drug prescription were men.30 In the study by Zinman, men accounted for 71.5% of the candidates.26 Overall, these findings may reflect a higher prevalence of cardiovascular risk factors in men at the community level compared to women. Consequently, considering the higher prevalence of certain risk factors in men, empagliflozin prescription may be more frequently indicated in these individuals.
ConclusionThe findings of the current study demonstrated that a significant percentage of individuals with type2 diabetes had indications for the prescription of empagliflozin based on clinical and laboratory criteria. Therefore, paying attention to cardiovascular risk factors in patients with type 2 diabetes and subsequently prescribing gliflozins, should be considered to achieve therapeutic goals in controlling the glycemic status of the patient and reducing diabetes-related cardiovascular complications.
Strengths and limitationsThe strengths of this study can be mentioned as follows: in this research used a large sample size of 398 patients with type 2 diabetes, which increases the statistical power and generalizability of the results. This study also used objective and standardized criteria to determine the indication for empagliflozin prescription, which reduces the bias and subjectivity of the assessment. Our study examined the association of empagliflozin prescription with various demographic and clinical factors, which provides a comprehensive and detailed picture of the target population. The limitation of this study was the cross-sectional nature of the study, which limits the ability to establish causality and temporality between empagliflozin prescription and cardiovascular outcomes.
FundingThis research has not received any fund.
Conflict of interestAll authors of this manuscript say that they have no conflicts of interest to disclose.
SMKH, GY, FB and AD designed, read, and approved the final version of the manuscript. The authors would like to thank Kerman University of Medical Sciences for concerning this manuscript.