Division of labour among cells of the immune system is a prevailing theme in the orchestration of immune responses. Contrary to this paradigm, a particular leukocyte population in the mouse seems to be equipped with the ability to kill transformed and virally infected cells and with the capability to mediate antigen processing and presentation to T cells. Those two functions are classically ascribed separately to Natural Killer (NK) cells and Dendritic cells (DC). IKDC (interferon-producing killer dendritic cell) were defined in mice as cells expressing CD11c, although to a lesser extent than conventional DCs (cDCs), while coexpressing B220, NK1.1, CD49b (VLA-2) and MHC Class II molecules. Absence of CD3, CD19 and Gr1(Ly49c) expression also featured this minor subset upon multicolour FACS characterization. These cells kill a variety tumor cell lines in a TRAIL-dependent fashion. Furthermore, IKDC produce high amounts of Interferon (IFN) gamma (IFN-γ) and type I IFN upon activation by combined interleukins (IL-18, IL-2, IL-12 or IL-15), tumor cells, or TLR-9 nucleotide agonists. Importantly, these cells may present antigen to prime CD4 and CD8 T cells. The discovery of this subset in mice was reported in two back to back articles published in Nature Medicine in 2006, along with a comment that highlighted how such a cell may provide the ability to kill cells in an innate fashion while starting T-cell adaptive immunity by means of cross-presentation of antigens collected from the apoptotic remains of their victim cells. However, two years later three articles in a single issue of the Journal of Experimental Medicine contended that such a population did not exist and that IKDC were actually nothing more than activated NK cells. The arguments were based on the inability of IKDC to present antigen and prime T cells, and on their dependency on IL-15 for differentiation and survival. Later on, the groups that first described IKDC have provided evidence that such a subpopulation can mediate antigen presentation and have also stressed several characteristics that discriminate activated NK cells and IKDC. The reasons for some of these experimental discrepancies are obscure. Yet, the core of the controversy is to establish wether the hybrid abilities proposed for such minor leukocyte population are relevant to physiology, pathology or therapy. To date, no equivalent population has been described in human lymphoid tissues.
La división de tareas entre las células del sistema inmunitario es un denominador común en la organización de las diferentes respuestas que lleva a cabo. Como excepción a este paradigma, una subpoblación leucocitaria de ratones parece estar equipada para matar células transformadas e infectadas con virus y, a la vez, con la capacidad de procesamiento antigénico y presentación a linfocitos T. Estas funciones se adscriben clásicamente de forma separada a linfocitos NK y a células dendríticas. Las IKDC (”Interferon-producing killer dendritic cells”) fueron definidas en ratón como células que expresan en su superficie CD11c con menor intensidad que las células dendríticas convencionales mientras que coexpresan B220, NK1.1, CD49b (VLA-2) y MHC de Clase II. La ausencia de CD3, CD19 y Gr1 (Ly49C) caracteriza también esta subpoblación cuando se estudia mediante citometría de flujo multicolor. Las IKDC ejercen funciones citolíticas mediadas por TRAIL frente a varias líneas celulares tumorales. Tras su activación in vitro con una combinación de citocinas (IL-18, IL-2, IL-12 o IL-15), células tumorales, o agonistas nucleotídicos de TLR-9, estas células producen cantidades elevadas de IFN-γ. De forma característica, las IKDC presentan antígeno y estimulan funcionalmente a linfocitos T CD8 y CD4. El descubrimiento de estas células fue publicado en 2006 en dos artículos aparecidos en el mismo número de “Nature Medicine”, junto con un comentario que subrayaba las capacidades de estas células para matar diferentes dianas celulares y presentar antígenos captados a partir de los restos apoptóticos de sus víctimas. Sin embargo, en 2007 aparecieron tres artículos en un mismo número del “Journal of Experimental Medicine” que rebatían estas conclusiones, proponiendo que las células IKDC no eran sino linfocitos NK activados. Sus argumentos se basaban en la necesidad de IL-15 para su desarrollo y supervivencia, y -sobre todo- en su capacidad para presentar antígeno a los linfocitos T. Más tarde, en 2009, los grupos que propusieron esta nueva estirpe celular han presentado evidencias de que estás células pueden mediar presentación antigénica, y han enfatizado diferencias transcriptómicas y funcionales que discriminan las células NK activadas de las IKDC. Las razones para algunas de las discrepancias experimentales continúan sin resolverse. El centro de la controversia es determinar si estas células minoritarias con funciones aparentemente híbridas desempeñan algún papel importante bien en la fisiología del sistema inmunitario, bien en alguna entidad patológica o en su terapia. Por el momento no se ha descrito la presencia de un tipo celular equivalente en órganos linfoides humanos.
Accumulating experimental evidences coincide in indicating that a bidirectional crosstalk is frequent between DC and NK cells in lymphoid organs(1-4). In vivo microscopy has shown that DCs in lymphoid organs reside in close proximity to NK cells, suggesting their organization in pairs and trios(5). On one hand, NK cells provide means to license DCs for T lymphocyte activation(6). Intriguingly, NK cells can also kill DCs at an immature state upon recognition by the NKp30 receptor(7). DCs have the regulated ability to produce NK cell-activating cytokines such as type I IFN, IL-12(8), IL-15, and IL-2(9). DCs may also express membraneattached ligands for activatory NK receptors. Classical and non-classical MHC molecules are expressed on DCs and provide cognate interactions for NK cell inhibitory receptors of the immunoglobulin and C-type lectin super-families(1).
Therefore, a set-up of cross-regulation is envisaged: (i) NK cells receive homeostatic maintenance by IL-15-IL15Rα trans-presented at the DC surface(10); (ii) an activated cytocydal state in NK cells is induced by DC-derived cytokines; (iii) NK cells practise an active surveillance of DC for signs of productive viral infection possibly in order to eliminate those DC co-opted by the viral intruder; (iv) but instead they activate/mature those DC that are likely to cross-present viral antigens.
The intricacy and diversity of the DC-NK relationship probably comes from ancient evolutionary reasons in the interactions of the immune system with species-specific viral pathogens. Having two different cells equipped with distinct functions in the innate response makes the system a less easy prey for viral strategies deployed to mock the immune response. Both cell types are endowed with molecular sensors to detect biomolecules denoting the presence of pathogens and/or abundant cell death under stressfull conditions. A minor subset of DCs seems specialized in sounding the alarm if viremia takes place by sensing viral nucleic acids(11,12). These cells produce high amounts of type I IFNs that alert and activate NK cells(13). Such cells were termed plasmacytoid DCs (pDC) and regardless of their scanty numbers account for most of the IFN-α/β biosynthesis upon viral infections(12). The ability of these cells to perform as truly professional antigen presenting cells for T lymphocytes in vivo can be observed in vitro and in vivo(14). Interestingly, pDCs are the main mediators of the therapeutic effects of the TLR-7/TLR-8 agonist ointment imiquimod in basal cell carcinoma of the epidermis, apparently because of their direct TRAIL-mediated cytocydal effects rather than because of antigen presentation(15).
If division of labour is a safety backing mechanism of the immune system one wonders why DCs should be endowed with killer functions, or why NK cells should exhibit professional ability to present antigens. Lineage differentiation among leukocytes involves coordinated transcription factors, epigenetic nuclear reprogramming and possibly distinct microRNA profiles. In spite of these apparently stringent mechanisms, some degree of plasticity between subsets is frequently reported(16). To what extent this is truly physiologically relevant for the defence of the organism remains debatable.
DESCRIPTION OF IKDC AND SOME ANTECEDENTSPrevious reports had proposed that certain DCs could mediate spontaneous cytolytic functions on typical NK targets in both humans and rodents(17-21). In rats, a DC that expresses the NKRP-1 receptor were able to actively kill the NK target cell line Yac-1(19). Furthermore, evidence was found that such killer cells were able to phagocytose the apoptotic bodies of the target cells(22). Interestingly, it was known that when exposed to type I interferon or IFN-γ, human DCs develop the ability to kill targets via induction of surface TRAIL(23), a function also induced in undiferentiated monocytes(24). Even the prototypic Langerhans cells develop cytolytic potential when stimulated via CD40 due to induction of FAS-Ligand(25). In mice, the group of Dimatteo(26) had identified in 2005 a population that co-expressed NK-1.1 and CD11c. This population was able to kill and mediate antigen presentation, however the level of cell purity in this study could have permitted contamination of CD11c+ NK cells and be the result of more than one cell type.
In 2006, a couple of papers were published in Nature Medicine(27,28) that proposed a new cell type that had functional properties astride NK cells, cDCs and pDCs. This multitask cell type was very difficult to work with, as its percentage in mouse spleens and lymph nodes is frequently below 1%, near the limit of cell separation credibility.
The group of Drew Pardoll and Frank Housseau(27) set out investigating pDCs and came to a cell that was capable of both NK-type killing of targets and subsequent processing and presentation to T cells. Their quest led them to isolate a distinct population of cells expressing similar levels of CD11c, B220 and MHC-II molecules as pDC, but that were Gr1 (Ly-6C) negative. Such cell co-expressed CD49b, which corresponds to VLA-2, an integrin commonly found on NK cells, and the high molecular weight isoform of CD45, termed B220, for it was originally characterised as a B cell marker. In their hands, none of the CD11chigh cDC expresses CD49b. Under electron microscopy those cells resembled more granular lymphocytes than pDCs. The authors went on to demonstrate that such isolated cells were able of producing IFN-γ and IFN-α upon stimulation with CpG olygonucleotides and that they killed Yac-1 cells. The acronym IKDC was chosen to describe such a population. These authors also correctly reported that these cells were absent in IL-2R,-deficient mice, which are also devoid of NK cells, but where cDC are present.
Most importantly, when these cells were isolated from mice systemically infected with Listeria expressing ovalbumin (OVA) as a model antigen, they productively stimulated CD4 T cells from a transgenic T-cell receptor mouse that recognized an OVA epitope (DO11.10). It is worth mentioning that these authors also observed upregulation of MHC-II on IKDCs and downregulation of NKG2D and its adaptors Dap10 or Dap12 after in vitro activation with CpG, resulting in loss of cytotoxic activity.
The group of Lawrence Zitvogel(28) followed a different path that took them to the same cell phenotype. These authors were working on the immunotherapeutic properties of the tyrosine kinase inhibitor imatinib mesylate (Gleevec) which inhibits c-Kit, Abl and PDGFR-α. This compound promotes antitumor effects mediated by NK-1.1+ cells in vivo when combined to IL-2. Notably, regressing tumors were infiltrated by a novel subset of DCs that secreted high levels of IFN-γ when in contact with tumor cells and killed B16 melanoma cells in a TRAIL-dependent fashion. They termed these cells IFN-γ-producing killer DCs (IKDCs), presumably following a consensus agreement with the group of Pardoll and Housseau. The group of Zitvogel confirmed that these CD11c+B220+ DCs exhibited a unique morphology and co-expressed the NK markers CD49b, NK- 1.1 and NKG2D. IKDCs expanded during therapy with imatinib mesylate plus IL-2, and infiltrated transplanted tumors. Moreover, intratumoral administration of these IKDC inside B16 melanomas produced antitumor effects even if the recipient mice were Rag−/− and therefore devoid of T and B cells.
An intriguing observation was made as follows: "Although IKDCs possess NK markers and exert NK functions, they diverge from canonical NK cells in their developmental origin. Thus, Rag2−/−Il2rγ−/− mice lack NK cells but do possess functional B220+NK-1.1+CD11c+ splenocytes". This point was very surprising giving the fact that such animals cannot respond to IL-15 (critical for the development of NK cells), IL-2 and IL-7 and was in contrast with the need for IL-2R, described by the other group.
Further studies documented that IL-15 activated and induced proliferation of these cells both in vitro(29) and in vivo(30). Supportive ontogeny differences were described in mouse experiments defining the cell fate of myeloid precursors sustaining that IKDC had slightly different progenitors in the bone marrow defined in transplantation experiments(31). They also concluded that the Id-2 transcription factor was crucial for the development of IKDCs and NK cells(31).
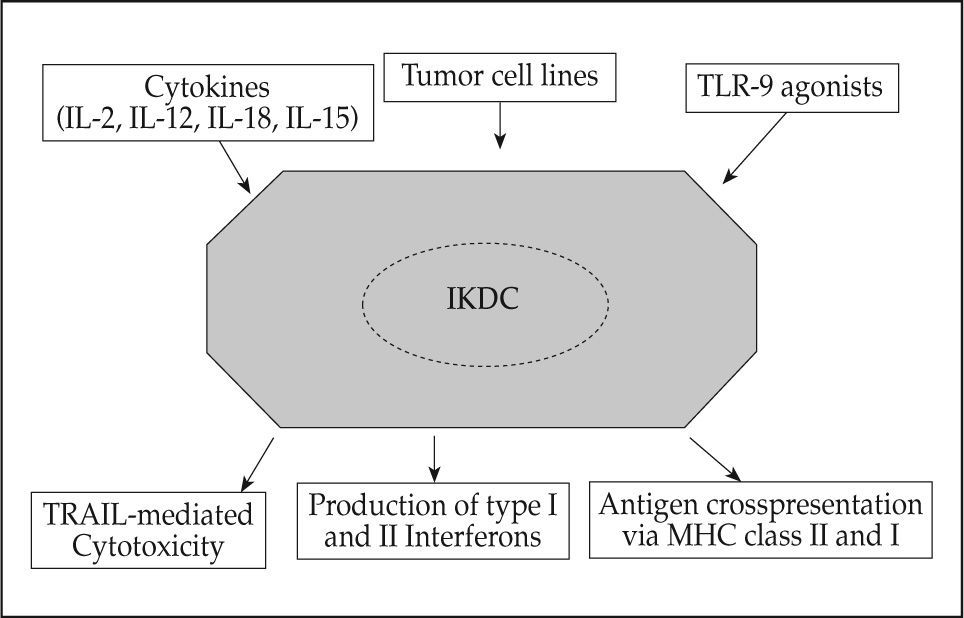
As a summary, Figure 1 portrays IKDC as a cell that constitutively kills in TRAIL dependent fashion and acts as an integrator of innate and adaptive immunity. It was shown to be stimulated by cytokines (IL-2, IL-12, IL-15, IL-18), tumor cells most likely in an NKG2D dependent fashion and once activated these cells produced type I and II IFNs. The remarkable point is that these very cells seemed simultaneously capable of presenting antigen to T cells.
View of IKDC as an integrator of signals that result in different functional capabilities mediated by the very same cell. It should be kept in mind that each of the functional capabilities of IKDC might be differentially regulated. For instance, IL-15 seems to foster cytotoxicity while decreasing antigen crosspresentation.
Two back-to-back papers in Nature Medicine do not pass unnoticed. Exactly one year later a perspective article in Immunity by Hergen Spits and Lewis Lanier(32) reminded that the topic could be very slippery reflecting on what was under the new name of IKDC. Making a comparison on what was known about the phenotypic characteristics of DCs, pDCs, IKDC and NK cells, the authors warned about two points: the MHC-II molecule is expressed on human activated NK cells (as opposed to mouse counterparts) and minute cross-contaminations of DC and NK cells could result in the wrong attribution of hybrid functionalities. Importantly, the presence of such cells in animals unable to respond to IL-15 described by the group of Zitvogel was considered the main point to differentiate IKDC to an activated phenotype of NK cells. Such a finding was eventually contradicted.
In October 2007 three papers were published in the same issue of the Journal of Experimental Medicine that contended that there were no differences between activated NK cells and IKDC.
The group of James di Santo(33) showed that the cytokine requirements for development of NK cells and IKDC matched perfectly well and were unable to find the described phenotype in Rag2−/−Il2rg−/− mice. They also claimed that such cells expressed the NK activation receptor NK46 using knock-in mice, and that the B220 CD45 isoform used as surface marker can be expressed on NK upon activation in vitro and in vivo and correlated with proliferation. However, IL-15-induced in vivo proliferation of purified cells showing that the IKDC phenotype does not automatically result in the acquisition of surface B220 in other hands(34).
The group of Marco Colonna showed experiments that contended several of the previous findings. They confirmed that IL-15 was absolutely required for the development of the putative IKDC, indicated that they were poor producers of type I IFNs in side-by-side comparisons with pDCs. Remarkably, in their hands such cells failed to express MHC-II, and Colonna's group postulated that the subset resembled the CD56brightCD16dim human NK cells that tend to reside in lymphoid organs.
The group of Ken Shortman(35) also provided contending experimental observations. In their hands, IKDC did not activate T cells, or if they did the performance as antigen presenting cells was minimal when compared to pDC and cDC. With these ideas in mind, they checked again that the cytokine requirements for development and survival of IKDC completely matched those of NK cells, including the need of IL-15 and IL-2Rγ. In their hands, IKDC and NK proliferated in the presence of IL-15 with expression of MHC-II in both cases.
At this point it became clear that quite possibly the data on the absence of IKDC requirement for IL-15 was wrong as originally reported by the group of Zitvogel, and that the core of the controversy was if IKDC were able to present antigens from the cells they kill. In addition, it could be perceived that methods were not straightforward and surface markers had the sufficient plasticity to permit a nonambioguous identification and reliable cell separations.
FUNCTIONAL PECULIARITIES OF IKDC THAT MAY CONTRIBUTE TO DEFINE A DISTINCT CELLULAR IDENTITYThe topic was horns racked. The original reporting groups on IKDC got to work, and in August 2009 two papers appeared in "Cancer Research" showing important functional peculiarities to be ascribed to the IKDC phenotype.
The group of Drew Pardoll and Frank Housseau(36) characterized the ability of these cells to mediate antigen cross-presentation. To this end they first showed that IKDC were equipped with the MHC-II presentation pathway at least at the transcriptional level. Using a experimental model of fibroblasts infected with murine cytomegalovirus they went on to show that these cells acquired MHC-II expression upon contact with the infected cells and presented viral antigens to T cells both in vitro and in vivo" An accompanying paper by the group of John Anderson(37) coincided that incubation of IKDC sorted from bone marrow with tumor cells upregulated MHCII molecules and were able to present antigen both via the MHC-I and -II routes from killed cells. Moreover, these authors found that IKDC transferred into RAG–/–IL-2Rγ–/– mice bearing tumors showed a tendency to migrate to the tumor tissue by an undefined homing/chemotactic mechanism.
The group of Zitvogel also presented in the same issue of "Cancer Research" interesting functional experiments using sorted IKDC that expressed the CD11b integrin chain(38). They found that the interaction with tumor cells induced the expression of MHC-II and licensed them to present antigen to CD4+ T cells in vivo. T cell priming was dependent on CD80 and CD86, as evidenced by CTLA-4-Ig blockade of this co-stimulatory pathway. Importantly, cross-presentation of OVA via MHC-I was also documented sorting-out IKDC from mice vaccinated 12 hours before with a fusion protein of OVA and a cholera toxin that favours delivery. Harvested and purified IKDC, if exposed to antigen-specific OT-I cells induced proliferation.
KEY POINTS FOR FUTURE RESEARCH ON THIS TOPICWe believe that it is still early to draw a moral from this complex story. The main point is that a very minor leukocyte population seems to be capable of killing tumor and virusinfected targets and eventually cross-presents their antigens. As a therapeutic tool it could be very useful for example for intratumoral injections. However, the paucity of the cell subset and our lack of technology to differentiate such a cell in vitro precludes its use at the present point of time. In addition, a cell fulfilling killing and cross-presentation ability is yet to be defined among human leukocytes.
Two points can be considered with the available information: First, even the describing groups acknowledge that antigen presentation and killing are reciprocally regulated. For instance, IL-15 upregulates killing but constraints antigen presentation ability. Second, the published wrong observation on the presence of IKDC in animals that do not respond to IL-15 has been the fodder of a controversy that otherwise should have been centered on the ability to kill and present antigen by the very same cell.
All in all, the information on a minor leukocyte population published in a high profile translational research journal created over-expectations. The controversy was mainly ignited by a wrong observation on knock-out strains of mice which were probably mishandled. The difficult experimental conditions working with an oddity population under FACS sorting purifications have probably done the rest to explain the functional discrepancies. Unless the Swiss knife-like multiple uses of this cell type find their way to exploitation in human therapy, in time we will remember this story as too much ado about very little advance.
CONFLICT OF INTERESThe authors declare no financial conflict of interest.