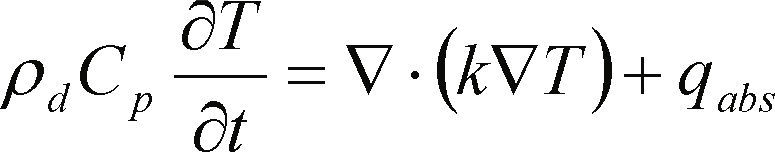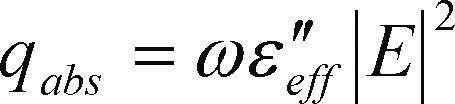Microwave range had been widely used for heating processes. In this work theoretical and experimental results on manufacturing of expandable polystyrene using specific frequencies of 2.45GHz are presented. Simulations of temperature distribution were performed using the software Comsol Multiphysics®. In order to increase the material absorption to microwaves, several solvents as ethanol, a mixture of ethanol/water and hydrogen peroxide were tested. Temperature distribution inside material was measured with an infrared laser sensor. Microwave heating allows Expanded Polystyrene Styrofoam (EPS) molding in 180seconds, reaching a considerable manufacturing time reduction compared with traditional processes where drying time may take up to one day.
El rango de microondas se ha utilizado ampliamente para procesos de calentamiento. En este trabajo se presentan estudios teóricos y prácticos de la manufactura de poliestireno expandible mediante calentamiento por microondas a 2.45GHz. Se realizaron simulaciones de incremento de temperatura y su distribución en el material utilizando el software Comsol Multiphysics®. Con la finalidad de incrementar la absorción del material a microondas, se realizaron experimentos con diferentes solventes tales como etanol, agua oxigenada y agua purificada. Se midió la distribución de temperatura dentro del material mediante un sensor de láser infrarrojo. El calentamiento por microondas del material permite el espumado del poliestireno expandible (EPS) en 180 segundos, alcanzando una reducción considerable del tiempo de fabricación en comparación con procesos tradicionales en los cuales el tiempo de secado puede durar hasta un día.
Polystyrene foam is a polymer produced from styrene monomer known as styrofoam; their use in food and electronics packaging, airplane and automotive parts, sporting equipment, among others, it has become a trend last years due to its different advantages as light weight (reduced bulk density), easy to form, low thermal conductivity, and low cost of production (Warsiki et al., 2012; Tsivintzelis et al., 2007). Commercial interest in polymeric foams has been increased due to new emerging applications and the ability to foam a variety of polymeric materials or composites. Despite their success, the continuous growth in research of foamed polymers into new markets depends on the ability to enhance control over its mechanical structure and performance (Emami et al., 2014).
Conventional polymer foaming process usually consist of polymer matrix saturation with a fluid (solvent) and its volatilization by increasing temperature, which induce phase separation, resulting in formation (nucleation) and growth of pores inside the polymer matrix (Tsivintzelis and Panayiotou, 2013). Polystyrene foaming has been extensively studied, for example, recently Nistor et al. (2013) prepared micro-cellular PS foams and studied the influence of toluene residua on the foam structure, using CO2 as high-pressure inductor. They observed that foam heat insulation properties improve with increasing porosity, and that toluene residua increased the porosity by increasing the cell sizes and lowering the thickness of the compact skin at the film surface. Gutiérrez et al. (2014), foamed polystyrene using limonene solutions as solvent and CO2 as foaming agent. They studied the effect of pressure, temperature, concentration of the solution, contact time and vent time over the diameter of cells, its standard deviation and the cells density, observing that the most suitable conditions to foam polystyrene from limonene solutions were 90bar, 30°C, 0.1g of Polystyrene/ml limonene, 240min contacting and 30min venting. Thus, foaming process strongly depends on solvent type and how temperature is raised. Usually, natural gas or fossil fuels are used in this process. However, constant increase in their prices and the pollution factor leads to finding new and clean (non-toxic) manufacturing techniques. Electromagnetic waves ranging from radiofrequency to microwaves could be an efficient alternative option for processes involving heating increase in polymers (Mallakpour and Rafiee, 2011). Sen et al. (2011), used microwave irradiation as the source of energy for expansion process and 2-propanol as expansion agent, however, they did not studied the thermal effects which have a direct impact in the quality of the product.
This work present thermal characterization profile of EPS manufacturing using microwaves as the source of heating throughout polystyrene foaming, and using ethanol, a mixture of ethanol/water and hydrogen peroxide as expansion agents. Advantages of this method includes fast heating, low-cost process, and independence of fossil fuels.
Methods and proceduresIn order to characterize thermal profile, experimental and numerical simulations using Comsol Multiphysics® were performed.
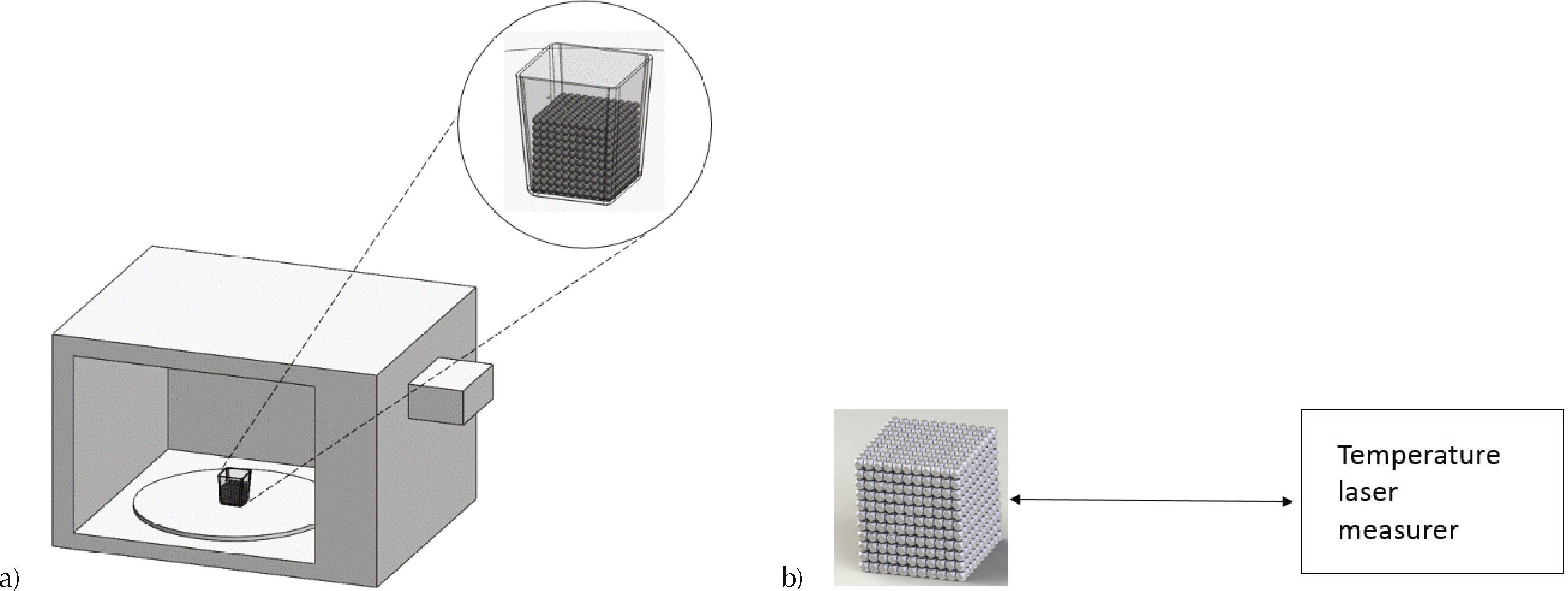
Experimental foaming processSamples of 130ml commercial expandable polystyrene with a density of 20kg/m3 and thermal conductivity of 0.35W/m·K were utilized. To increase the radiation absorption, experiments in conjunction with three solvents: ethanol, a mixture 25:75wt./wt. of water/ ethanol and hydrogen peroxide were performed. Purity of ethanol utilized was 96%. Solvents were applied directly to pre-expanded EPS beads with a syringe. After that, samples were heated for 180seconds into a commercial microwave oven with maximal power P = 950W and frequency f = 2.45GHz as shown in Figure 1a. In order to determinate thermal distribution, measurements of foamed material were performed using an infrared laser measurer fabricated by Extech Instruments model 42510A. Temperature measurements were taken at the center, top, bottom and lateral side (Figure 1b).
Mathematical modeling and simulationSince EPS is transparent to microwave wavelength, a heat transfer mechanism is required. Polar molecules as water present favorable conditions to be heated when they are irradiated by microwave. Eq. (1) and (2) describe the process of microwave heating (Warren et al., 2012).
From eq. (1)
ρd = material density
Cp = specific heat at a given temperature
k = thermal conductivity
qabs = absorbed volumetric power density
From eq. (2)
w = angular frequency
¿”eff = (s/ω¿0) = imaginary part of the complex permittivity from the material which is also known as loss tangent (tgd)
s = material conductivity
¿0 = free space permittivity
E = electric field
Steam produced above boiling point allows thermal energy transfer to the materials such as polystyrene to achieve thermal expansion required for its manufacturing process. The property that determines the absorption of electric field produced by microwave and its conversion to heat is the complex dielectric constant. This parameter is composed by the dielectric constant of the material that represents the capacity to store electric charge, and the loss tangent (tgδ), which is the absorption level of radiation that is transformed to heat. Table 1 shows microwave absorption of some solvents (Shadpour, 2011). It is observed that water provides a medium absorption level. Hence, uses of solvents of high loss tangent are expected to be more efficient for EPS manufacturing with microwave.
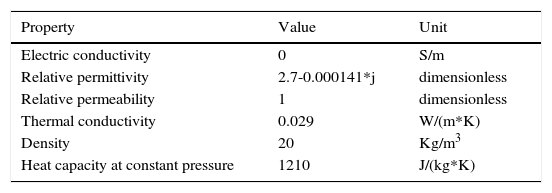
In order to estimate the response time and 3D thermal distribution simulations were performed in the software COMSOL Multiphysics 4.3. Parameters utilized are shown in Table 2.
From Table 2, the value of electric conductivity is cero since EPS is a dielectric material. Relative permittivity is a complex number where the real part represents the dielectric constant of material and the complex number means material absorption or loss tangent which is in the range of 5x10-7 to 5x10-3 (Jerzy et al., 1998). Relative permeability of 1 means that the material is not affected by magnetic fields. Thermal conductivity is the quantity of heat transmitted through a unit thickness in a direction normal to a surface of unit area, due to a unit temperature gradient. According to Askeland (2001) its value for foam polystyrene is 0.029. Density is the material mass per unit volume, its from 10 to 50kg/m3 (EPS, 2003). Finally, heat capacity is the amount of heat needed to raise the temperature of the system by one degree. For expanded polystyrene its value is 1210J/(kg*K) (Incropera and DeWitt, 1999).
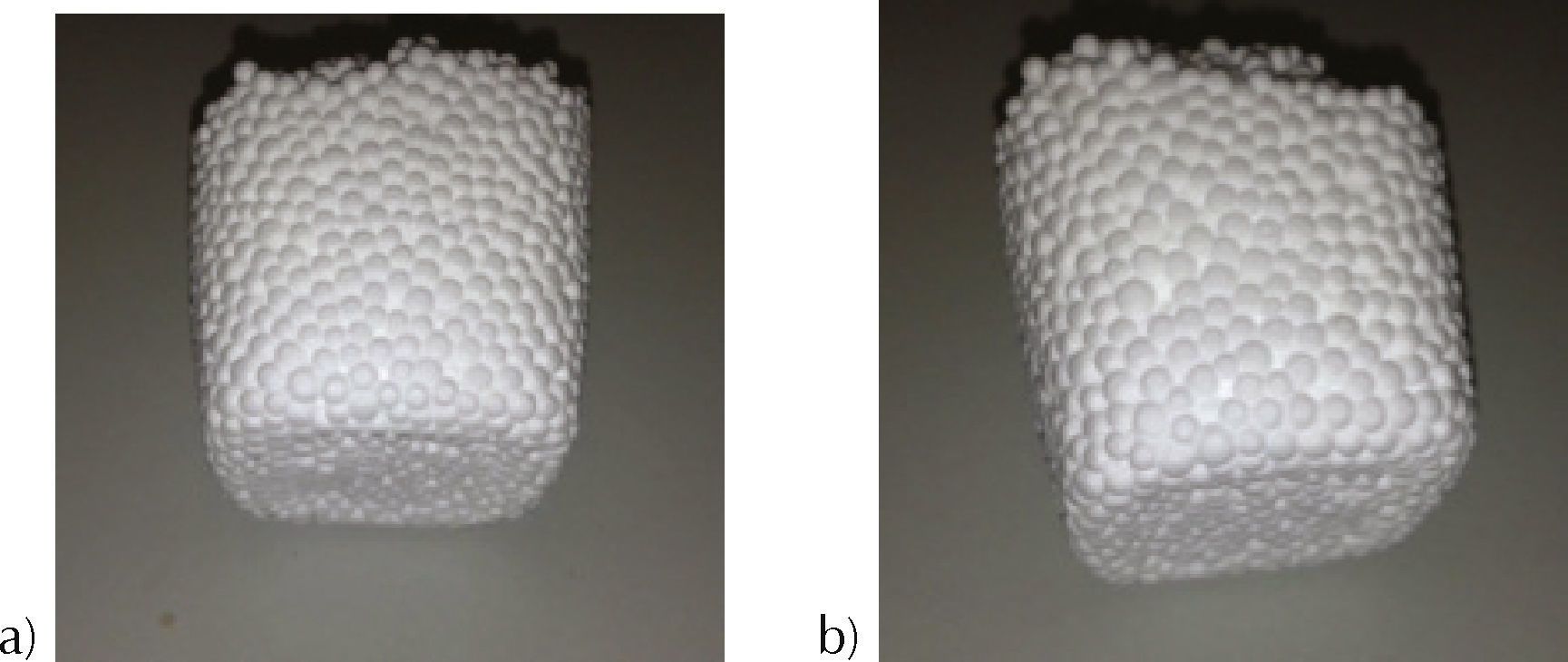
Results and discussionExperimental results of EPS fabrication using solventsIn order to study the repeatability of manufacturing process, each experiment was made six times. Obtained results of EPS mixed with ethanol, below 120seconds of microwave heating presented expansion as required in the manufacturing process. However, moisture was also present which is undesirable. At 150seconds, humidity was eliminated, but the material presented easy detachment of polystyrene pearls. At 180seconds of microwave radiation, only small detachment on the top of material was observed (Figure 2a). Tests were also performed for a mixture of water/ethanol with a volume ratio of 4:3. In the range of 120 to 180seconds humidity and material detachment was observed (Figure 2b).
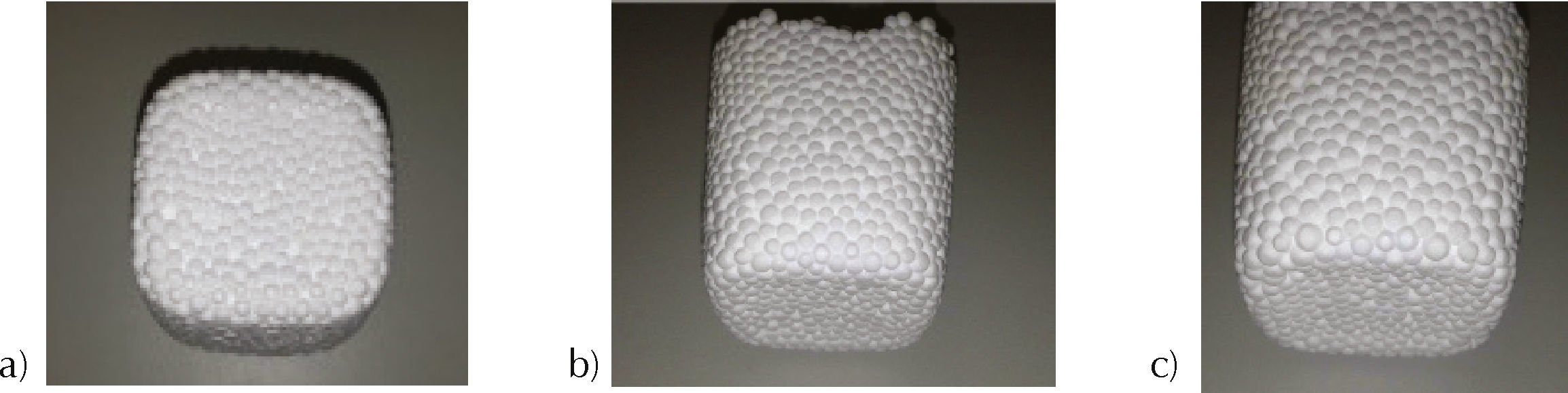
Better results were achieved when hydrogen peroxide was used as solvent. In this case, at 180seconds, expanded EPS did not present detachment, nor humidity (Figure 3).
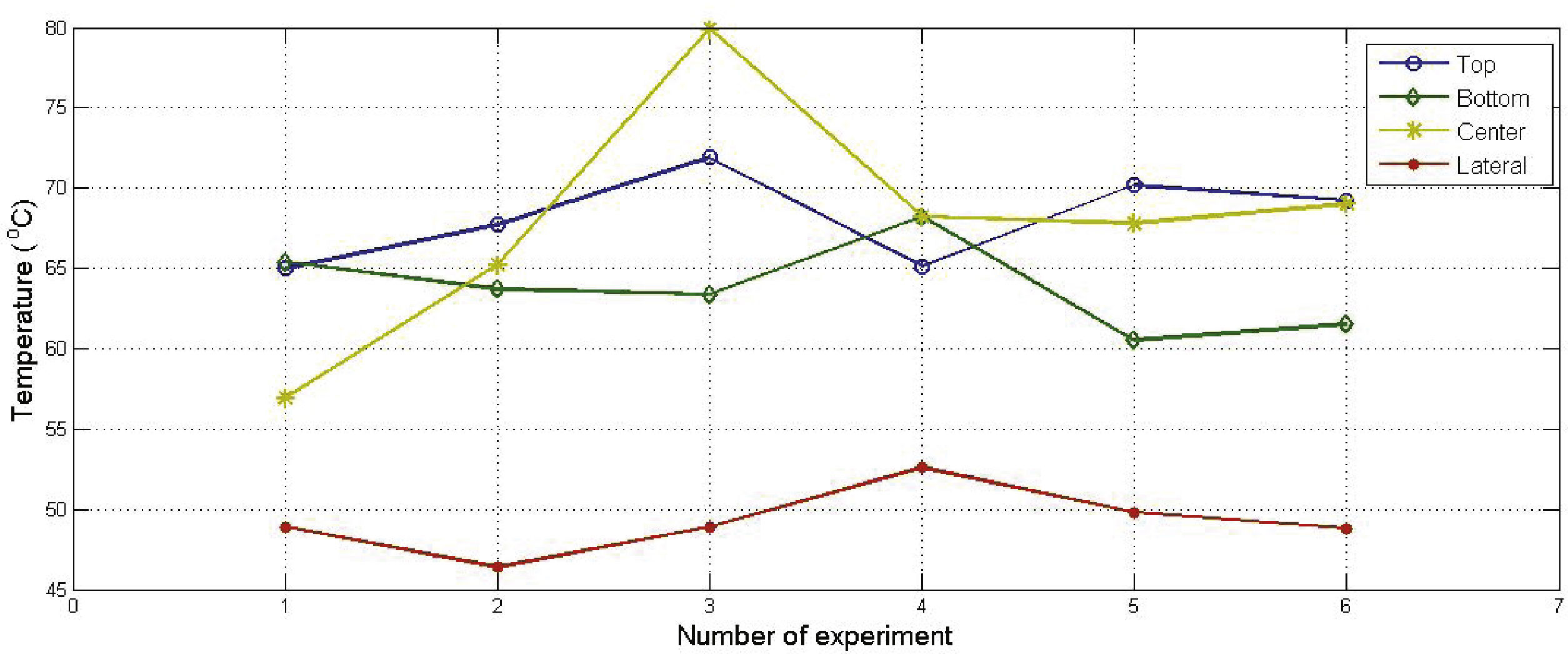
Due favorable results achieved with hydrogen peroxide, quantitative studies were performed in this particular case. Experimental thermal distributions of EPS at top, bottom, center and lateral side after microwave heating with hydrogen peroxide are shown in Figure 4. Results showed an accuracy of ±6°C at top, bottom and lateral sides. However, accuracy at center was ±23°C.
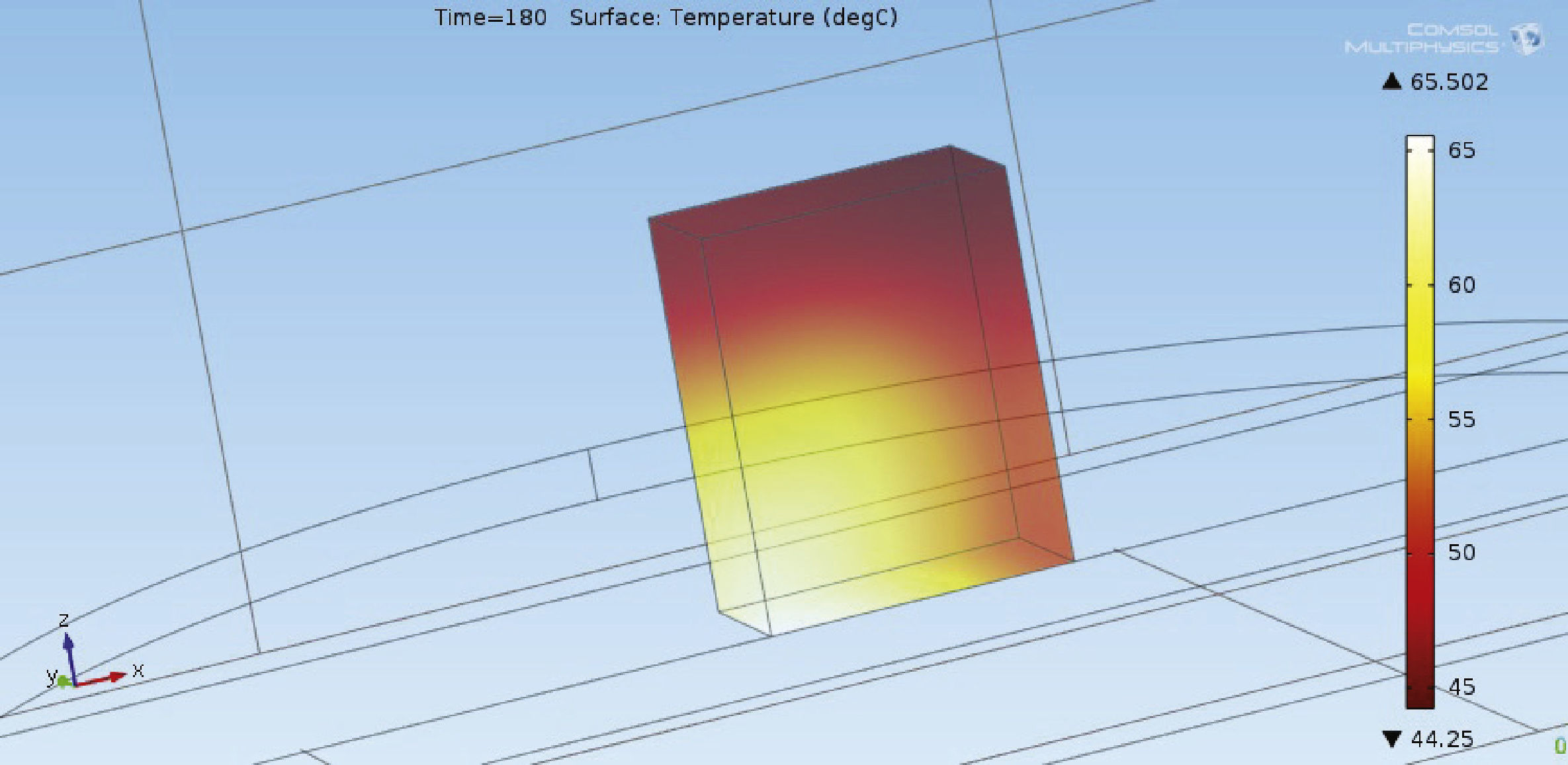
Numerical simulation resultsResult of numerical simulation performed in COMSOL Multiphysics solving eqs. (1) and (2) is shown in Figure 5. Input parameters utilized were: f = 2.45GHz, t = 180 s and e = 2.7-141x10-6 j. As it can be seen from Figure 5, simulated thermal distribution varies in a range of 21oC. Theoretically, this is mostly originated by the electric field distribution of microwaves. Simulations behavior on top sections of lateral sides showed agreement with experimental results in the range of 45 to 52°C.
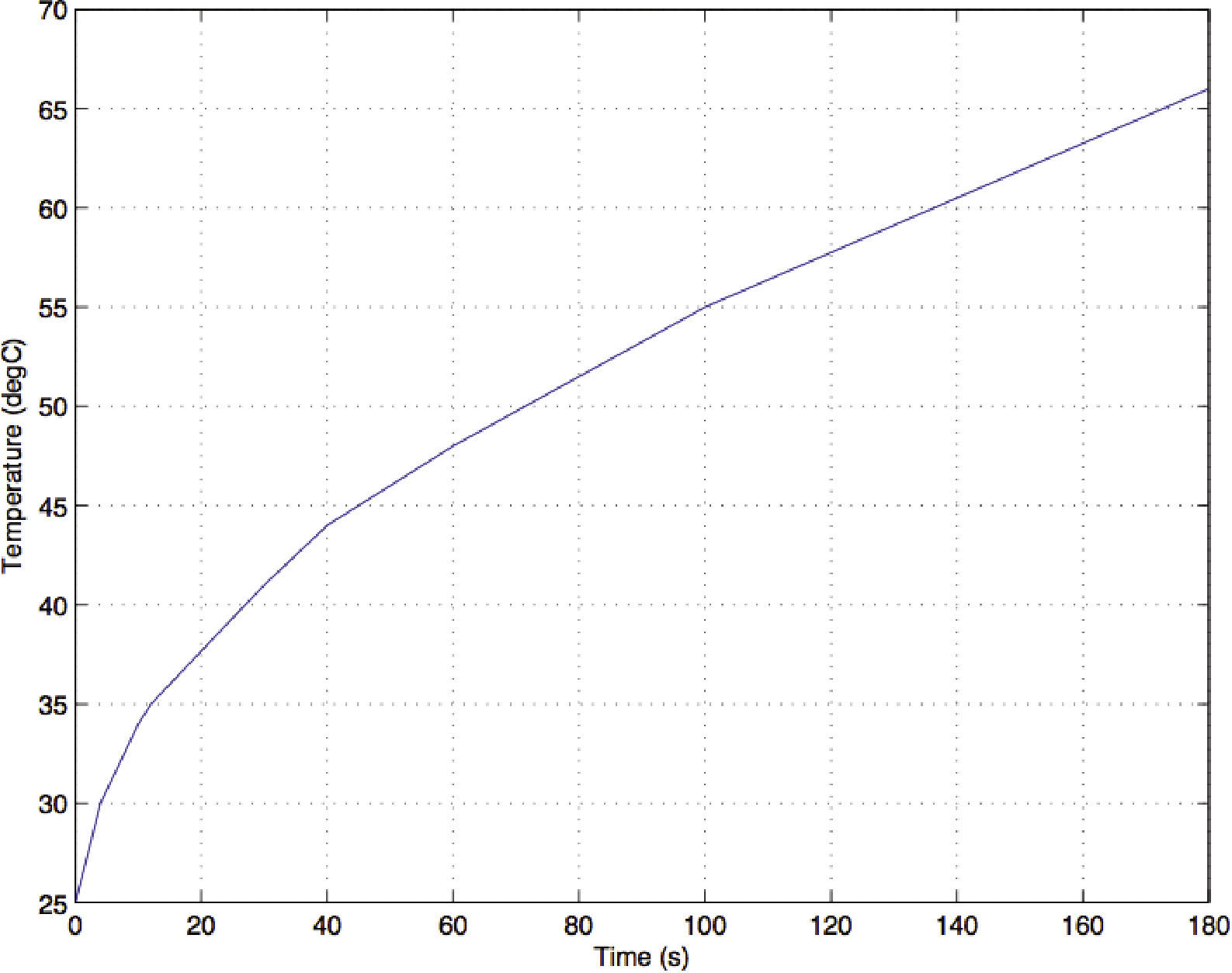
Thermal distribution shown in Figure 6 corresponds to a final time of simulation at 180seconds. The evolution of temperature with respect to time is depicted in Figure 6. Initial condition of temperature was 25°C. As it can be seen from Figure 6, increase of temperature is exponential below 80 s. From 80 to 180seconds temperature behavior is linear with a slope of 0.14.
Discussion of resultsExperimental results showed that the use of ethanol as solvent for heating EPS with microwaves in spite of a fast increase in temperature, it produces moisture or easy detachment of pearls in EPS which could represent an issue in industrial processes. The same problem was observed for mixture of ethanol and water. Moisture problem can be solved increasing time exposure to microwaves from 180 to 300seconds. However, this represent an increase of 60% of kWh which also represents an increase in cost production. In the other hand, the use of hydrogen peroxide showed elimination of moisture and lack of detachment of EPS. Numerical simulations showed a temperature range on the system (EPS and solvents) from 45 to 65oC, whereas experimental results showed an overall range from 46 to 80oC. This variation may be associated with the conditions of measurements at the center of the material. In this case temperatures were taken by destructive tests. On the other hand, measurements in lateral sides, top, and bottom are more uniform as can be seen in Figure 4.
ConclusionsFabrication of EPS with microwaves at 2.45GHz and three different solvents, i.e. ethanol, hydrogen peroxide and a mixture of water/ethanol were demonstrated. The better result was achieved with hydrogen peroxide. Elimination of moisture and solid EPS fabrication was observed for 180seconds of manufacturing process. Simulation and experimental results showed non-uniform thermal distribution, which depends of microwave wavelength, and the electrical field distribution. Future studies could include the implementation of longer wavelengths to attenuate temperature variations. This work could potentially benefit polystyrene manufacturing industry, specifically in energy and time saving.
Authors would like to thanks M.Sc. Eddie Nahúm Armendariz-Mireles for his contribution in this work.
Carlos Adrián Calles-Arriaga. Was born in Tamaulipas, México on September 2nd, 1979. He received the B.E. degree in electronics and communications engineering from the Universidad Autónoma de Nuevo León, Nuevo León, México, in 2002, the M.Sc. and Ph.D. degrees in industrial physics engineering from the Universidad Autónoma de Nuevo León, México in 2005 and 2009 respectively. His research interests include lasers, fiber optic, optical sensors and electromagnetism.
Juan López-Hernández. Was born in Veracruz, México. He received the B.E. degree in electronics engineering from the Instituto Tecnológico de Veracruz, México, in 2000, the M.Sc. and Ph.D. degrees in electronics sciences from the Instituto Nacional de Astrofísica, Óptica y Electrónica, México, in 2003 and 2008. His research interests include chaotic systems, hybrid intelligent systems and VLSI Design.
Martín Hernández-Ordoñez. Was born in Veracruz, México on March 2nd, 1976. He received the B.E. degree in electronics engineering from the Instituto Tecnológico de Veracruz, Veracruz, México, in 1999, the M.Sc. and Ph.D. degrees in electrical engineering from the Universidad Autónoma de San Luis Potosí, México in 2004 and 2007 respectively. His research interests include mathematical modeling, biomedical, fuzzy systems and differential geometric control and robotics.
Rodolfo Arturo Echavarría-Solís. Was born in Tampico, Mexico. He received the B. Sc. degree in electronics engineering from the Instituto Tecnológico de Ciudad Madero, in 1991, and the M. Sc. and Ph. D. degrees in electronics engineering from the Centro Nacional de Investigación y Desarrollo Tecnológico, CENIDET, Cuernavaca, Mexico, in 1995 and 2002, respectively. He completed a postdoctoral stay at the Department of Electrical Engineering, Texas A&M University, USA, in 2003-2004. He was a professor with the Electronics Department of CENIDET, from 1996 to 2004. He was the design manager of VOGAR, working on tap changing regulators and UPS, from 2005 to 2008. Currently, he is a professor of the Universidad Politécnica de Victoria, México. His areas of interest are on-load tap changing regulators, power quality, and history of electrical engineering.
Víctor Manuel Ovando-Medina. Was born in Chiapas, Mexico, in 1978. He graduated from Instituto Tecnológico de Celaya in Guanajuato (Mexico) in 2003 with a M.Sc. in chemical engineering, and he obtained his Ph.D. in the Center of Research in Applied chemistry (Saltillo, Mexico) in 2007 under supervision of Prof. René D. Peralta in the synthesis of polymeric nanoparticles by microemulsion polymerization. Since 2007 he moved to Autonomous University of San Luis Potosi where he was promoted as full-time professor. His current research focuses on the synthesis of composites of nanoparticles of semiconducting polymers and inorganic photocatalysts with enhanced performance on degradation of organic dyes. Also, he has been working on the study and optimization of heterophase polymerization of styrenic and vinyl monomers. He has published 17 peer-reviewed papers, and he is member of the editorial board of the Journal of Materials Science Research (Canada).