Protective antibody responses require cognate interaction between B cells and T helper cells in the germinal center of lymphoid follicles. This interaction leads to the formation of plasma cells that secrete high-affinity antibodies of different classes with distinct effector functions. Growing evidence shows that B cells receive additional helper signals from a variety of cells of the innate immune system, including dendritic cells, macrophages, follicular dendritic cells and epithelial cells. Granulocytes are a fundamental component of the innate immune system, as they are the first leukocytes that infiltrate infection and inflammation sites in order to clear invading microbes and necrotic cells. Granulocytes utilize opsonizing antibodies to enhance their phagocytic and killer functions, but recent studies indicate that granulocytes also optimize antibody diversification and production. In this article, the mechanisms by which different subsets of granulocytes deliver helper signals to B cells and plasma cells are discussed.
Las respuestas de anticuerpos requieren la interacción de linfocitos B y T helper en los centros germinales de folículos linfoides. Esta interacción induce la formación de células plasmáticas que secretan anticuerpos de alta afinidad y con distintas funciones efectoras. Recientes avances demuestran que los linfocitos B reciben señales adicionales de una variedad de células del sistema inmune innato, incluyendo células dendríticas, macrófagos, células dendríticas foliculares y células epiteliales. Los granulocitos representan un componente fundamental del sistema inmune innato, ya que son los primeros leucocitos que se infiltran en los sitios de infección e inflamación para eliminar microbios invasores y células necróticas. Los granulocitos utilizan anticuerpos opsonizantes para mejorar sus funciones fagocíticas y líticas, pero estudios recientes indican que también pueden optimizar la diversificación y producción de anticuerpos. En esta revisión discutiremos los mecanismos por los cuales los granulocitos proporcionan señales de ayuda a las células B y células plasmáticas.
Granulocytes are innate immune cells characterized by the presence of a multilobulated nucleus and a variety of cytoplasmic granules that permit the identification of three morphologically and functionally distinct granulocyte populations known as neutrophils, eosinophils and basophils.1 Similar to other innate immune cells, granulocytes sense the presence of microbes by detecting highly conserved microbial molecular signatures through a broad array of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs). These nonspecific microbial sensors deliver activation signals that stimulate the phagocytic and cytotoxic functions of granulocytes, thereby promoting the initial containment and clearance of invading microbes.2 In addition to containing cytotoxic and inflammatory compounds, neutrophils, eosinophils and basophils release cytokines, chemokines and other immune mediators that promote the recruitment and activation of monocytes and dendritic cells (DCs).3–5 These innate immune cells internalize microbial antigens and process them in the context of major histocompatibility class-II (MHC-II) complexes that are presented to CD4+ T cells to initiate highly specific adaptive immune responses, including antibody production by B cells. Granulocytes can further modulate adaptive immune responses by acquiring DC-like MHC-II-dependent antigen-presenting function and by releasing cytokines that modulate the activation and differentiation of T cells.3,5 The role of granulocytes in B cell responses is less understood. Here we discuss recent evidence that indicates the existence of unexpected functional interactions between granulocytes and B cells in lymphoid organs such as the bone marrow, lymph nodes and the marginal zone of the spleen.
B cell responsesMature B cells located in the follicles of lymph nodes and spleen generate specific antibody-mediated immune protection and memory upon activation by antigen. Mature B cells originate from bone marrow B cell precursors that generate a primary antibody repertoire following V(D)J recombination, an antigen-independent process mediated by recombination-activating gene (RAG) endonucleases that assemble antigen-binding immunoglobulin (Ig) variable regions from individual V (variable), D (diversity) and J (joining) gene segments.6 Transitional and mature B cells emerging from the bone marrow co-express IgM and IgD receptors capable of recognizing virtually any antigen present in the environment. These B cells enter the circulation and colonize secondary lymphoid organs to initiate immune responses against intruding antigens.7
Protein antigens initiate T-cell-dependent (TD) Ig responses in lymphoid follicles, a microenvironment that favors the cognate interaction of follicular B cells (also known as B-2 cells) with CD4+ T helper (Th) cells expressing the tumor necrosis factor (TNF) family member CD40 ligand (CD40L, or CD154).8 This initial Th cell-B cell interaction is followed by a germinal center (GC) reaction that further involves interaction of B cells with T follicular helper (Tfh) cells expressing CD40L and the cytokine interleukin-21 (IL-21).9 TD responses usually generate long-lived memory B cells and plasma cells that secrete high-affinity antibodies, but can also activate an alternative extrafollicular pathway that elicits short-lived plasma cells secreting low-affinity antibodies.10
Importantly, the GC reaction involves the up-regulation of activation-induced cytidine deaminase (AID), a DNA-editing enzyme that is essential for the induction of Ig somatic hypermutation (SHM) and class switch recombination (CSR).11 SHM introduces point mutations at high-rates in recombined V(D)J genes encoding the antigen-binding variable regions of Ig molecules, thereby providing the structural correlate for selection of high-affinity antibody mutants by antigen.12 CSR involves the replacement of constant μ (Cμ) and Cδ genes encoding IgM and IgD with Cγ, Cα or C¿ genes encoding IgG, IgA or IgG, providing novel effector functions without changing antigen specificity.13 A non-canonical form of CSR from Cμ to Cδ has also been described in humans’ lymphoid structures associated with the upper respiratory tract for the generation of specialized IgD producing B cells.14,15
Memory B cells enter the circulation and patrol lymphoid organs to react against recall antigens, whereas plasma cells home to the bone marrow and populate survival niches that promote the continuous release of high-affinity antibodies into the circulation.16 While follicular B-2 cells mediate slow-appearing but high-affinity antibody responses against TD protein antigens, extrafollicular B cells such as B-1 cells and splenic marginal zone (MZ) B cells induce fast-appearing but low-affinity antibody responses against T-cell-independent (TI) carbohydrate and glycolipid antigens.17
NeutrophilsNeutrophil biologyNeutrophils are the most abundant leukocytes in our circulation and become rapidly mobilized to eliminate microbes and necrotic cells in areas of infection or inflammation.18 Despite having a brief half-life and lacking proliferative potential, neutrophils have the ability to synthesize and release immunoregulatory factors, thereby helping the recruitment of DCs and monocytes that not only complete innate clearance of invading microbes, but also initiate more specific adaptive immune responses.4 Neutrophils are generated in the bone marrow from specific granulocyte–monocyte precursors that undergo proliferation and maturation in response to granulocyte–colony stimulating factor (G-CSF).18 This cytokine is produced by local stromal cells and promotes the release of mature neutrophils in the circulation by opposing the action of CXC-chemokine ligand 12 (CXCL12 or SDF-1), a stroma-derived chemokine that retains neutrophils in the bone marrow.18
Neutrophils are characterized by the presence of a heterogeneous set of cytoplasmic granules. Primary (or azurophilic) granules predominate in early stages of neutrophil maturation and are less capable of exocytosis than secondary (or specific) granules, which are generated in later developmental stages. Primary granules contain a variety of proteases, including myeloperoxidase (MPO), which are important for the digestion of phagocytosed material.4,18 In contrast, secondary granules contain a broad array of compounds such as lactoferrin and gelatinase, which are important to degrade the extracellular matrix, exert antimicrobial activity and initiate inflammation.4,18 In addition to undergoing degranulation, neutrophils generate a respiratory burst by activating an enzymatic complex known as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which generates reactive oxygen species involved in microbial killing.18
Neutrophils are recruited to sites of infection or inflammation by undergoing diapedesis through endothelial cells lining blood vessels. This complex process is activated by microbial chemotactic factors as well as CXCL1, CXCL2 and CXCL8 (or IL-8) chemokines released by activated endothelial cells. Following migration into inflamed tissues, neutrophils activate defensive programs that promote phagocytosis, intracellular killing and inflammation.18 Of note, neutrophils can also form neutrophil extracellular traps (NETs), which are cellular projections capable of trapping and killing bacteria.19 These structures contain decondensed chromatin embedded with cytoplasmic and granular proteins with powerful antimicrobial functions, including serin proteases and antimicrobial peptides such as cathelicidin.19
Immunoregulatory functions of neutrophilsIn addition to playing a key role in the containment and clearance of intruding microbes, neutrophils provide signals to multiple cells of the innate and adaptive immune systems (Fig. 1). Neutrophils communicate with these cells by releasing cytokines, chemokines and growth factors.4,20 In the initial phases of the innate response, neutrophils promote the recruitment of monocytes and immature DCs by releasing CCL3, CCL4, CCL5 and CCL10 chemokines as well as alarmins, properdin and defensins. Additional factors such as TNF promote the differentiation of monocytes into macrophages, a professional phagocyte that enhances microbial clearance.21 Similar factors promote the activation of immature DCs and their differentiation into maturation DCs.22 Contact-dependent interaction between neutrophils and DCs also occurs and involves the binding of CD11b (Mac-1) and carcinoembryonic antigen-related cell adhesion molecule (CEACAM1 or CD66) on neutrophils by DC-specific ICAM-3-grabbing non-integrin 1 (DC-SIGN or CD209) on DCs.4
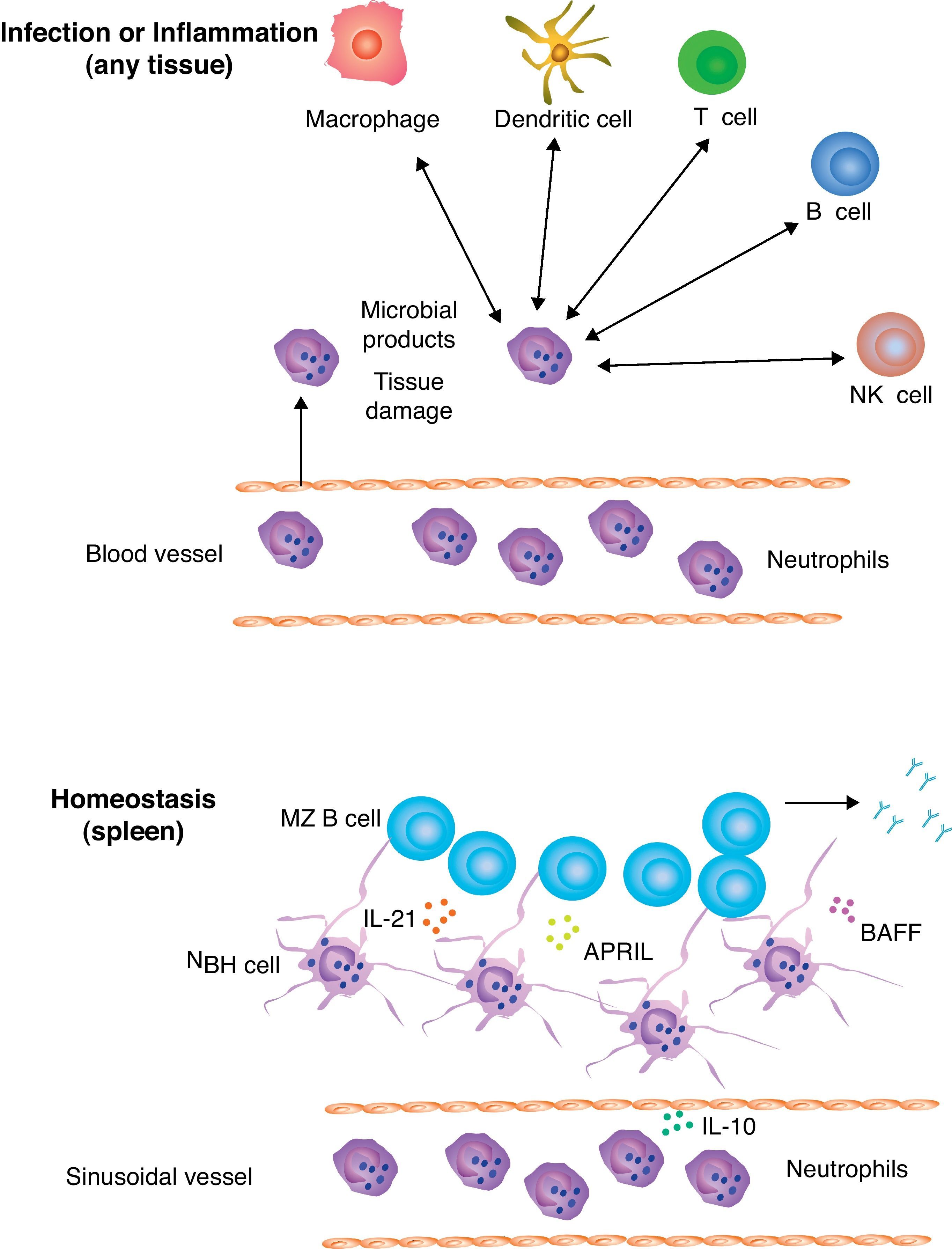
Neutrophils and B cells. Circulating neutrophils undergo transendothelial migration to infiltrate sites of infection or inflammation. In addition to clearing and killing invading microbes, neutrophils interact with macrophages, dendritic cells, NK cells, B cells and T cells, which are either already resident in the injured tissue or get recruited by neutrophils via cytokines, chemokines and alarmins. This complex process allows neutrophils to initiate both innate and adaptive immune responses. In the absence or infection of inflammation, neutrophils home to perifollicular areas of the spleen, where they undergo reprogramming in response to cytokines such as IL-10 released by local sinusoidal cells. Reprogramming involves neutrophil acquisition of MZ B cell-helper activity (hence their name of NBH cells) through up-regulation of IL-21, APRIL and BAFF as well as formation of antigen-trapping NET-like structures. NBH cells help MZ B cells to generate innate (natural) antibodies against common microbial antigens.
Together with contact-independent signals generated by neutrophil-derived soluble factors, this crosstalk results in DC maturation, which includes the upregulation of antigen-presenting MHC-II molecules and T cell-co-stimulatory CD80 (B7-1) and CD86 (B7-2) molecules as well as the release of inflammatory IL-12 and TNF cytokines.4 IL-12 supports the development of inflammatory Th1 cells that enhance the protective function of macrophages through the release of interferon-γ (IFN-γ), whereas TNF supports the activation of endothelial cells as well as the maturation of monocytes and DCs into efficient antigen-presenting cells.
In addition to inducing T cell differentiation, neutrophils release granular factors such as cathepsin G, azurocidin and defensins as well as CXCL9, CXCL10 and CXCL11 chemokines that promote the recruitment of Th1 and Th17 cells.23 Conversely, Th17 cells enhance the recruitment of neutrophils by releasing the cytokine IL-17 and the chemokine CXCL8.23 Recent studies also show that, in the presence of inflammatory signals, neutrophils up-regulate the expression of MHC-II as well as the secretion of IL-1β, IL-6 and IFN-γ, thereby acquiring the ability to present antigen to CD4+ T cells and to induce their differentiation into inflammatory Th1 and Th17 cells.24 Moreover, neutrophils are important for the peripheral maturation and function of natural killer (NK) cells, an innate cell type with powerful killing activity.25
In certain models, neutrophils have also been shown to dampen inflammatory responses via the release of elastase and other factors that impair the maturation of DCs.4 A subset of neutrophil-derived myeloid cells suppresses T cell responses in the context of pregnancy and cancer. T cell inhibition by these myeloid-derived suppressor cells (MDSCs) involves the enzyme arginase I, which depletes extracellular arginine required for T cell proliferation.26 T cell suppression by MDSCs is also mediated by the NADPH oxidase nitric oxide synthase, involved in the release of reactive oxygen species and nitric oxide, which impair T cell proliferation after antigen stimulation.26 Finally, neutrophils produce the cytokine IL-10 upon sensing bacteria though PRRs such as TLRs and C-type lectin receptors, at least in mice.4 By counteracting the action of IL-12 and IFN-γ, IL-10 exerts a powerful anti-inflammatory function on DCs, macrophages and T cells. Thus, neutrophils can initiate adaptive immune responses by influencing the early recruitment, activation and maturation of macrophages, DCs and T cells. Neutrophils would also contribute to the cessation of adaptive immune responses by inhibiting the activation of DCs, macrophages and T cells.
Crosstalk of neutrophils with B cellsBy modulating the activation of DCs and T cells, neutrophils are likely to have an enhancing effect on B cell activation and antibody production. In mice, neutrophil release of cathepsin G activates T cells that promote antigen specific IgG production by B cells.27 Interestingly, human neutrophils produce the TNF-related cytokine B cell-activating factor of the TNF family (BAFF or BLyS) and its homolgue a proliferating-inducing ligand (APRIL), two B cell-stimulating factors that are crucial for the survival, maturation and differentiation of B cells. In inflamed mucosal tissues and some tumors, neutrophil secretion of APRIL plays an important role in the activation of B cells and the survival of antibody-secreting plasma cells.4 To establish functional interactions with T and B cells, neutrophils first migrate to secondary lymphoid organs such as lymph nodes and spleen in response to locally generated inflammatory signals.28,29 However, neutrophils can also home to the perifollicular area surrounding the MZ of the spleen under non-inflammatory conditions, that is, in the absence of infection or inflammation.30
Splenic peri-MZ neutrophils are present in healthy mice, primates and humans and function as professional helper cells for marginal zone B cells to facilitate the generation of antibodies against highly conserved microbial antigens under homeostatic conditions.30 These B cell-helper (NBH) neutrophils mediate activation of MZ B cells through a mechanism involving the expression of B cell-stimulating factors such as BAFF, APRIL, IL-21 and CD40L.30 Conversely, NBH cells suppress the activation of T cells like MDSCs do.30 By exerting this dual B cell-helper T cell suppressor function, NBH cells may promote extrafollicular responses to TI antigens while minimizing follicular B cell responses to TD antigens and inflammation. Accordingly, NBH cells enter splenic follicles only under inflammatory conditions, perhaps to activate T cells.30 In vivo observations in patients with neutropenia further indicate the specific involvement of NBH cells in antibody production, as these patients produce fewer antibodies to some common microbial antigens. Overall, the crosstalk between NBH cells and MZ B cells may be instrumental to generate a second line of innate (or natural) antibody defense against systemic invasion by commensal antigens and microbes that break the first line of defense at the mucosal barrier.
BasophilsBasophil biologyBasophils are the least abundant granulocytes in our circulation, representing usually less than 1% of leukocytes under steady state conditions. For many years basophils have remained an enigmatic immune cell type, mostly because of the lack of proper phenotypic markers for their identification and their short lifespan, which is estimated to be in the range of 1-2 days.3 The identification of a specific c-Kit (CD117)−c¿RI (high-affinity IgE receptor)+CD11b+CD123 (IL-3R)hi phenotype has greatly helped the identification and functional characterization of basophils as well as their distinction from mast cells, which are tissue-resident leukocytes very similar to basophils. Basophil depletion by monoclonal antibodies and the generation of basophil-deficient transgenic mice have further increased our knowledge of the role of basophils in host immunity.3
Basophils originate from multi-potent lineage-restricted granulocyte-monocyte progenitors located in the bone marrow. In spite of their resemblance with mast cells, basophils represent a cell lineage distinct from mast cells and indeed follow a different differentiation pathway.3 In the presence of inflammatory signals and IL-3, basophil progenitors rapidly expand and fully differentiate into mature basophils, which represent the main source of histamine and other vasoactive compounds during allergic reactions.3 In addition to vasoactive factors, basophils produce immunomodulating factors such as platelet-activating factor (PAF), leukotriene C4, granzyme B and retinoic acid as well as antibody-inducing and Th2-differentiating cytokines, including IL-4, IL-6 and IL-13.3 Among basophil-tropic cytokines, IL-3 enhances basophil recruitment into lymphoid tissues, augments basophil secretion of IL-4 and promotes basophil expansion after parasite infection.3 However, some studies show that IL-3 is not required for the maintenance of basophils in vivo, probably because this function is also covered by the IL-7-like cytokine thymic stromal lymphopoietin (TSLP).31
Basophils exert an important function in allergic reactions and in host defense against parasite infections.3 Basophils bind the IL-4-induced antibody IgE by expressing the high-affinity IgE receptor (Fc¿RI), which triggers degranulation and release of a host of inflammatory factors upon cross-linking of pre-bound IgE by allergens or parasite antigens.3 Basophils also express a variety of receptors for cytokines, chemokines, IgG (FcγRIIB) and adhesion molecules, which regulate their migration, activation and function in ways that remain poorly understood.
Immunoregulatory functions of basophilsGrowing evidence shows that basophils establish complex interactions with cells of the adaptive immune system (Fig. 2). Basophils release IL-4 and facilitate the differentiation of Th2 cells producing IL-4 in response to signals from IgE-binding antigens, cytokines (IL-3, GM-CSF, IL-33 or IL-18), microbial receptors (TLR2 and TLR4), and allergenic proteases.32,33 Consistent with this notion, basophilia and persistent Th2 polarization have been described in mice lacking critical negative regulators of basophil activation and expansion, including the transcription factor IFN-regulated factor 2 (IRF2) and the kinase Lyn.34,35 Some studies show that basophils function as professional antigen-presenting cells for the development of Th2 responses against a variety of antigens.33,36,37 However, this conclusion remains somewhat controversial.38 Basophils are also important to generate immunity against helmints39 and ectoparasites such as ticks.40 Moreover, basophils can induce the production of IL-10 by cytotoxic CD8+ T cells through a mechanism involving basophil release of IL-4 and IL-6.41 The effector functions of basophils may be further modulated by complement receptors, TLRs and leukocyte immunoglobulin-like inhibitory receptors (LIRs).
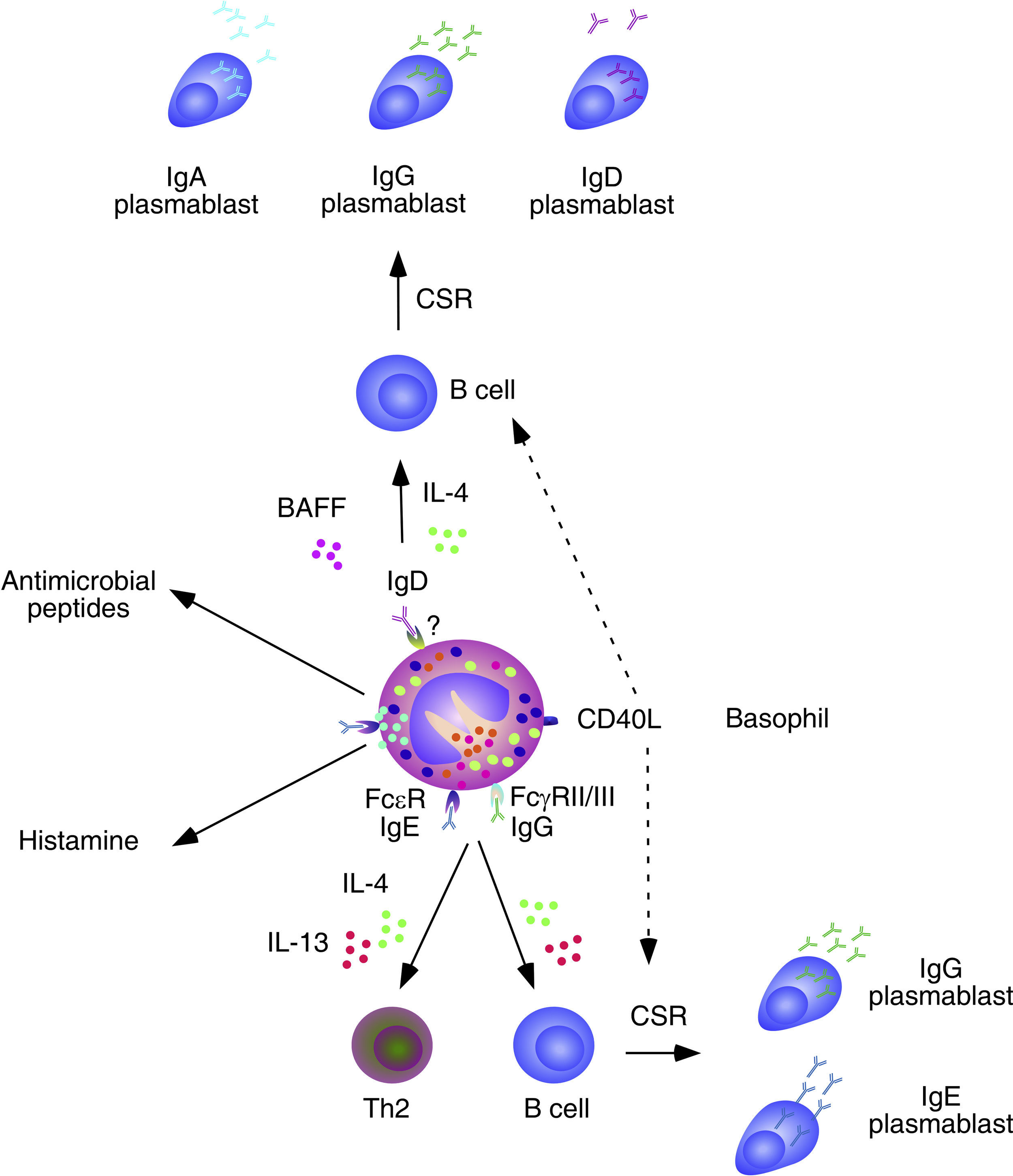
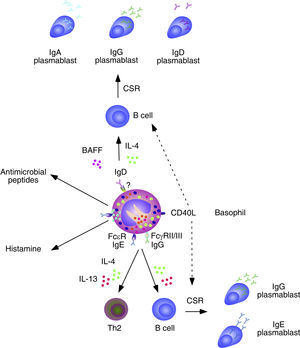
Basophils and B cells. In the presence of allergic inflammation or infections by parasites, circulating and mucosal basophils undergo degranulation and trigger inflammation by releasing histamine and other vasoactive factors upon engagement of Fc¿RI-bound high-affinity IgE by allergen or antigen. The concomitant release of chemokines and cytokines, including IL-4 and IL-13, facilitates the recruitment of additional inflammatory leukocytes, Th2 differentiation and B cell class switching from IgM to IgE. In immunized individuals, circulating basophils migrate to lymph nodes to enhance memory B cell responses by inducing CD40L expression as well as IL-4 and IL-6 secretion after engagement of Fc¿RI-bound low-affinity IgE by a recall antigen. In addition to directly activating B cells, IgE-activated basophils facilitate the formation of antibody-inducing Th2 cells. FcγRIIB-bound IgG may elicit similar responses. Circulating and mucosal basophils also bind IgD through an unknown receptor. Crosslinking of IgD by antigen causes secretion of several B cell-stimulating cytokines, including IL-4 and BAFF, which elicit class switching from IgM to IgG or IgA. IgD-activated basophils also release antimicrobial peptides.
Over the past few years, growing evidence has shown the participation of basophils in B cell antibody responses. In addition to presenting antigen–MHC-II complexes to CD4+ T cells, basophils release IL-4 and IL-6 and thereafter become capable to induce the formation of Th2 cells with B cell helper activity. Consistent with these findings, the frequency of basophils in the spleen is compatible with that required for the activation of B cells.42 Of note, depletion of basophils not only impairs memory B cell responses, including the production of Th1-type (IgG2) and Th2-type (IgG1) antibodies, but also augments the susceptibility of mice to sepsis.42
In spite of lacking intrinsic somatically recombined antigen receptors, basophils efficiently capture soluble antigens through IgE antibodies pre-bound on surface Fc¿RI receptors. Antigen recognition by low-affinity IgE causes no histamine release, but rather upregulation of CD40L expression and release of IL-4 and IL-6 by basophils. These factors enhance antibody responses by inducing Th2 cells that secrete B cell-activating cytokines such as IL-4, IL-5, IL-6, IL-10 and IL-13.39 Remarkably, basophils can also activate B cells directly, as exemplified by their ability to trigger class switching from IgM to IgE and IgG4 upon stimulating B cells via IL-4, IL-13 and CD40L.43
In mice lacking the basophil-regulating kinase Lyn, activation of basophils by immunocomplexes containing autoreactive IgE increases the expression of MHC-II molecules and elicits release of BAFF and IL-4. These effects stimulate the activation of autoreactive CD4+ T helper cells by basophils as well as the survival of autoreactive B cells, leading to an increased TD production of pathogenic IgG1 and IgE that cause lupus-like nephritis.44 In addition to IgE, basophils have IgD molecules pre-bound on their surface, although the receptor for IgD remains unknown. IgD is an enigmatic antibody isotype that appears early in evolution. Transitional and mature B-cells emerging from the bone marrow express both IgM and IgD receptors on their surface, but IgD expression is usually downregulated after B cell encounter with antigen. However, some antigen-activated B cells of the human upper respiratory mucosa downregulate IgM instead of IgD expression through a process of non-conventional class switching from IgM to IgD that leads to the formation of IgD-secreting plasma cells.
In spite of being heavily hypermutated, IgD antibodies produced by upper respiratory plasma cells are largely polyreactive and bind multiple antigenic determinants on commensals and pathogens to promote mucosal protection.14,15,45 In addition to crossing the epithelial barrier to reach the mucosal surface, IgD enters the circulation to interact with basophils as well as monocytes and neutrophils.45 In basophils, IgD cross-linking induces the release of antimicrobial peptides (cathelicidin), opsonization factors (Pentraxin-related protein PTX3), inflammatory cytokines (TNF and IL-1β), Th2-inducing cytokines (IL-4 and IL-13), and B-cell stimulating factors (BAFF and APRIL).45 Consistent with these findings, IgD-activated basophils increase in autoinflammatory disorders associated with dysregulated TNF and IL-1β production and stimulate B cells to secrete IgM and undergo class switching from IgM to IgG and IgA.45
EosinophilsEosinophil biologyEosinophils are the second most frequent granulocyte subset in the circulation, ranging from 1% to 4% of total peripheral blood leukocytes. Eosinophils are mainly implicated in cytotoxic responses against parasites and play an important role in the pathogenesis of allergic disorders through the release of a broad array of inflammatory and cytotoxic molecules pre-stored in intracellular granules.5 However, in recent years eosinophils have also been shown to modulate adaptive immunity as a result of their ability to up-regulate the expression of MHC-II molecules and secrete cytokines, chemokines, lipid mediators and growth factors.5,46 The cytokines IL-3, IL-5 and GM-CSF play a key role in the development, maturation, activation and recruitment of eosinophils. In particular, IL-5 induces eosinophil differentiation and eosinophil release from the bone marrow to the circulation. Accordingly, a humanized anti-IL-5 antibody reduces the number of circulating eosinophils.5
Eosinophils contain numerous cytotoxic proteins in their granules, including major basic protein (MBP), eosinophil cationic protein (ECP), and the eosinophil-derived neurotoxin (EDN). All these cationic proteins cause inflammation and microbial killing upon their release by eosinophils.5 In healthy individuals, eosinophils are mainly found in mucosal tissues such as the small intestine, where they may serve as an innate line of defense against commensals and pathogens. Yet, eosinophils can also be found in the bone marrow under homeostatic conditions and circulating neutrophils may home to the lymph nodes and spleen after immunization.
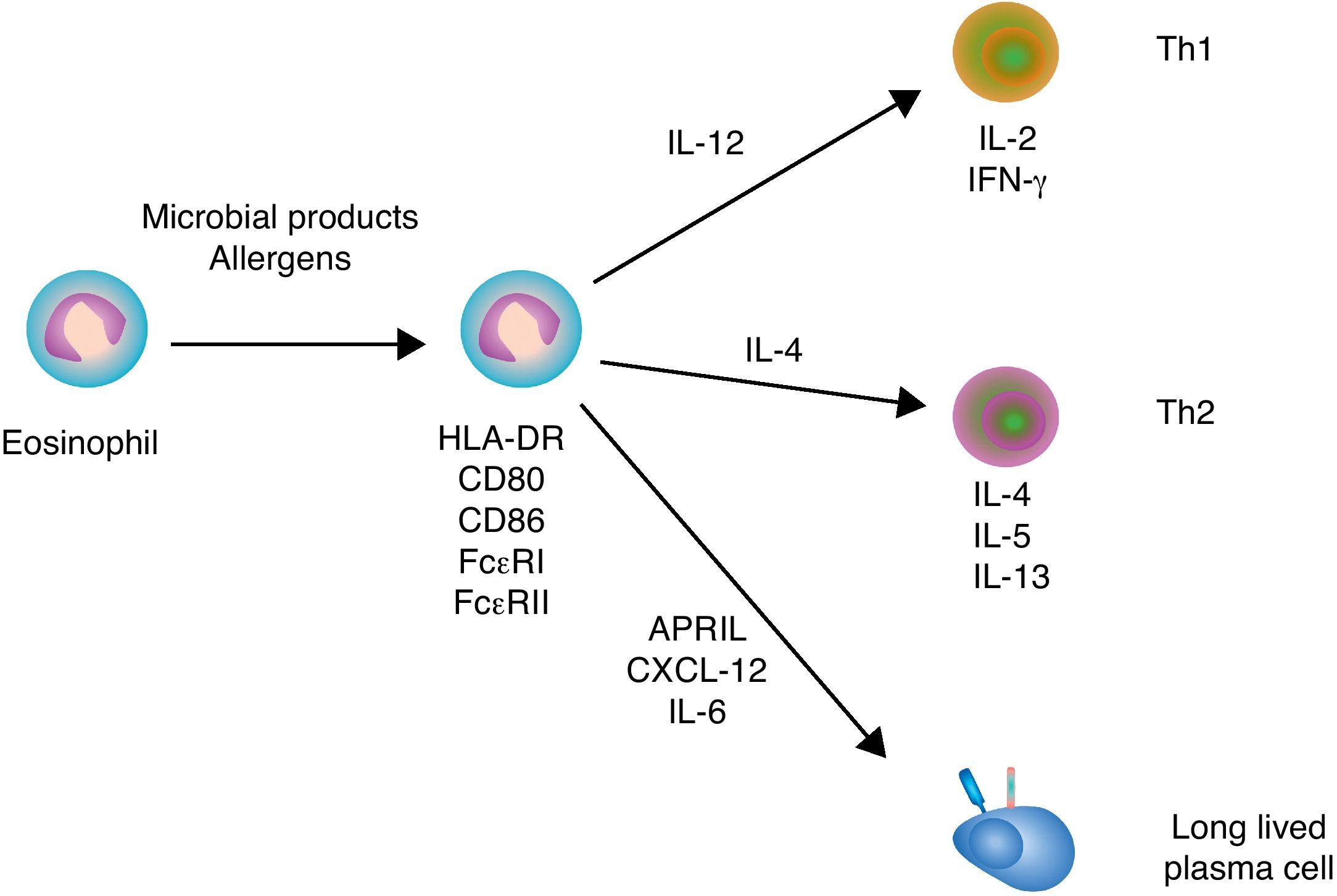
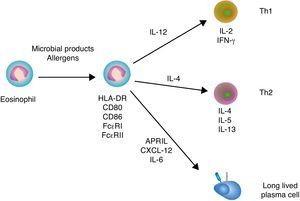
Immunoregulatory functions of eosinophilsSimilar to neutrophils and basophils, eosinophils can regulate both innate and adaptive immune responses (Fig. 3). Eosinophils modulate innate immune responses by regulating the activation of mast cells, basophils and neutrophils through MBP.5 In addition, eosinophils induce the expression of antigen-loading MHC-II and T cell costimulatory molecules after undergoing transendothelial migration and in the presence of appropriate cytokines.47 Eosinophil production of chemokines and cytokines such as TNF, IL-4 and IL-12 not only influences the recruitment and maturation of DCs, but also induces the differentiation of Th1 and Th2 cells. Moreover, eosinophils constitutively express Notch ligands, which signal in both autocrine and paracrine manners to modulate the function of eosinophils as well as other cells of the immune system.48 By expressing MHC-II and releasing IL-4, eosinophils may further function as antigen-presenting cells to promote the activation of antigen-specific T cells and induce their differentiation into antibody-inducing Th2 cells.5
Eosinophils and B cells. In the presence of allergic inflammation or infection by parasites, circulating and mucosal eosinophils up-regulate antigen-loading MHC-II molecules as well as T cell costimulatory CD80 and CD86 molecules, thereby promoting the activation of antigen-specific T cells. By releasing IL-12 and IL-4, eosinophils may also facilitate Th1 and Th2 differentiation, respectively. Th1 cytokines such as IFN-γ amplify Th1 responses and trigger activation of inflammatory cells, including macrophages, whereas Th2 cytokines such as IL-4, IL-5 and IL-13 amplify Th2 responses, including B cell IgE production, and augment the recruitment, activation and survival of eosinophils. In healthy individuals, eosinophils release CXCL12, APRIL and IL-6 to promote the recruitment and sustain the survival of antibody-secreting plasma cells in the bone marrow.
Eosinophils express surface FcγRII receptors that bind allergen-specific IgG1 and IgG3 but not IgG4 antibodies. Interaction of esosinophil-bound IgG wih antigen triggers degranulation and release of EDN, which is a cytotoxic and inflammatory factor.5 Moreover, eosinophils express an FcαRI receptor (or CD89) that binds IgA. Recognition of IgA coating the surface of intestinal bacteria by FcαRI elicits eosinophil secretion of various antimicrobial and immunomodulating proteins.5 Although poorly studied, this response may contribute to intestinal homeostasis and immunity, as eosinophils are relatively abundant in the small intestine of healthy individuals. Whether these eosinophils also participate in local IgA responses remains unknown.
Additional studies show that eosinophils support antibody production in the bone marrow. Indeed, eosinophils release APRIL, which promotes the long-term survival of plasma cells in specific niches of the bone marrow. Given that bone marrow plasma cells usually emerge from the GC reaction, the plasma cell survival activity of eosinophils appears to be essential for the continuous release of high-affinity antibodies in the circulation.49 Of note, APRIL elicits survival signals by engaging the receptor BCMA (B cell maturation antigen) on plasma cells.50 Bone marrow eosinophils also secrete IL-6, another critical cytokine in plasma cell differentiation and survival.49 Upon antigen priming, bone marrow eosinophils further release IL-4 and IL-10, which enhance plasma cell stimulation and survival.
The survival signals provided by eosinophils are critical for the maintenance of the plasma cell pool in the bone marrow, because depletion of eosinophils rapidly results in plasma cell apoptosis.49 Remarkably, bone marrow eosinophils are in close contact with stromal cells that release CXCL12, a chemokine that binds to the CXCR4 receptor on plasma cells.49 CXCR4 is also expressed by eosinophils, which provides a mechanistic explanation for the colocalization of eosinophils with plasma cells.49 Given the key role of eosinophils in plasma cell migration to and retention in the bone marrow, it is not suprising that depletion of eosinophils augments the number of plasma cells in both spleen and lymph nodes.49
The plasma cell helper function of eosinophils not only may contribute to the antibody-enhancing function of known vaccine adjuvants such as alum, but could also be exploited to develop novel vaccine strategies.49 By causing stable activation of eosinophils in the bone marrow, immunization stimulates eosinophils to produce a broader spectrum of plasma cell survival factors, including IL-4 and IL-10, thereby eliciting more sustained plasma cell survival.51 In addition to stimulating plasma cells in the bone marrow, alum can trigger homing of eosinophils to the spleen, particularly after intraperitoneal injection. This effect augments the priming of splenic B cells by antigen as well as early IgM production.52
Concluding remarksOver the past decade, a growing recognition of the importance of neutralizing antibodies in host defense combined with the success of B cell-depletion therapies in treating autoimmune disorders has led to an increased focus on better understanding the pathways underpinning B cell antibody production. In general, B cells require cognate interaction with T helper cells in the germinal center of lymphoid follicles to generate protective antibodies. However, recent evidence shows that B cells receive additional help from granulocytes, including neutrophils, eosinophils and basophils. These innate immune cells enhance T cell-dependent antibody responses by delivering helper signals to B cells located in both pre-germinal center and post-germinal center lymphoid sites, including the bone marrow. In addition to enhancing and complementing the B cell helper activity of canonical T cells, granulocytes can deliver B cell helper signals in a TI manner to initiate rapid antibody responses at the mucosal interface and in the MZ of the spleen. The B cell helper activity of granulocytes could be harnessed to enhance the induction of protective antibodies by vaccines. Conversely, granulocytes could be therapeutically targeted to dampen the release of pathogenic autoantibodies in autoimmune disorders.
Conflict of interestThe authors declare no competing financial interests.
This work was supported by the Ministerio de Ciencia e Innovación grant SAF 2008-02725 (A.C) and Juan de la Cierva program (I.P).