We present a case of liver abscess in a 34-week preterm infant after umbilical venous catheterization (UVC). The infant had clinical symptoms of intestinal sepsis, with an encapsulated hypodense hepatic focal lesion with air inside, as observed by abdominal tomography. Staphylococcus aureus was isolated in blood cultures. Liver abscess is a rare complication associated with umbilical catheterization and can be prevented by implementing an appropriate prevention program.
Presentamos un caso de un absceso hepático en una prematura de 34 semanas. La niña presentó síntomas clínicos de sepsis intestinal con una imagen de lesión hipodensa conteniendo aire en hígado en la tomografía de abdomen. Se aisló Staphylococcus aureus en cultivos de sangre. El absceso secundario a colocación de catéter venoso umbilical es infrecuente y puede ser prevenido con un programa preventivo adecuado.
Liver abscess secondary to umbilical catheterization is an infrequent, preventable, and highly lethal complication, which increases hospital stays, morbidity and mortality. It is generally caused by bacteria and fungi, but in some cases there is not microbiological isolation.1,2 Its usual location is in the right hepatic lobe.1–7 Fewer than 60 cases have been reported between 1972 and 2013, most of them in the United States (USA), India, France and Venezuela.1,2 Associated risk factors are prematurity, immunodeficiency, malposition of the umbilical catheter and infusion of hypertonic fluids.1–7
Description of the caseIn February, 2011, an 8-day-old, 34-week preterm infant originating from Aruba was admitted to the neonatal intensive care unit (NICU). She was the product of the second pregnancy of a healthy 23 years old mother with prenatal care, delivered by emergency C-section due to preeclampsia. She received 2 doses of pulmonary maturation; the dose and type of steroid were not reported. Birth weight was 1654g, length 42cm, head circumference 31cm, Apgar 9-9-10/10. The referring site began early per os (by mouth) and placed a umbilical venous catheter (UVC) for total parenteral nutrition (TPN). Hypoglycemia was corrected and indirect hyperbilirubinemia was treated with phototherapy for two days. Brain ultrasound showed grade I intraventricular hemorrhage and the echocardiogram was reported as normal. Continuous positive airway pressure (CPAP) was begun due to persistent apneas, and ampicillin was started and later replaced with flucloxacillin due to isolation of Staphylococcus aureus in blood. She developed abdominal distension without necrotizing enterocolitis (NEC) images, and per os was suspended. She developed anemia which required transfusions, and thrombocytopenia without repercussions or transfusions. Antibiotic treatment was stepped up to meropenem for 24h, after which, she was referred to a tertiary level due to suspected NEC.
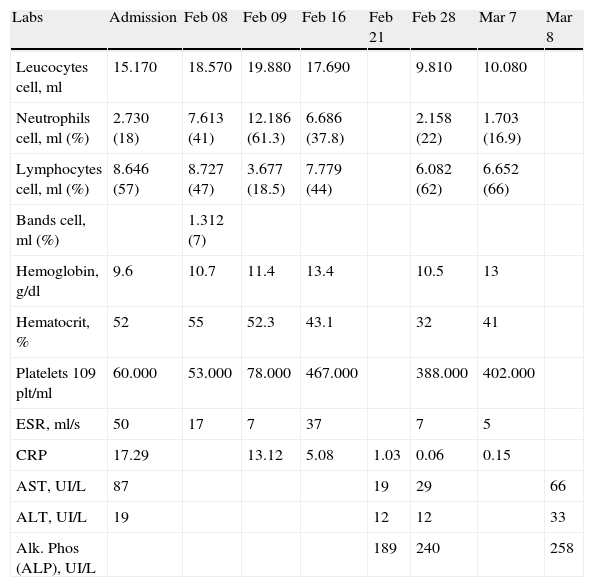
On admission to our NICU she was pale, with respiratory distress, without tachycardia, with systolic heart murmur, audible bowel sounds, and soft and distended abdomen with a visible venous network and a painful hepatomegaly 4cm below the right costal margin, abdominal circumference of 32cm, weight 2090g and length 43cm. She continued nothing by mouth, with intravenous fluids, oxygen and open orogastric tube. She was admitted with anemia and thrombocytopenia without clinical repercussions and with elevated levels of erythroid sedimentation rate (ESR), C-reactive protein (CRP) and aspartate transferase (AST) enzymes. The renal and thyroid function was within normal limits for age, but she presented severe hypoglycemia and hypercalcemia, which were corrected. After 24h of hospitalization, she developed bands on the CBC with an increase in the severity of thrombocytopenia without repercussion (see Table 1). Congenital infection with syphilis, Toxoplasma, rubella, cytomegalovirus, herpes I and II or human immunodeficiency virus type 1 and 2 was ruled out. Two blood cultures were drawn and piperacillin, tazobactam and vancomycin were started.
Labs drawn during her NICU stay.
| Labs | Admission | Feb 08 | Feb 09 | Feb 16 | Feb 21 | Feb 28 | Mar 7 | Mar 8 |
| Leucocytes cell, ml | 15.170 | 18.570 | 19.880 | 17.690 | 9.810 | 10.080 | ||
| Neutrophils cell, ml (%) | 2.730 (18) | 7.613 (41) | 12.186 (61.3) | 6.686 (37.8) | 2.158 (22) | 1.703 (16.9) | ||
| Lymphocytes cell, ml (%) | 8.646 (57) | 8.727 (47) | 3.677 (18.5) | 7.779 (44) | 6.082 (62) | 6.652 (66) | ||
| Bands cell, ml (%) | 1.312 (7) | |||||||
| Hemoglobin, g/dl | 9.6 | 10.7 | 11.4 | 13.4 | 10.5 | 13 | ||
| Hematocrit, % | 52 | 55 | 52.3 | 43.1 | 32 | 41 | ||
| Platelets 109 plt/ml | 60.000 | 53.000 | 78.000 | 467.000 | 388.000 | 402.000 | ||
| ESR, ml/s | 50 | 17 | 7 | 37 | 7 | 5 | ||
| CRP | 17.29 | 13.12 | 5.08 | 1.03 | 0.06 | 0.15 | ||
| AST, UI/L | 87 | 19 | 29 | 66 | ||||
| ALT, UI/L | 19 | 12 | 12 | 33 | ||||
| Alk. Phos (ALP), UI/L | 189 | 240 | 258 |
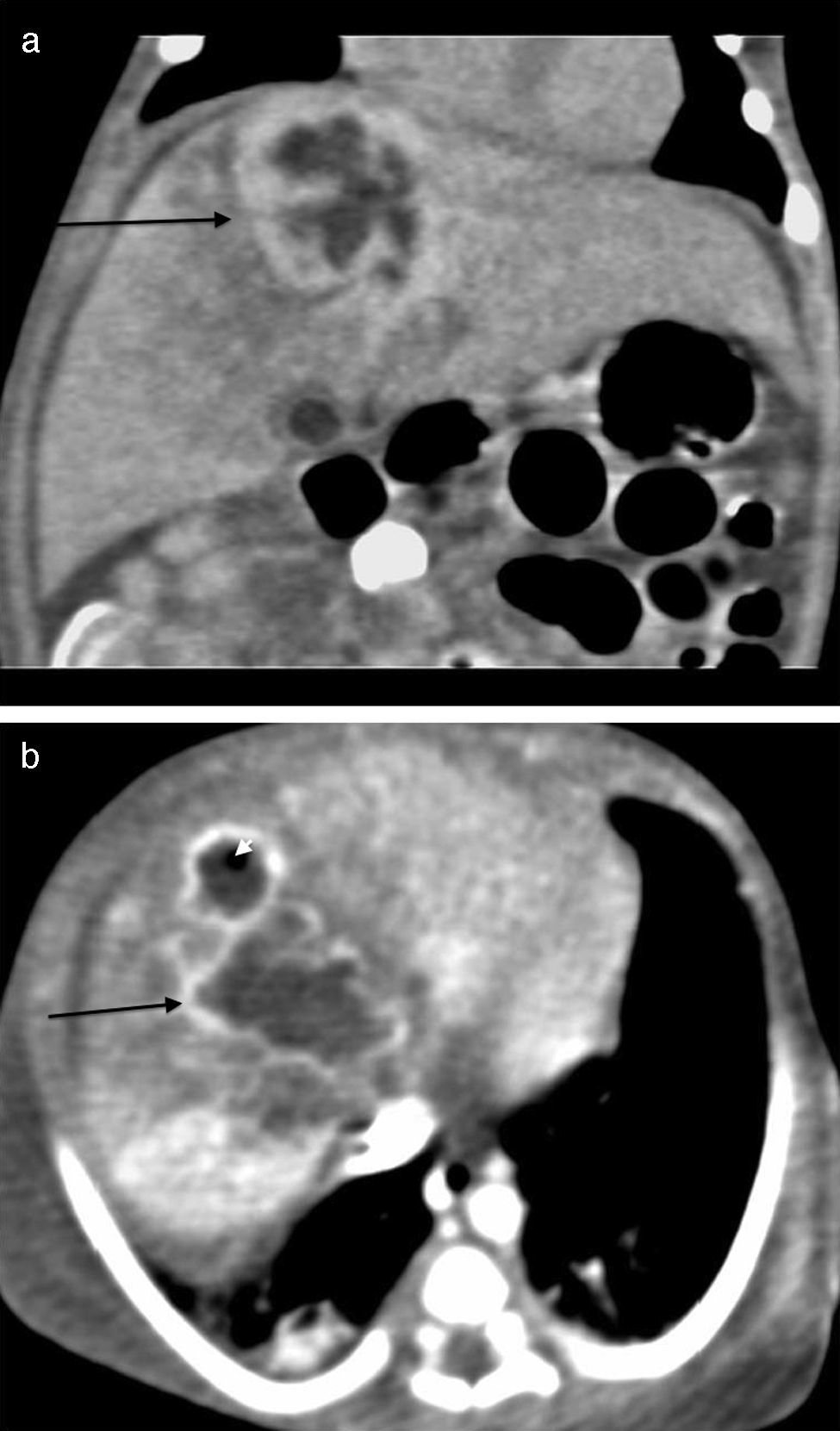
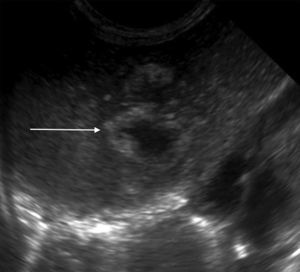
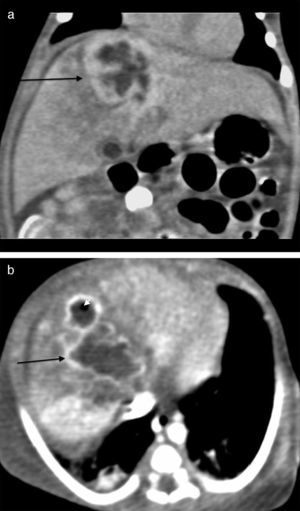
The initial abdominal ultrasound showed a focal hepatic lesion in segment IVA and VIII (see Fig. 1) and the abdominal scan with contrast showed an image compatible with an encapsulated hepatic abscess (see Fig. 2a and b). Methicillin sensitive S. aureus was isolated in blood and oropharynx (Bac/ALERT FA Plus machine; USA). Oxacillin scheme was included and subsequent negative blood cultures were obtained 72h later. She showed good clinical and paraclinical progress with resolution of the anemia and thrombocytopenia, normalization of AST, ESR, and CRP (see Table 1), and she continued with IV antibiotics without percutaneous drainage of the abscess.
Abodminal ultrasound. Multiple focal, confluent, echogenic lesions, in segments IVA and VIII, with a thick echogenic wall and hypoechoic center, with a combined 29mm diameter, corresponding to the abscess (arrow); and a satellite lesion is visible (arrowhead), without an increased interior flow on echo doppler color.
Abdominal scan with contrast. (A) Axial plane acquisition in portal phase: Focal lesion in segment VIII (arrow) with a 23mm×10mm diameter, hypodense liquid content with capsular highlighting and interior air (arrowhead) associated with adjacent hepatic parenchymal hypodensity due to altered perfusion. (B) Delayed coronal reconstruction which shows the thick capsule.
The brain ultrasound, electrocardiogram and follow-up echocardiogram were normal. In addition, primary cellular immunodeficiency (PI), hypogammaglobulinemia and chronic granulomatous disease were ruled out in the patient and the mother. After finishing the 21-day IV antibiotic cycle, she completed six weeks with cephalexin. The clinical, paraclinical and imaging progress was adequate, with a decrease in the size of the hepatic lesion to 16mm in segment VIII. The lesion disappeared in the next follow-up ultrasound.
DiscussionHepatic abscess secondary to umbilical venous catheterization is an infrequent and lethal complication produced by an ascending infection which enters the portal system and usually affects the right lobe of the liver, due to its segmental vascular distribution. Fifty percent are solitary lesions.1,2 They can be homogenous, solitary, well-defined circular masses or heterogenous, multiloculated, poorly defined, septated masses, with detritus or even air inside.1–7 The reported etiologic agents are 37% gram positive cocci (distributed in S. aureus 21% and Staphylococcus epidermidis 16%) with a latent clinical evolution, such occurred in our case; gram negative bacilli in 36% with greater systemic involvement and higher mortality; fungi in 12.5% associated with a fatal outcome, and approximately 10% of cases without microbial isolation, with a subacute evolution.1–7 Isolation in blood was performed by colorimetry on Bact/ALERT equipment, which has high analytical sensibility, growth efficiency and microbiologic recovery. Clinically, they develop abdominal distension, painful hepatomegaly and nonspecific signs of sepsis such as irritability, altered heart rate, and dysthermias related to altered acute phase reactants, leukocyte counts and platelet counts, as we observed in our patient.
The majority of cases reported until now were from USA, India, France and Venezuela, which reflects a greater reporting culture rather than a higher incidence.1–7 The literature lists as risk factors: prematurity, low birth weight, immune lability secondary to prematurity, NEC, primary or acquired immunodeficiency. Chronic granulomatous disease should be ruled out by using the flow cytometric dihydro-rhodamine 123 test,8 as well as invasive procedures, such as TPN administration, infusion of hypertonic solutions and antibiotics.1–7
This case report coincides with the literature in two risk factors: 34-week gestation prematurity and placement of a UVC with TPN and antibiotic infusion, with prematurity being non modifiable in the NICU. Precautions such as the isolation of the catheter emplacement and a correct location at the vena cava, above the ductus venosus and suprahepatic veins and below the level of the right atrium,9 with radiologic control to avoid hepatic intraparenchymal migration and infections related to the device, should be taken. These recommendations are in accord with the guidelines published in 2011 by the IDSA (Infectious Diseases Society of America)10 and includes adequate hand washing,11 and washing of surfaces and medical equipment in the NICU with 4% clorhexidine prior to the procedure, given the increased incidence in the NICU of colonization and infection by methicillin resistant S. aureus (MRSA) of intra or extra-institutional origin, which could be a cause of neonatal bacterial endocarditis.12,13
Regarding the treatment received by our patient, IV antibiotic treatment for three weeks was considered due to her high risk secondary to prematurity, weight, and latent clinical, paraclinical and imaging involvement on admission. She completed a total of 6 weeks abx treatment per os with strict follow-up since she was not a candidate for percutaneous drainage due to an adequate response to medical management, abscess size less than 6cm, location in the right lobe of the liver, no perforation and lack of associated immunodeficiency.14
In conclusion, literature review and our experience with present case, indicate that hepatic abscess related to umbilical venous catheterization is an infrequent but lethal situation where clinician should be aware. A precise indication for catheter placement along with precautions such as a correct location of the catheter and strict radiologic follow-up, will decrease potential complications.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare not to have any conflict of interest.
The authors would like to thank the Department of Radiology from the “Fundación Cardio Infantil” who kindly gave access to the images of this case.