The aim of this study was to determine HLA associations with progression to end-stage renal disease (ESRD) in the mixed Zulian population in Venezuela, regardless of other factors. A retrospective study to determine HLA Class I association was performed on 188 patients with ESRD due to different types of glomerulonephritis, and 202 healthy controls. Patients and control groups were serologically typed by Terasaki microlymphocytotoxicity technique using commercial Class I plates including 26 HLA-A and 48 HLA-B specificities. The antigens positively associated to the ESRD were: HLA-B38, B51, B53 and B62. Negatively associated antigens were: HLA-A9, B12, B17, B40 and B48. The haplotypes positively associated were: HLA-A2-B51, A2-B53, A23-B38 and A68-B38. The negatively associated haplotypes were: HLA-A2-B12, A2-B48, A9-B35 and A28-B40. The high Odds ratio observed and its statistical corroboration reflect the strength of the described association between HLA antigens and ESRD. Further molecular studies should clarify types and subtypes of the HLA class I alleles involved in the progression to ESRD.
El objetivo principal de este trabajo fue determinar las posibles asociaciones de los antígenos HLA con la progresión a enfermedad renal terminal (ESRD por las siglas en inglés), independientemente de otros factores, en la población Zuliana de Venezuela. Se trata de un estudio retrospectivo, analizando los resultados obtenidos de la tipificación de los antígenos HLA clase I (A y B) en 188 pacientes con enfermedad renal crónica en etapa terminal y con indicación de trasplante renal, en comparación con 202 controles sanos de la misma población. Los antígenos HLA fueron tipificados por la técnica de microlinfocitotoxicidad de Terasaki, utilizando placas comerciales para clase I incluyendo 26 especificidades HLA-A y 48 HLA-B. Los antígenos HLA clase I asociados a ESRD fueron: HLA-B38, B51, B53, B62; y los asociados negativamente resultaron HLA-A9, B12, B17, B40, B48. Los haplotipos asociados positivamente fueron: HLA-A2-B51, A2-B53, A23-B38 y A68-B38. Los haplotipos asociados negativamente resultaron: HLA-A2-B12, A2-B48, A9-B35, A28-B40. Los altos riesgos relativos observados y su corroboración estadística reflejan la fortaleza de las asociaciones descritas. Estos resultados podrían orientar los estudios moleculares que permitirán determinar y confirmar de manera más específica cuáles son los alelos HLA clase I asociados con esta patología.
Progression of chronic renal failure (CRF) leads, in the majority of instances to end-stage renal disease (ESRD) at which point renal replacement therapy is irremediable. Since the mid-1980s, there has been a marked increase in the incidence and prevalence of ESRD around the world.1
Gender, genetic, race, lipids, hypertension, and smoking are the associated factors among others to the ESRD.2 The role of the immune system in renal diseases must also be considered, because it could be the origin or cause of the disease and its progression.3 These observations resulted in the search for positive associations between human leukocyte antigens (HLA) and a wide range of renal diseases. Nevertheless, it is only in glomerulonephritis where a convincing rationale for such studies has been demonstrated. The studies on HLA and renal diseases show some striking associations with HLA Class I and Class II alleles. The approach to the analysis of HLA associations with renal disease has evolved since the late 1980s, mainly because of the much deeper understanding of Class I and Class II molecules structure and function. HLA phenotypes are correlated with an increased or decreased risk of alloantibody sensitization (PRA) in end-stage renal disease candidates for first or repeat kidney transplantation.4 This study provides evidence that HLA-linked genes influence the anti-HLA alloantibody sensitization in ESRD. Various pathologies may be associated to HLA alleles and/or haplotypes such as idiopathic membranous nephropathy, minimal change nephrotic syndrome, immunoglobulin A (IgA) nephropathy, post-streptococcal glomerulonephritis and familial focal glomerulosclerosis, among others.5-12 Family studies of the HLA Class II system in acute post-streptococcal glomerulonephritis in the population of Zulia State confirm the association between HLA alleles and the disease.13
The purpose of this study was to determine if specific HLA Class I antigens or combinations of antigens are associated with progression to ESRD, regardless of other factors, in patients of the population of Zulia state, Venezuela.
Materials and methodsA retrospective study was conducted using results of serological microlymphocitotoxicity Terasaki test, obtained from samples submitted to the HLA and Immunology Laboratory of the Zulia State Blood Bank, between 1985 and 2003. A total of 188 patients with ESRD, in their majority reported as post-streptococcal or other glomerulonephritis origin and 202 healthy controls of Zulian population were evaluated. For this study, ESRD was defined as a moment when long-term treatment with dialysis was initiated and a transplant was required for survival. The patients were referred from the Nephrology Service of the University Hospital of Zulia State. The control group includes both sex non-related subjects with ages between 20 and 72 years old. The results obtained with serological commercial test from HLA Class I (BIOTEST Diagnostics, One Lambda or C six Diagnostics, INC) following the manufacturers recommendations were analyzed; they included 26 HLA-A and 48 HLA-B specificities. Antigenic frequencies were determined for each subject's HLA14 and haplotypes frequencies were calculated by the Mattiuz method.15 The results were analyzed by the Chi square statistical method, corrected by Yates [pc=1−(1−p)n], Fisher's exact; p-value (1-tail) or Bonferroni test when necessary; p<0.05 was considered statistically significant.16 Odds ratio (O.R.) was used to estimate the association's strength between HLA antigens and ESRD.17,18 Preventive Fraction (PF) indicates how much protection was assigned to the marker.16
ResultsThe age range of the 188 patients was between 20 and 44 years, with a median age of 34.3 years. A total of 117 (62.5%) patients were males and 71 (38.5%) females. In the controls, 107 (52.97%) were females and 95 (47.03%) were males.
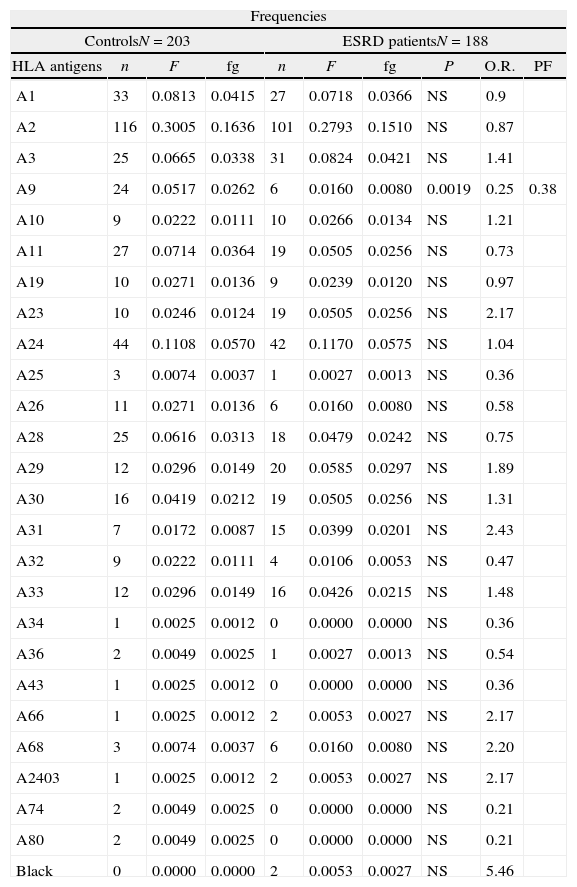
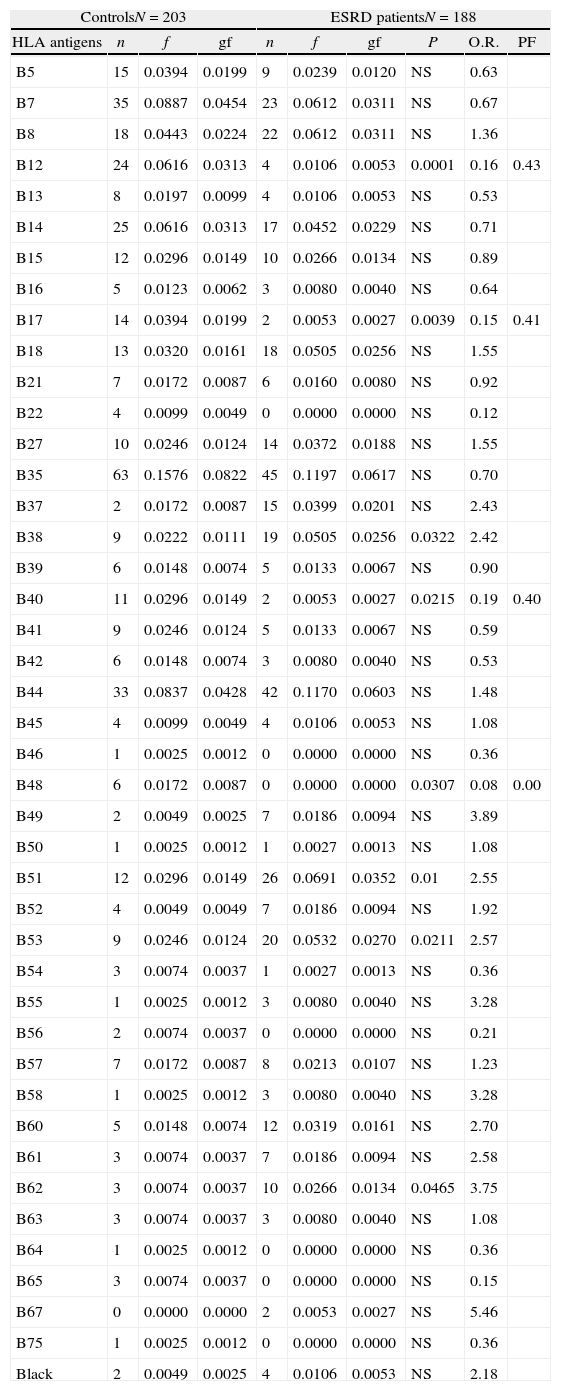
HLA-B38, B51, B53 and B62 antigens were positively associated to the ESRD. The HLA-A9, B12, B17, B40 and B48 antigens showed a negative association to the ESRD (Tables 1 and 2). Only HLA-A9 and HLA-B12 negative association with ESRD remained after Bonferroni correction applied (Table 3).
HLA-A class I antigen frequencies in patients with ESRD and control group of Zulian population.
| Frequencies | |||||||||
| ControlsN=203 | ESRD patientsN=188 | ||||||||
| HLA antigens | n | F | fg | n | F | fg | P | O.R. | PF |
| A1 | 33 | 0.0813 | 0.0415 | 27 | 0.0718 | 0.0366 | NS | 0.9 | |
| A2 | 116 | 0.3005 | 0.1636 | 101 | 0.2793 | 0.1510 | NS | 0.87 | |
| A3 | 25 | 0.0665 | 0.0338 | 31 | 0.0824 | 0.0421 | NS | 1.41 | |
| A9 | 24 | 0.0517 | 0.0262 | 6 | 0.0160 | 0.0080 | 0.0019 | 0.25 | 0.38 |
| A10 | 9 | 0.0222 | 0.0111 | 10 | 0.0266 | 0.0134 | NS | 1.21 | |
| A11 | 27 | 0.0714 | 0.0364 | 19 | 0.0505 | 0.0256 | NS | 0.73 | |
| A19 | 10 | 0.0271 | 0.0136 | 9 | 0.0239 | 0.0120 | NS | 0.97 | |
| A23 | 10 | 0.0246 | 0.0124 | 19 | 0.0505 | 0.0256 | NS | 2.17 | |
| A24 | 44 | 0.1108 | 0.0570 | 42 | 0.1170 | 0.0575 | NS | 1.04 | |
| A25 | 3 | 0.0074 | 0.0037 | 1 | 0.0027 | 0.0013 | NS | 0.36 | |
| A26 | 11 | 0.0271 | 0.0136 | 6 | 0.0160 | 0.0080 | NS | 0.58 | |
| A28 | 25 | 0.0616 | 0.0313 | 18 | 0.0479 | 0.0242 | NS | 0.75 | |
| A29 | 12 | 0.0296 | 0.0149 | 20 | 0.0585 | 0.0297 | NS | 1.89 | |
| A30 | 16 | 0.0419 | 0.0212 | 19 | 0.0505 | 0.0256 | NS | 1.31 | |
| A31 | 7 | 0.0172 | 0.0087 | 15 | 0.0399 | 0.0201 | NS | 2.43 | |
| A32 | 9 | 0.0222 | 0.0111 | 4 | 0.0106 | 0.0053 | NS | 0.47 | |
| A33 | 12 | 0.0296 | 0.0149 | 16 | 0.0426 | 0.0215 | NS | 1.48 | |
| A34 | 1 | 0.0025 | 0.0012 | 0 | 0.0000 | 0.0000 | NS | 0.36 | |
| A36 | 2 | 0.0049 | 0.0025 | 1 | 0.0027 | 0.0013 | NS | 0.54 | |
| A43 | 1 | 0.0025 | 0.0012 | 0 | 0.0000 | 0.0000 | NS | 0.36 | |
| A66 | 1 | 0.0025 | 0.0012 | 2 | 0.0053 | 0.0027 | NS | 2.17 | |
| A68 | 3 | 0.0074 | 0.0037 | 6 | 0.0160 | 0.0080 | NS | 2.20 | |
| A2403 | 1 | 0.0025 | 0.0012 | 2 | 0.0053 | 0.0027 | NS | 2.17 | |
| A74 | 2 | 0.0049 | 0.0025 | 0 | 0.0000 | 0.0000 | NS | 0.21 | |
| A80 | 2 | 0.0049 | 0.0025 | 0 | 0.0000 | 0.0000 | NS | 0.21 | |
| Black | 0 | 0.0000 | 0.0000 | 2 | 0.0053 | 0.0027 | NS | 5.46 | |
n: number of antigens, F: antigen frequencies, gf: gene frequencies, P: probability, NS: no significant, O.R.: Odds ratio, N: number of individuals, ESRD: end stage renal disease, PF: preventive fraction.
HLA-B class I antigen frequencies in patients with ESRD and control group of Zulian population.
| ControlsN=203 | ESRD patientsN=188 | ||||||||
| HLA antigens | n | f | gf | n | f | gf | P | O.R. | PF |
| B5 | 15 | 0.0394 | 0.0199 | 9 | 0.0239 | 0.0120 | NS | 0.63 | |
| B7 | 35 | 0.0887 | 0.0454 | 23 | 0.0612 | 0.0311 | NS | 0.67 | |
| B8 | 18 | 0.0443 | 0.0224 | 22 | 0.0612 | 0.0311 | NS | 1.36 | |
| B12 | 24 | 0.0616 | 0.0313 | 4 | 0.0106 | 0.0053 | 0.0001 | 0.16 | 0.43 |
| B13 | 8 | 0.0197 | 0.0099 | 4 | 0.0106 | 0.0053 | NS | 0.53 | |
| B14 | 25 | 0.0616 | 0.0313 | 17 | 0.0452 | 0.0229 | NS | 0.71 | |
| B15 | 12 | 0.0296 | 0.0149 | 10 | 0.0266 | 0.0134 | NS | 0.89 | |
| B16 | 5 | 0.0123 | 0.0062 | 3 | 0.0080 | 0.0040 | NS | 0.64 | |
| B17 | 14 | 0.0394 | 0.0199 | 2 | 0.0053 | 0.0027 | 0.0039 | 0.15 | 0.41 |
| B18 | 13 | 0.0320 | 0.0161 | 18 | 0.0505 | 0.0256 | NS | 1.55 | |
| B21 | 7 | 0.0172 | 0.0087 | 6 | 0.0160 | 0.0080 | NS | 0.92 | |
| B22 | 4 | 0.0099 | 0.0049 | 0 | 0.0000 | 0.0000 | NS | 0.12 | |
| B27 | 10 | 0.0246 | 0.0124 | 14 | 0.0372 | 0.0188 | NS | 1.55 | |
| B35 | 63 | 0.1576 | 0.0822 | 45 | 0.1197 | 0.0617 | NS | 0.70 | |
| B37 | 2 | 0.0172 | 0.0087 | 15 | 0.0399 | 0.0201 | NS | 2.43 | |
| B38 | 9 | 0.0222 | 0.0111 | 19 | 0.0505 | 0.0256 | 0.0322 | 2.42 | |
| B39 | 6 | 0.0148 | 0.0074 | 5 | 0.0133 | 0.0067 | NS | 0.90 | |
| B40 | 11 | 0.0296 | 0.0149 | 2 | 0.0053 | 0.0027 | 0.0215 | 0.19 | 0.40 |
| B41 | 9 | 0.0246 | 0.0124 | 5 | 0.0133 | 0.0067 | NS | 0.59 | |
| B42 | 6 | 0.0148 | 0.0074 | 3 | 0.0080 | 0.0040 | NS | 0.53 | |
| B44 | 33 | 0.0837 | 0.0428 | 42 | 0.1170 | 0.0603 | NS | 1.48 | |
| B45 | 4 | 0.0099 | 0.0049 | 4 | 0.0106 | 0.0053 | NS | 1.08 | |
| B46 | 1 | 0.0025 | 0.0012 | 0 | 0.0000 | 0.0000 | NS | 0.36 | |
| B48 | 6 | 0.0172 | 0.0087 | 0 | 0.0000 | 0.0000 | 0.0307 | 0.08 | 0.00 |
| B49 | 2 | 0.0049 | 0.0025 | 7 | 0.0186 | 0.0094 | NS | 3.89 | |
| B50 | 1 | 0.0025 | 0.0012 | 1 | 0.0027 | 0.0013 | NS | 1.08 | |
| B51 | 12 | 0.0296 | 0.0149 | 26 | 0.0691 | 0.0352 | 0.01 | 2.55 | |
| B52 | 4 | 0.0049 | 0.0049 | 7 | 0.0186 | 0.0094 | NS | 1.92 | |
| B53 | 9 | 0.0246 | 0.0124 | 20 | 0.0532 | 0.0270 | 0.0211 | 2.57 | |
| B54 | 3 | 0.0074 | 0.0037 | 1 | 0.0027 | 0.0013 | NS | 0.36 | |
| B55 | 1 | 0.0025 | 0.0012 | 3 | 0.0080 | 0.0040 | NS | 3.28 | |
| B56 | 2 | 0.0074 | 0.0037 | 0 | 0.0000 | 0.0000 | NS | 0.21 | |
| B57 | 7 | 0.0172 | 0.0087 | 8 | 0.0213 | 0.0107 | NS | 1.23 | |
| B58 | 1 | 0.0025 | 0.0012 | 3 | 0.0080 | 0.0040 | NS | 3.28 | |
| B60 | 5 | 0.0148 | 0.0074 | 12 | 0.0319 | 0.0161 | NS | 2.70 | |
| B61 | 3 | 0.0074 | 0.0037 | 7 | 0.0186 | 0.0094 | NS | 2.58 | |
| B62 | 3 | 0.0074 | 0.0037 | 10 | 0.0266 | 0.0134 | 0.0465 | 3.75 | |
| B63 | 3 | 0.0074 | 0.0037 | 3 | 0.0080 | 0.0040 | NS | 1.08 | |
| B64 | 1 | 0.0025 | 0.0012 | 0 | 0.0000 | 0.0000 | NS | 0.36 | |
| B65 | 3 | 0.0074 | 0.0037 | 0 | 0.0000 | 0.0000 | NS | 0.15 | |
| B67 | 0 | 0.0000 | 0.0000 | 2 | 0.0053 | 0.0027 | NS | 5.46 | |
| B75 | 1 | 0.0025 | 0.0012 | 0 | 0.0000 | 0.0000 | NS | 0.36 | |
| Black | 2 | 0.0049 | 0.0025 | 4 | 0.0106 | 0.0053 | NS | 2.18 | |
n: number of antigen, f: antigen frequencies, gf: gene frequencies, P: probability, NS: no significant, O.R.: Odds ratio, N: number of individuals, ESRD: end stage renal disease, PF: preventive fraction.
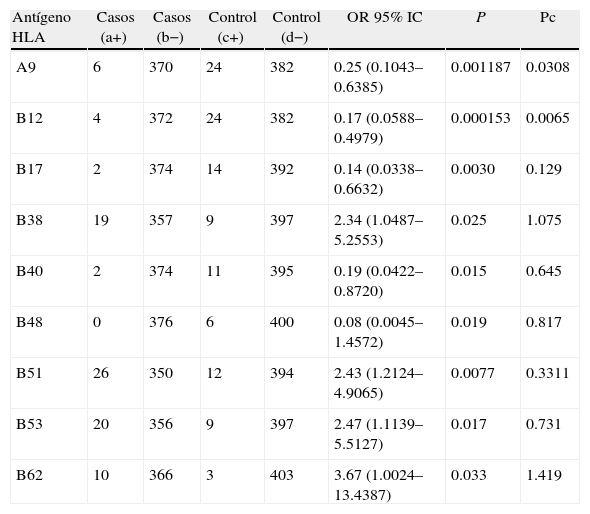
HLA-A9 and HLA-B12 negative association with ESRD remained after Bonferroni correction applied.
| Antígeno HLA | Casos (a+) | Casos (b−) | Control (c+) | Control (d−) | OR 95% IC | P | Pc |
| A9 | 6 | 370 | 24 | 382 | 0.25 (0.1043–0.6385) | 0.001187 | 0.0308 |
| B12 | 4 | 372 | 24 | 382 | 0.17 (0.0588–0.4979) | 0.000153 | 0.0065 |
| B17 | 2 | 374 | 14 | 392 | 0.14 (0.0338–0.6632) | 0.0030 | 0.129 |
| B38 | 19 | 357 | 9 | 397 | 2.34 (1.0487–5.2553) | 0.025 | 1.075 |
| B40 | 2 | 374 | 11 | 395 | 0.19 (0.0422–0.8720) | 0.015 | 0.645 |
| B48 | 0 | 376 | 6 | 400 | 0.08 (0.0045–1.4572) | 0.019 | 0.817 |
| B51 | 26 | 350 | 12 | 394 | 2.43 (1.2124–4.9065) | 0.0077 | 0.3311 |
| B53 | 20 | 356 | 9 | 397 | 2.47 (1.1139–5.5127) | 0.017 | 0.731 |
| B62 | 10 | 366 | 3 | 403 | 3.67 (1.0024–13.4387) | 0.033 | 1.419 |
O.R.: Odds ratio, P: probability, Pc: P Bonferroni corrected.
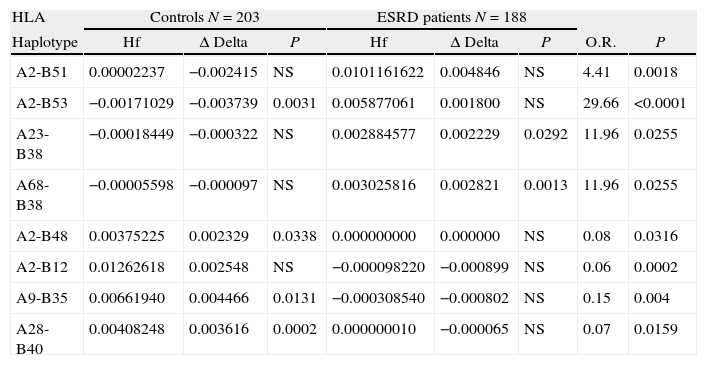
The haplotypes positively associated with ESRD were: A2-B51, A2-B53, A23-B38 and A68-B38. The A2-B48, A2-B12, A9-B35 and A28-B40 haplotypes were negatively associated with ESRD, expressing relative risks lower to 1 (Table 4).
Association of HLA-A-B two loci haplotypes class I in patients with ESRD and control group of the Mestizos Zulian population.
| HLA | Controls N=203 | ESRD patients N=188 | ||||||
| Haplotype | Hf | Δ Delta | P | Hf | Δ Delta | P | O.R. | P |
| A2-B51 | 0.00002237 | −0.002415 | NS | 0.0101161622 | 0.004846 | NS | 4.41 | 0.0018 |
| A2-B53 | −0.00171029 | −0.003739 | 0.0031 | 0.005877061 | 0.001800 | NS | 29.66 | <0.0001 |
| A23-B38 | −0.00018449 | −0.000322 | NS | 0.002884577 | 0.002229 | 0.0292 | 11.96 | 0.0255 |
| A68-B38 | −0.00005598 | −0.000097 | NS | 0.003025816 | 0.002821 | 0.0013 | 11.96 | 0.0255 |
| A2-B48 | 0.00375225 | 0.002329 | 0.0338 | 0.000000000 | 0.000000 | NS | 0.08 | 0.0316 |
| A2-B12 | 0.01262618 | 0.002548 | NS | −0.000098220 | −0.000899 | NS | 0.06 | 0.0002 |
| A9-B35 | 0.00661940 | 0.004466 | 0.0131 | −0.000308540 | −0.000802 | NS | 0.15 | 0.004 |
| A28-B40 | 0.00408248 | 0.003616 | 0.0002 | 0.000000010 | −0.000065 | NS | 0.07 | 0.0159 |
N: number of individuals, Hf: haplotype frequency, P: probability (exact Fischer or Yates corrected testing), O.R.: Odds ratio, ESRD: end-stage renal disease, Δ Delta: linkage disequilibrium.
The range of age of the majority of patients with ESRD of Zulia state was located between 20 and 44 years of age (the median age is 34.3 years), belonging to the most productive labor force segment of the population. The US RENAL DATA SYSTEM (USRDS) has reported that the majority of patients with ESRD have a range of age between 45 and 64 years. The median age of the prevalent ESRD population in USA has increased 3.2 percent since 2000, reaching 59.4 in 2008.19 This age difference between our patients and those reported in USA could be because in our patients, the proliferative glomerulonephritis was the main cause of ESRD, this glomerular injury may be secondary to endemic infections including streptococcal infection, schistosomiasis, etc., frequent infections to younger ages and which have low frequency in developed countries, where it was indicated as the main cause of diabetes and hypertension, diseases characteristic of older age. In Zulia population, the men with ESRD are 1.65 times the number of women, not reflecting significant differences.
HLA-B38, B51, B53 and B62 antigens were positively associated in patients with ESRD in Zulia population while the HLA-A9, B12, B17, B40 and B48 antigens were shown as protective antigens. The HLA-B53 antigen has been reported previously, associated with an increased risk for alloantibodies sensitization and with decreased graft survival in patients with ESRD (PRA response, Panel Reactive Antibodies), while, the HLA-B12 and B40, associated negatively in this study, have been reported associated with a significantly decreased risk for sensitization in these patients.4 However, the presence of anti-HLA alloantibodies was not evaluated in this study.
Since the early 1980s, the USRDS has reported racial and ethnic discrepancies in ESRD. The rates in the African American and Native American populations were 3.6 and 1.8 times greater, respectively, than the rate among whites.19 Perhaps this is due, at least in part, to hypertensive nephrosclerosis, nephropathy secondary to diabetes mellitus types I and II, and chronic glomerulonephritis, occur more frequently in African Americans. In this population, the existence of a first-degree relative with ESRD raises nine times the risk of developing this condition.20 It should be noted that the Venezuelan population is a mixture of native Indigenous with Caucasian immigrants from different regions of Europe and Africans blacks.21 Of positively associated antigens in this study, only HLA-B53 was present in previous studies in healthy Zulian population with a frequency of 2.1%.22 In the town of Curiepe, Miranda state, inhabited mainly by people of Negroid origin, this antigen reported a frequency of 7%,23 meanwhile, was not present in indigenous Wayuu.22 The Black Colombians show equally HLA-B53 high frequency (11.3%),22 and also, in the Cuban population, the HLA-B*5301 allele (10.7%).24 In another research in our laboratory, in the Zulia population, the HLA-B*5301 allele was the unique subtype found (1.82%).25 Apparently, the black and mixed populations with a high compound of the HLA-B53 are most affected regarding the presence HLA genes associated to ESRD, which coincide with high prevalence feature of the disease.
Previous studies identified several HLA supermotifs (HLA-A and B supertype), each of which corresponds to the ability of peptide ligands to bind several different HLA alleles.26,27 HLA-B51, positively associated with ESRD in this study and B*5301, reported in our healthy population, belong to B7-supertype. Equally, B44, negatively associated with ESRD, and B4001, also reported in our healthy population, belong to the B44-supertype. Furthermore B62 and A9 antigens are included in the B62-supertype and A24-supertype, respectively. Nevertheless, definition of HLA-supertypes requires DNA-based typing because some HLA specificities are difficult to type by serological methods. Notwithstanding, it will be possible to identify some peptides associated to progression or protection to ESRD based on HLA-supertyping molecular definition.
Many studies have described association between HLA and a variety of kidney diseases and with ESRD due to a certain disease, but are few studies evaluating the association with progression to ESRD. This HLA association with progression has been studied in other diseases such as diabetes and chronic lymphocytic leukemia.28-30 In the case of the association with progression to ESRD, these studies have shown an increased HLA-A78 and decreased HLA-B14 in Brazilian patients.31 Others have reported increased HLA-A33, -A11 and -B49.32 These findings provide support to the idea that HLA polymorphism may influence susceptibility to renal disease progression. Both studies were carried out without regard to primary renal disease leading to ESRD. Karahan et al. studied the association of HLA with the primary diseases leading to ESRD in Turkish patients reporting a negative association between almost all A locus antigens and chronic glomerulonephritis and hypertensive nephrosclerosis, meanwhile, found a positive association between the HLA-B58 and HLA-DRB1*03 with amyloidosis and diabetic nephropathy, respectively.33 Studies such as these suggest that the determination of HLA may be useful in preventing both the progression to ESRD as the primary disease recurrence post-transplantation.
Finally, the differences in the results published by different authors may be due to the large variability in the frequency of HLA alleles present in different populations or ethnic groups. Other explanation may be linkage disequilibrium between these alleles HLA and other nearby genes involved in regulating the immune response. For example, it has been reported that polymorphism in genes encoding certain cytokines, including IL-6, IL-4 and TNF may be involved in the progression to ESRD.34
This study confirms the existence, in our population, of HLA Class I antigens and/or haplotypes directly associated, with the progression or protection of the ESRD. Finally this could orientate the physician to get a prognosis and treatment. Furthermore, molecular studies will allow for the classification of types, subtypes and/or supertypes of those alleles involved in progression to ESRD.
Conflict of interestThe authors declare no conflict of interest.
We are grateful to Zulay Layrisse for critical review of the manuscript.